Abstract
Cigarette smoking has long been tied to a multitude of poor health outcomes; however, in reproductive biology, smoking has shown several unintuitive findings. Smoking is associated with significantly decreased rates of endometriosis and endometrial cancer. Here, we show that treatment with cigarette smoke extract leads to increased mRNA and protein expression of homeobox A10 (HOXA10) and progesterone receptor (PGR) as well as more rapid decidualization of endometrial stromal cells in vitro. In vivo, mice exposed to cigarette smoke similarly showed increased expression of HOXA10 and PGR in the endometrium. Both HOXA10 and PGR drive endometrial differentiation and are suppressed in endometrial tumors and in endometriosis. The increased expression found upon exposure to cigarette smoke may provide a protective effect, mediating the decreased incidence of endometrial disease among smokers. This mechanism contrasts with the accepted paradigm that the effects of smoking on the uterus are secondary to ovarian alterations rather than direct effects on endometrium as demonstrated here.
Keywords: endometrium, environment, female reproductive tract, tobacco, uterus
Exposure to cigarette smoke results in increased expression of PGR and HOXA10 in vitro and in vivo; this could explain the decreased prevalence of endometriosis and endometrial cancer among smokers.
INTRODUCTION
Beginning in 1950 with Doll and Hill's groundbreaking study linking cigarette smoking to lung cancer [1], mounting evidence over the past 60 years that smoking is detrimental to human health has become undeniable. Cigarette smoking has been shown to have a multitude of deleterious health effects. Intriguingly, however, women who smoke cigarettes have also been shown to have decreased rates of endometrial cancer [2] and endometriosis [3, 4].
Smoking has well-known negative reproductive consequences. Women who smoke have decreased fertility overall [5]. Smokers have been shown to have decreased oocyte competence during in vitro fertilization [6] and to require nearly double the number of embryo transfer cycles as their nonsmoking peers to become pregnant [7]. Additionally, women who smoke are more likely to have ectopic pregnancies, have higher rates of twins and multiples [8], and are more prone to preterm delivery [5].
Smokers also generally have decreased serum levels of estrogen [9]. Lower estrogen levels may result in decreased endometrial stimulation and potentially impact the development of both endometriosis and endometrial cancer, because both diseases are estrogen-dependent. Similarly, progesterone is a steroid hormone that counteracts the proliferative effects of estrogens in the uterus. Progesterone acts through progesterone receptor (PGR), and both are essential for implantation and are necessary for endometrial differentiation. Changes in the levels of sex steroids, their receptors, and other downstream genes that mediate their action may influence normal endometrial development as well as endometrial proliferative diseases, such as endometriosis and endometrial cancer.
Homeobox A10 (HOXA10) is a member of the HOXA gene cluster and encodes a DNA-binding transcription factor that is essential for tissue differentiation and development of the genitourinary tract [10]. HOXA10 expression is necessary for development in all animal species; however, HOXA10 is also expressed in adults and is essential for normal fertility [11, 12]. HOXA10 expression in the endometrium is driven by sex steroids, and its expression peaks in the midluteal phase as a result of progesterone expression. HOXA10 is a well-characterized marker of endometrial receptivity. Deficiency in expression of HOXA10 has been linked to endometriosis [13] and endometrial cancer [14].
In the present study, we demonstrate that mice exposed to cigarette smoke have increased endometrial HOXA10 and PGR expression. We confirm a direct effect of cigarette smoke extract (CSE) on the expression of these genes in endometrial stromal cells in vitro. Furthermore, we demonstrate an altered rate of decidualization in CSE-exposed endometrial stromal cells. Cigarette smoke may affect endometrium not as a secondary consequence of ovarian damage but, rather, through a direct effect on endometrial differentiation that may lead to the decreased risk of endometriosis and endometrial cancer seen in smokers.
MATERIALS AND METHODS
Cell Culture
Human endometrial stromal cells (HESCs) are a telomerase immortalized endometrial cell line (gift of Dr. Charles Lockwood, Yale University School of Medicine, New Haven, CT) that were maintained in Dulbecco modified Eagle medium (high-glucose) medium containing 10% fetal bovine serum and 1% 100× antibiotic-antimycotic (Invitrogen). At 80% confluence, cells were trypsinized and plated in 6-cm plates. Nearly confluent cells were treated for 24 or 48 h in medium with 0.2 mg/ml of CSE (Arista Laboratories, Inc.) or with vehicle alone (dimethyl sulfoxide [DMSO]). CSE is manufactured by combusting cigarettes without filters, with the smoke bubbled through serum-free media; the resulting suspension is filtered (pore size, 0.20 μm) to remove bacteria and large particles. Culture media were changed daily.
To study the decidualization of HESCs, cell cultures were treated with either DMSO vehicle (mock), 0.5 mM 8-Bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP; Sigma-Aldrich) and 0.1 μM progesterone (8-Br-cAMP/P4; progesterone from Sigma), or with 0.5 mM 8-Br-cAMP and 0.1 μM progesterone plus 0.1 μg/μl of CSE (8-Br-cAMP/P4/CSE). Media were changed every 48 h, and cultures were evaluated for the extent of decidualization via light microscopy daily. Additionally, real-time RT-PCR was performed to evaluate prolactin mRNA expression as a marker of decidualization.
Immunoblotting
For analysis of HOXA10 and PGR proteins, HESCs were lysed using a nuclear extract kit (Active Motif) according to the manufacturer's instructions. Nuclear extract samples were mixed 1:1 with a loading buffer containing SDS, denatured for 5 min at 95°C, and run on a 4–20% polyacrylamide gel (Bio-Rad). The proteins were then transferred to a nitrocellulose membrane. The membrane was washed and blocked for 1 h in 5% milk in phosphate-buffered saline with Tween 20 (PBST). The primary antibodies polyclonal HOXA10 (N-20) or rabbit polyclonal PGR (C-19; both from Santa Cruz Biotechnology) were used at a dilution of 1:200, and rabbit polyclonal glyceraldehyde phosphate dehydrogenase (GAPDH) antibody (Cell Signaling) was used at a dilution of 1:1000, with all incubated overnight at 4°C. The membranes were then washed and blotted with an anti-rabbit horseradish peroxidase antibody (Vector Laboratories) for 1 h at room temperature. The membranes were further washed, and then chemiluminescent signal was developed using Western Lightning Enhanced Chemiluminescent Substrate (PerkinElmer). The membranes were then exposed to x-ray film and developed. Band densitometry was performed using Bio-Rad Quantity One 1-D Analysis Software. The densities for HOXA10 and PGR bands were normalized with the GAPDH bands.
Total RNA Isolation and Quantitative Real-Time RT-PCR
Total RNA from all cell cultures was purified using the Qiagen RNeasy Plus Mini kit according to the manufacturer's instructions. In all, 200 ng of purified RNA were converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad). Real-time PCR was performed using the LightCycler SYBR Green RT-PCR kit from Roche. PCR for HOXA10 and PGR was performed for 40 cycles of 95°C for 15 sec, 58.7°C for 20 sec, and 72°C for 25 sec. Primers used were as follows: HOXA10: sense, 5′-AGGTGGACGCTGCGGCTAATCTCTA-3′; antisense, 5′-GCCCCTTCCGAGAGCAGCAAAG-3′; PGR: sense, 5′-TGGAAGAAATGACTGCATCG-3′; antisense, 5′-TAGGGCTTGGCTTTCATTTG-3′; prolactin: sense, 5′-CATCAACAGCTGCCACACTT-3′; antisense, 5′-CGTTTGGTTTGCTCCTCAAT-3′; β-actin: sense, 5′-CGTACCACTGGCATCGTGAT-3′; antisense, 5′-GTGTTGGCGTACAGGTCTTTG-3′.
Animals
C57BL/6 male and female mice were obtained from Charles River Laboratories. Twenty females were divided into two groups: Ten were exposed to room air, and 10 were exposed to smoke from nonfiltered research cigarettes using a smoking apparatus [15] for 8 wk at one cigarette per session, three sessions per day, 7 days per week.
Vaginal smear cytology was used to determine the estrous-cycle phases before and after exposure to cigarette smoke or room air. Twenty mice in estrus were chosen for this experiment. After exposure to cigarette smoke or room air, these mice were again tested for estrous-cycle phase. Mice were grouped by phase of estrous cycle and killed, and uterine tissue was collected for immunohistochemical analysis. All experiments were approved by the Yale University Institutional Animal Care and Use Committee.
Immunohistochemistry
Immunohistochemical analysis of HOXA10 and PGR expression was performed. Uterine horns obtained from mice were fixed in formalin, embedded in paraffin, sectioned (thickness, 5 μm), and mounted onto glass slides. Slides were deparaffinized and rehydrated through a series of xylene and ethanol washes. Antigen retrieval was performed by steaming in 0.01 M sodium citrate buffer for 20 min, followed by a 20-min cooling period. Then, the slides underwent three times 5-min washes in PBS. Endogenous peroxidases were quenched with 3% hydrogen peroxide for 10 min, followed by three 5-min PBS washes. Slides were circumscribed with a hydrophobic pen, and nonspecific binding was blocked with 5% normal horse serum or 5% goat serum in PBST for 1 h at room temperature. Slides were incubated in the primary antibody overnight at 4°C. Normal goat immunoglobulin G (Santa Cruz Biotechnology) was used as a negative control. Horse anti-goat or goat anti-rabbit biotinylated secondary antibody (Vector Laboratories) was applied for 30 min at room temperature. After washing in PBS, the slides were incubated in ABC Elite (Vector Laboratories) for 15 min at room temperature, then washed and incubated for 3 min in diaminobenzidine (Vector Laboratories). A 16-sec exposure to hematoxylin was used as a counterstain. Slides were rehydrated through 5-min ethanol and 20-min xylene washes and mounted with Permount. All slides were processed simultaneously. The mice were grouped by estrous-cycle phase at the time of euthanization, and slides were compared only against tissue in the same estrous-phase group. The in vivo expression of HOXA10 and PGR protein by immunohistochemistry was quantified using the H-scoring method by two independent evaluators blinded to the treatment group [16, 17]. Intensity of nuclear staining was indicated by a value of 1, 2, or 3 (weak, moderate, or strong, respectively).
Statistical Analysis
The data obtained from the real-time quantitative RT-PCR were evaluated using the 2−ΔΔCt method [18] and analyzed by Student t-test. H-score data was compared using the Mann-Whitney U-test.
RESULTS
Cigarette Smoke Increased HOXA10 Expression
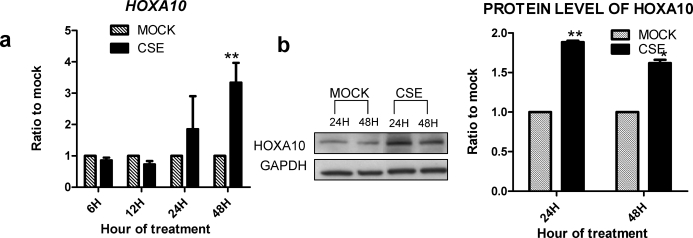
To determine if cigarette smoke had a direct effect on markers of endometrial cell differentiation, HESCs were treated with CSE or vehicle control. HESCs are a telomerase immortalized benign endometrial stromal cell line [19]. As shown in Figure 1, quantitative real-time PCR revealed a 3.2-fold increase in HOXA10 mRNA expression in CSE-treated HESCs compared vehicle-treated control cells after 48 h (P < 0.005). Likewise, protein levels of HOXA10 were 1.8-fold increased in CSE-treated HESCs compared to vehicle-treated control cells as determined by Western blot analysis (P < 0.05).
FIG. 1.
CSE enhances HOXA10 expression in HESCs. Cells were treated with 0.2 mg/ml of CSE for 24 or 48 h. a) mRNA levels of HOXA10 were measured by quantitative real-time PCR and expressed as the ratio of CSE-treated to vehicle control (mock) using the 2−ΔΔCt method [16]. b) Protein was detected using specific antibodies by Western blot analysis and normalized with GAPDH. HOXA10 mRNA and protein expression was increased by CSE within 24 h. *P < 0.05, **P < 0.005 (Student t-test).
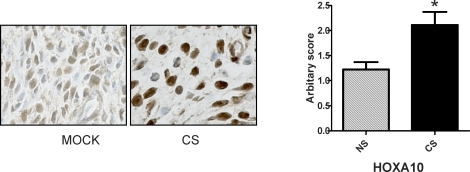
To confirm that the effects observed in vitro also occurred in mice, we exposed them to cigarette smoke daily for 8 wk. Those mice exposed to cigarette smoke similarly showed an increase in endometrial HOXA10 expression compared to those exposed to normal room air only. Immunohistochemical staining for HOXA10 in the uterine stroma showed a marked increase in staining versus the control (Fig. 2). H-scoring showed HOXA10 staining was 1.8-fold higher in cigarette smoke-exposed mice compared to controls (P < 0.05).
FIG. 2.
Cigarette smoke enhances HOXA10 expression in the mouse uterus. Mice were exposed to cigarette smoke (CS) or room air (mock) for 8 wk as described in Materials and Methods. HOXA10 expression in the uterus was detected by immunohistochemistry (left) in the nuclei of endometrial stromal cells. Semiquantitative H-scores (right) demonstrated a nearly 70% increase in the CS-exposed mice compared to those exposed to room air (NS) (n = 9 per group). *P < 0.05 (Student t-test).
Cigarette Smoke Increased PGR Expression
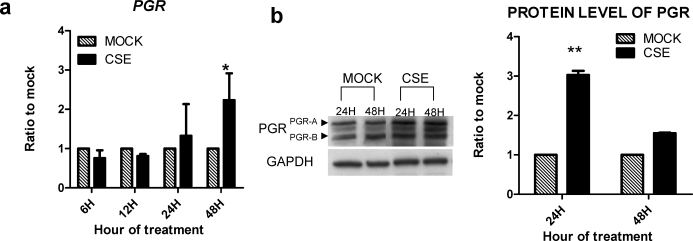
The PGR mediates the antiproliferative and differentiating effects of progesterone on endometrial stroma. Quantitative real-time PCR on HESCs showed a 2.5-fold increase in mRNA expression of PGR in CSE-treated cells versus vehicle-treated control cells at 48 h (P < 0.05) (Fig. 3). Protein levels of PGR were also shown to be 3.1-fold increased versus control as determined by Western blot analysis (P < 0.005).
FIG. 3.
CSE treatment of HESCs increases PGR expression. Cells were treated with 0.2 mg/ml of CSE for 24 or 48 h. a) The mRNA levels of PGR were measured by quantitative real-time PCR and expressed as the ratio of CSE-treated to vehicle control (mock) using 2−ΔΔCt method [16]. b) Protein was detected using specific antibodies by Western blot analysis and normalized with GAPDH. The PGR mRNA and protein expression were increased by CSE exposure within 24 h. *P < 0.05, **P < 0.005 (Student t-test).
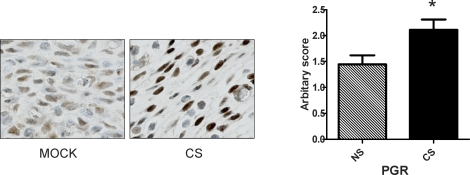
A similar increase in PGR expression was demonstrated in vivo using mice exposed to cigarette smoke versus controls exposed to room air only. As shown in Figure 4, uterine stromal cells in cigarette smoke-treated mice showed a marked increase in staining for PGR versus controls as demonstrated by immunohistochemistry. H-scoring showed PGR staining was 1.5-fold higher in cigarette smoke-exposed mice compared to controls (P < 0.05).
FIG. 4.
Cigarette smoke enhances PGR expression in the mouse uterus. Mice were exposed to cigarette smoke (CS) or room air (mock) for 8 wk. PGR expression in the uterus was detected by immunohistochemistry and H-scored. Semiquantitative H-scores (right) demonstrated a nearly 50% increase in the CS-exposed mice compared to those exposed to room air (NS) (n = 9 per group). *P < 0.05 (Student t-test).
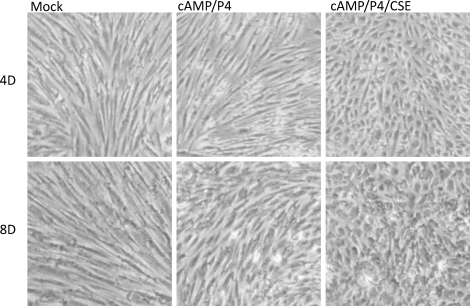
CSE Enhanced Decidualization of HESCs
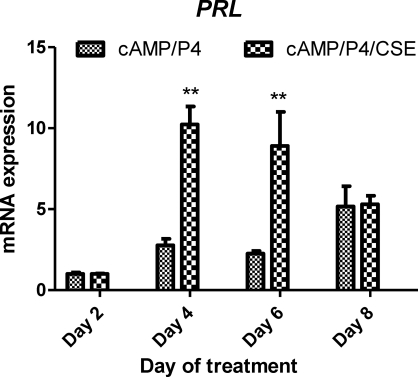
Because CSE increased expression of HOXA10 and PGR, which typically promotes endometrial stromal cell differentiation, we next examined whether CSE treatment could lead to altered decidualization of HESCs. As shown in Figure 5, in the absence of CSE, 8-br-cAMP/P4 induced significant morphological changes in HESCs by Day 8. However, in the presence CSE, HESC decidualization was obvious as early as Day 4. These data indicate that CSE significantly enhances the rate of HESC differentiation. Corresponding to these morphological changes, prolactin mRNA expression was measured as a marker of decidualization in 8-br-cAMP/P4-treated HESCs, as shown in Figure 6. Prolactin mRNA levels were similar on Day 2 with or without the addition of CSE to culture media. On Days 4 and 6, the 8-br-cAMP/P4/CSE-treated cells expressed significantly more prolactin mRNA compared to those cells treated with 8-br-cAMP/P4 alone. By Day 8, prolactin mRNA expression was similar between CSE and non-CSE-treated cells. Together, these results demonstrate more rapid decidulization in the presence of CSE; however, both reach similar states of decidualization after 8 days of treatment.
FIG. 5.
CSE enhances decidualization of endometrial stromal cells (HESCs). HESCs were cultured in the presence of either vehicle control alone (mock), 8-Br-cAMP/P4, or 8-Br-cAMP/P4/CSE for 8 days. The medium was changed every 48 h. The micrographs were acquired using a light microscope with magnification of ×40. Results are representative of three independent experiments. No decidualization is seen in the vehicle-treated control cells (mock). Decidualization begins at 8 days (8D) in the 8-Br-cAMP/P4-treated cells but is obvious by Day 4 (4D) with the addition of CSE.
FIG. 6.
CSE enhances prolactin (PRL) mRNA expression. HESCs were cultured in the presence of 8-Br-cAMP/ P4 or 8-Br-cAMP/P4/CSE for 2–8 days. The medium was changed every 48 h. PRL mRNA was quantified by quantitative PCR and normalized against beta-actin. Results shown are fold-induction relative to Day 2 (n = 3). **P < 0.005 (Student t-test, CSE-treated vs. non-CSE-treated at same time point).
DISCUSSION
In uterine endometrium, estrogen induces proliferation without differentiation. Progesterone, through its activation of PGR, inhibits the action of estrogen, preventing proliferation and triggering differentiation. Similarly, HOXA10 induces endometrial differentiation. In both endometrial cancer and endometriosis, expression of HOXA10 and PGR is reduced. Thus, the increase in expression of these two genes and the more rapid decidualization in response to exposure to cigarette smoke may provide the protective effects that prevent the development of these proliferative diseases in women who smoke.
One commonly accepted theory for the reduced incidence of endometrial cancer among smokers is that smoking causes ovarian damage, diminished ovarian reserve, and decreased estrogen production. Endogenous or exogenous estrogens unopposed by progesterone can increase the mitotic activity in endometrial cells [20], leading to hyperplasia and malignancy [21]. However, even lower-than-average levels of estrogen can cause hyperplasia or cancer as seen in women with chronic anovulation and low but static estrogen levels. Similarly, menopausal women treated with estrogen in the absence of a progestin have a high incidence of endometrial hyperplasia despite estrogen exposure well below that of a reproductive-age woman [22]. It is unlikely that the small decline in estrogen production seen in reproductive-age smokers will prevent hyperplasia. Treatment with exogenous progesterone has been shown to have protective effects against development of endometrial cancer, as seen in the decreased incidence of endometrial cancer among users of combined estrogen and progestin oral contraceptives [23]. An increase in the expression of PGR in the endometrium of smokers may provide a protective effect against neoplasm by creating increased progesterone sensitivity and responsiveness. We speculate that increased PGR may also enhance endometrial development and explain the high pregnancy rate among young smokers before the onset of diminished ovarian reserve.
Likewise, lower HOXA10 expression has been correlated with increased tumor grade in endometrial carcinomas [24]. Moreover, methylation of the HOXA10 promoter has been found to lead to decreased expression and is associated with endometrial tumors [14]. Thus, the increased HOXA10 expression in the uterus upon exposure to cigarette smoke could confer a protective effect against the development of endometrial cancer, opposing the downregulation of this protein because of methylation.
Women with endometriosis have been shown to have decreased levels of HOXA10 in their ectopic and eutopic endometrium [13, 25]. Downregulation of HOXA10 may be required to decrease differentiation and allow the increased proliferation necessary for the development of endometriosis. As in endometrial cancer, hypermethylation of the gene is associated with diminished differentiation of endometrial cells, leading to endometriosis [26–28]. Cigarette smoke-induced increases in uterine HOXA10 expression could counter the effect of methylation on the HOXA10 promoter and provide a protective effect against the development of endometriosis in women who smoke. HOXA10 drives increased differentiation and also preserves endometrial receptivity; the increase may impede the development of endometriosis while still allowing embryo implantation.
Expression of PGR is also decreased in women with endometriosis. Specifically women with endometriosis have decreased expression of PGR-B, one of two isoforms of PGR, in both the ectopic and the eutopic endometrium [29]. Progesterone resistance is a prominent feature of this disease [30–32]. The increased PGR driven by tobacco use may similarly increase progesterone action and protect against the development of endometriosis.
Although cigarette smoke appears to provide protection against endometriosis and endometrial cancer, its numerous other deleterious effects found both in the reproductive system and elsewhere certainly prohibit the recommendation that any woman smoke. Instead, the present study seeks to establish the molecular mechanism explaining the seemingly paradoxical observation that smoking decreases the incidence of these two diseases. Perhaps among the over 4000 compounds found in cigarette smoke is one or more that can prevent endometrial proliferation and induce the expression of genes that direct endometrial differentiation. Isolation and characterization of these compounds may lead to the development of novel therapeutic agents.
ACKNOWLEDGMENT
We thank Dr. Min-Jong Kang for his excellent technical advice and assistance and for use of the smoking apparatus.
Footnotes
Supported by NIH U54 HD052668 and R01 ES010610.
These authors contributed equally to this work.
REFERENCES
- Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J 1950; 2: 739 748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Yang L, Sun Q, Cong R, Gu H, Tang N, Zhu H, Wang B. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. Am J Med 2008; 121: 501 508 [DOI] [PubMed] [Google Scholar]
- Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci 2002; 955: 11 22 [discussion 34, 36, 396 406 ]. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 1986; 255: 1904 1908 [PubMed] [Google Scholar]
- Anderson K, Nisenblat V, Norman R. Lifestyle factors in people seeking infertility treatment—a review. Aust N Z J Obstet Gynaecol 2010; 50: 8 20 [DOI] [PubMed] [Google Scholar]
- Gruber I, Just A, Birner M, Losch A. Effect of a woman's smoking status on oocyte, zygote, and day 3 pre-embryo quality in in vitro fertilization and embryo transfer program. Fertil Steril 2008; 90: 1249 1252 [DOI] [PubMed] [Google Scholar]
- Feichtinger W, Papalambrou K, Poehl M, Krischker U, Neumann K. Smoking and in vitro fertilization: a meta-analysis. J Assist Reprod Genet 1997; 14: 596 599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares SR, Simon C, Remohi J, Pellicer A. Cigarette smoking affects uterine receptiveness. Hum Reprod 2007; 22: 543 547 [DOI] [PubMed] [Google Scholar]
- Van Voorhis BJ, Dawson JD, Stovall DW, Sparks AE, Syrop CH. The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet Gynecol 1996; 88: 785 791 [DOI] [PubMed] [Google Scholar]
- Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod 1997; 57: 1338 1345 [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 1998; 101: 1379 1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot CN, Troy PJ, Taylor HS. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther 2000; 7: 1378 1384 [DOI] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 1999; 14: 1328 1331 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Broaddus R, Cheng W, Xie S, Naora H. Deregulation of the HOXA10 homeobox gene in endometrial carcinoma: role in epithelial-mesenchymal transition. Cancer Res 2006; 66: 889 897 [DOI] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997; 277: 2002 2004 [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab 1994; 79: 643 649 [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL, Ricke EA, Piva M, Horowitz GM. Differential expression and localization of de novo synthesized endometriotic haptoglobin in endometrium and endometriotic lesions. Hum Reprod 2000; 15: 2180 2185 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001; 25: 402 408 [DOI] [PubMed] [Google Scholar]
- Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology 2004; 145: 2291 2296 [DOI] [PubMed] [Google Scholar]
- Key TJ, Pike MC. The dose-effect relationship between ‘unopposed' estrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 1988; 57: 205 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmedkhanov A, Zeleniuch-Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspectives. Ann N Y Acad Sci 2001; 943: 296 315 [DOI] [PubMed] [Google Scholar]
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two-year substudy results. Fertil Steril 2003; 80: 1234 1240 [DOI] [PubMed] [Google Scholar]
- Sherif K. Benefits and risks of oral contraceptives. Am J Obstet Gynecol 1999; 180: S343 S348 [DOI] [PubMed] [Google Scholar]
- Lane DB, Rutherford TJ, Taylor HS. HOXA10 expression in endometrial adenocarcinoma. Tumour Biol 2004; 25: 264 269 [DOI] [PubMed] [Google Scholar]
- Browne H, Taylor H. HOXA10 expression in ectopic endometrial tissue. Fertil Steril 2006; 85: 1386 1390 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod 2007; 13: 323 332 [DOI] [PubMed] [Google Scholar]
- Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod 2009; 80: 79 85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol 2005; 193: 371 380 [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006; 1: 106 111 [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med 2010; 28: 75 80 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X, Lin Z, Imir G, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med 2010; 28: 36 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Pavone ME, Yin P, Imir G, Utsunomiya H, Thung S, Xue Q, Marsh EE, Tokunaga H, Ishikawa H, Kurita T, et al. 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin Reprod Med 2010; 28: 44 50 [DOI] [PMC free article] [PubMed] [Google Scholar]