Abstract
Hematopoietic stem cell (HSC) transplantation is a potentially curative treatment for numerous hematologic malignancies. The transplant procedure as performed today takes advantage of HSC trafficking; either egress of HSC from the bone marrow to the peripheral blood, i.e. mobilization, for acquisition of the hematopoietic graft, and/or trafficking of HSC from the peripheral blood to bone marrow niches in the recipient patient, i.e. HSC homing. Numerous studies, many of which are reviewed herein, have defined hematopoietic regulatory mechanisms mediated by the 20-carbon lipid family of eicosanoids, and recent evidence strongly supports a role for eicosanoids in regulation of hematopoietic trafficking, adding a new role whereby eicosanoids regulate hematopoiesis. Short-term exposure of HSC to the eicosanoid prostaglandin E2 (PGE2) increases CXCR4 receptor expression, migration and in vivo homing of HSC. In contrast, cannabinoids reduce hematopoietic progenitor cell (HPC) CXCR4 expression and induce HPC mobilization when administered in vivo. Leukotrienes have been shown to alter CD34+ cell adhesion, migration, and regulate HSC proliferation, suggesting that eicosanoids have both opposing and complimentary roles in the regulation of hematopoiesis. Since numerous FDA approved compounds regulate eicosanoid signaling or biosynthesis, the utility of eicosanoid based therapeutic strategies to improve hematopoietic transplantation can be rapidly evaluated.
Keywords: Eicosanoid, homing, mobilization, stem cell, prostaglandin, cannabinoid, leukotriene
Introduction
Stem cell migration is a common feature of hematopoiesis, occurring both during development and throughout life. Hematopoietic stem (HSC) and progenitor (HPC) cells continuously traffic to and from their bone marrow niche (1), and the egress of hematopoietic cells from bone marrow into the periphery can be facilitated by various agents, such as cytokines or chemotherapy, a process termed mobilization (2, 3). Circulating HSCs can home to the bone marrow and lodge within specific microenvironmental niches that support their survival, self-renewal and differentiation, and only HSC homing to this bone marrow niche are able to sustain hematopoiesis long-term (4, 5). These trafficking processes form the basis of modern hematopoietic transplantation, which is routinely used to treat patients with leukemias, cancer, and hematologic and genetic diseases. Sources of HSC for transplant include bone marrow, mobilized peripheral blood (MPB) and umbilical cord blood (UCB). While bone marrow was the traditional source of HSC for transplantation, MPB has become the preferred source for hematopoietic grafts (6, 7), and the use of UCB for transplants is steadily increasing (8). Hematopoietic reconstitution after transplantation is a multi-step process, but with some sources of HSCs, efficacy is limited by inadequate HSC number, inability to migrate/home to marrow niches, and poor engrafting efficiency and self-renewal (9–11).
Considerable research, much of which is discussed in this issue of Leukemia, has focused on pathways governing HSC trafficking, with the goal of understanding and translating these findings to improve hematopoietic transplantation. Research strategies designed to increase HSC mobilization from bone marrow to peripheral blood in order to obtain an enhanced MPB product, or to enhance homing of HSC into the recipient bone marrow for enhanced hematopoietic engraftment are being evaluated in pre-clinical and clinical models. While cytokines, chemokine/chemokine receptor interactions and adhesion molecules have been the major targets of attention for improvement of HSC mobilization and homing, recent evidence has begun to elucidate the role of bioactive lipids as regulators of HSC migration. In particular, an emerging role for the 20-carbon lipid eicosanoids in hematopoietic trafficking suggests new avenues and therapeutic strategies for clinical improvements in hematopoietic transplantation. Here we review what is known about eicosanoids and regulation of hematopoiesis and hematopoietic cell trafficking, present novel effects of eicosanoids on cell trafficking, and discuss potential strategies for improvement in hematopoietic stem cell transplantation based on modulation of eicosanoid signaling and/or synthesis.
Eicosanoids
The eicosanoid family of lipids include the prostaglandins along with prostacyclins and thromboxanes, leukotrienes and endocannabinoids, most of which are formed by oxidation of 20-carbon essential fatty acids. Eicosanoids affect all organs, tissues, and cells (12). Prostaglandin E2 (PGE2) is the primary metabolite of arachidonic acid and the most abundant eicosanoid. PGE2 is a known mediator of cancer, fever, inflammation, atherosclerosis, blood pressure and strokes, ovulation and numerous other physiological systems (reviewed in (13, 14).
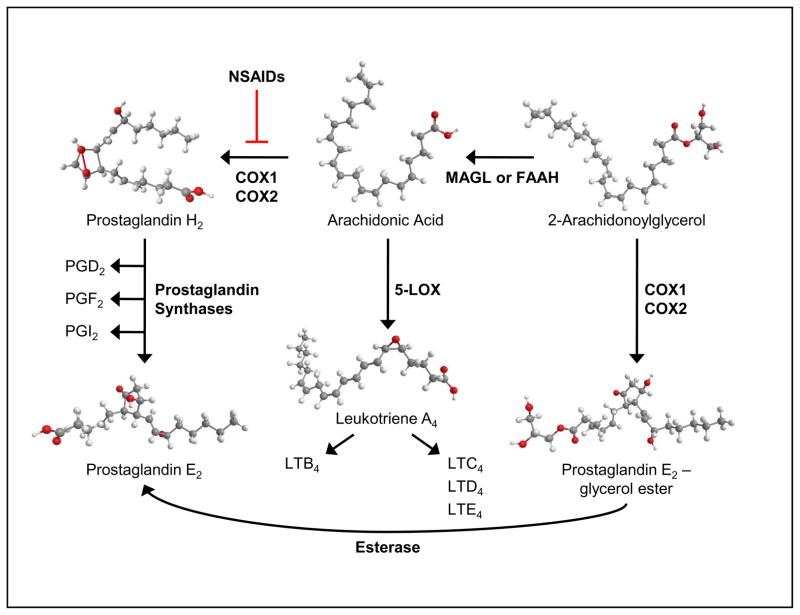
All nucleated cells can synthesize prostaglandins (14), which occurs in 3 steps: cleavage of arachidonic acid from phospholipids by phospholipase A2; oxidation by the cyclooxygenase enzymes (COX1 and COX2) forming the unstable intermediate PGG2, with subsequent reduction to form PGH2; and isomerization to mature prostaglandins by specific synthases (15–17) (Figure 1). Non-steroidal anti-inflammatory drugs (NSAIDs) have been developed that inhibit COX enzymes with selective specificities for COX1 and COX2 (18–20) and their primary therapeutic effect is due to reduced PGE2 biosynthesis. Based upon its chemical/metabolic instability, PGE2 is thought to act locally in autocrine or paracrine fashion (21). Within the bone marrow microenvironment, osteoblasts are a major source of PGE2 (22–24) and due to their physical proximity to hematopoietic cells in the niche, are likely a primary source of PGE2 for paracrine regulation of stem and progenitor function. Macrophages and monocytes also possess strong PGE2 biosynthetic capacity and we have previously shown that monocyte/macrophage-derived PGE2 plays a physiological role in hematopoiesis (25–27).
Figure 1. Schematic of eicosanoid biosynthesis.
Activation of c-phospholipase A2 releases arachidonic acid from phospholipids. Cyclooxygenase (COX) enzymes, which can be inhibited by non-steroidal anti-inflammatory drugs (NSAIDs), convert arachidonic acid into the Prostaglandin H2 intermediate, which is then converted to the various prostaglandins by distinct prostaglandin synthases . Similarly, 5- lipoxygenase (5-LOX) converts arachidonic acid into Leukotriene A4 (LTA4) and then synthesis proceeds to the various leukotrienes. Endocannabinoid (2-Arachidonoylglycercol) is catabolized by fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL) and can be converted into alternate prostaglandin forms.
The leukotrienes are biosynthesized by oxygenation of arachidonic acid by 5’-Lipoxygenase (LOX) and conversion to the unstable intermediate leukotriene A4 (LTA4), which is enzymatically hydrolyzed to LTB4 or conjugated to glutathione forming the cysteinyl leukotriene LTC4. Leukotriene C4 is subsequently converted to LTD4 and LTE4 (Figure 1). Leukotriene formation occurs predominantly in inflammatory cells, including granulocytes, mast cells and macrophages, dendritic cells, and B lymphocytes (28), however 12’-LOX in platelets can convert LTA4 produced by granulocytes to LTB4 and lipoxins (29). LTB4 is produced at sites of inflammation and stimulates inflammatory leukocyte function (30, 31). Like prostaglandins, the leukotrienes have a short half-life and are primarily involved in localized signaling.
The two main endocannabinoids, anandamide and 2-arachidonoyl glycerol (2-AG), are derivatives of arachidonic acid and are synthesized on demand (32–34). However, since they are structurally similar to arachidonic acid, they are also substrates for COX enzymes (35–37), resulting in alternative prostaglandins, with similar and novel effects (35, 38). In addition, alternative prostaglandins can be readily metabolized into traditional prostaglandins via esterases or by dehydration (38). Endocannabinoids can also be metabolized back to arachidonic acid through the action of fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL) (39) (Figure 1). Recycling of endogenous endocannabinoids for new endocannabinoid biosynthesis can also occur (40). Cannabinoids can stimulate the production of prostaglandins (41), and likewise, PGE2 can stimulate synthesis of 2-AG intermediates (42). In summary, it is clear that eicosanoid biosynthesis is highly interactive and changes in biosynthesis or cell signaling pathways can alter overall eicosanoid balance and produce eicosanoids with similar or opposing functions.
Prostaglandin E2: Inhibitory or Stimulatory to Hematopoiesis?
Numerous studies by us and others spanning a period of more than 20 years from the early 1970s to the mid 1990s demonstrated that PGE2 inhibits mouse and human myeloid progenitors cells, defined as colony forming units-granulocyte macrophage (CFU-GM) and macrophage (CFU-M), in vitro (25, 26, 43–46). A physiological role for PGE2 in regulation of hematopoiesis, particularly as a negative feedback regulator of myelopoiesis was further defined by studies in mice differing in PGE synthetic capacity (27, 47); demonstration of abnormal PGE2 responses in patients with leukemia (43, 44, 46, 48, 49); prognostic association of disordered PGE2 response in patients with myelodysplastic syndromes (MDS) (50); abnormal hematopoietic progenitor cell response in patients cured of germ cell tumors but progressing to acute leukemia (51); and association of HPC response to PGE2 with clinical response to Interferon-γ in patients with chronic myelogenous leukemia (CML), chronic lymphocytic leukemia (CLL), and Hodgkin's disease (52, 53).
Not all studies, however, demonstrated an inhibitory effect of PGE2. Studies by Fehrer and Gidali in 1974 showed that short-term PGE2 treatment of murine marrow cells in vitro increased the number of day 9 colony forming unit – spleen (CFU-S) in cell cycle (54). An increase in CFU-GM in S-phase was also seen after PGE pulse exposure of human marrow (55). However, early studies evaluating in vivo dosing of PGE2 in mice led to little or no increase in hematopoiesis (56). We later demonstrated that repetitive in vivo PGE2 administration inhibits CFU-GM frequency and cell cycle rate, and decreases marrow and spleen cellularity (57–60); whereas, short-term in vitro exposure to PGE2 stimulated proliferation and cell cycle of quiescent cells and an increase in HPC, suggesting a stimulatory effect of PGE2 on HSC (61, 62). Further studies identified that dose, timing and duration of exposure were critical factors that determined positive or negative effects of PGE2.
Hematopoietic Stem Cell Regulation by Prostaglandin E2
Interest in the regulatory roles of PGE2 on hematopoiesis has recently increased and new studies have provided further insights into hematopoietic regulation and possible therapeutic applications with PGE2. Studies by North et al. demonstrated that zebrafish embryos treated with a long-acting derivative of PGE2, 16,16 dimethyl-PGE2 (dmPGE2), had an increase in HSC production, while treatment with an NSAID or COX knockout decreased HSC number during embryogenesis (63). In a zebrafish kidney marrow irradiation-recovery assay, dmPGE2 increased kidney marrow repopulation, and ex vivo pulse exposure to dmPGE2 increased the repopulating capacity of murine bone marrow cells in a competitive repopulation assay.
While these studies demonstrated that PGE2 affects HSCs, the mechanisms of action of PGE2 on HSC were not determined. Wnt signaling, particularly in the contexts of hematopoietic stress, ageing or disease, play an important regulatory role on HSCs (reviewed in 64). Prostaglandin E2 signaling through the EP2 and EP4 receptors results in phosphorylation of GSK-3 and increased β-catenin signaling (65), which is downstream of the Wnt pathway and signaling specifically through the EP4 receptor has been shown to directly increase β-catenin suggesting synergistic cross-talk between COX2 and Wnt pathways (66). In a follow-up study to those by North et al., PGE2 signaling was shown to stabilize β-catenin in HSC and promote survival and proliferation during embryogenesis (67) demonstrating the in vivo significance of PGE2/Wnt interactions. Recently, we demonstrated that PGE2 increases survival and proliferation of HSC (68), and exposure to PGE2 increased the anti-apoptotic protein Survivin, which we have previously reported to be required for HSC to enter and progress through cell cycle (69, 70), providing further mechanistic insights into the regulation of HSC by PGE2. In the context of pulse exposure to enhance hematopoietic transplantation, an important clinical translation of these described studies, we suggest a model in which PGE2 facilitates enhancement of engraftment by a multipart mechanism. PGE2 increases survival and self-renewal of HSC, through regulation of Wnt/ β-catenin, Survivin and possibly unidentified pathways, and increases CXCR4 expression enhancing homing and tethering of HSC in the bone marrow. The effect of PGE2 on CXCR4 expression is critical to its ability to enhance HSC homing and engraftment and is described in further detail below.
Clinical trials are currently exploring the utility of ex vivo exposure to PGE2 to enhance HSC engraftment. However, the ability to clinically modulate PGE2 signaling in vivo may provide future therapeutic applications. An early study by Gidali and Feher in 1977, primarily evaluating CFU-S, suggested that in vivo dosing of PGE2 in mice led to little or no increase in hematopoiesis (56). Extensive studies by our laboratory in the 1980s showed that repetitive in vivo PGE2 administration inhibits CFU-GM frequency and cell cycle rate, and decreases marrow and spleen cellularity (57–60), however utilization of competitive repopulation assays, definitive assays of HSC function, were not performed. In a recent study by Frisch et al., repeated administration of PGE2 for 16 days in vivo, expanded short-term, but not long-term, HSC in transplantation models (71). This regimen resulted in changes in the bone marrow niche, suggesting that the in vivo effects of PGE2 on HSC and HPC may include indirect mechanisms. However, high doses of PGE2 (~120 μg/mouse) were administered for extended periods of time, and control vehicle alone showed a ~2-fold increase in the percentage of Lineageneg Sca-1+ c-kit+ (LSK) cells after 16 days, while PGE2 treatment showed ~3-fold increase over the same time period (71). The effects of vehicle may be suggestive of inadvertent stress effects on hematopoiesis in this system. Moreover, the effects of PGE2 on hematopoiesis at physiological concentrations may be different than those observed using pharmacologic doses, as previously suggested (56). Additional studies evaluating in vivo modulation of PGE2 signaling, both at a receptor level and on biosynthesis, are necessary to fully explore the therapeutic potential of the prostaglandin pathway.
Hematopoietic Regulation by other Eicosanoids
The cannabinoids have been implicated in positive and negative effects on mature cells of the immune system (72, 73); however, little is known about their effects on early hematopoietic cells. Anandamide can act as a synergistic growth factor for HPC (74) and has a pro-apoptotic effect on erythrocytes (75). The endocannabinoid 2-AG also stimulates proliferation of HPC (76), and has recently been shown to increase CFU-GEMM colony formation and cell migration (77). Furthermore, activation of cannabinoid CB receptors on murine embryonic stem cells promotes hematopoietic differentiation (78). In addition, Non-Hodgkin’s lymphoma cells have abnormally high levels of CB2 receptor expression (79), suggesting a potential proliferative role for cannabinoids.
Leukotrienes, like other eicosanoids, are produced in the marrow microenvironment (80), and 5’-LOX is found in HPC (81). While PGE2 inhibits HPC proliferation and COX inhibitors enhance HPC production in vitro, the leukotrienes LTB4, LTC4 and LTD4 increase mouse and human HPC (82–84), which is inhibited by 5'-LOX inhibitors (82, 83, 85). Moreover, PGE stimulates erythropoiesis (86, 87), while LTB4 and LTC4 inhibit early and late erythroid progenitor cells (88). Similarly, LOX inhibitors enhance erythropoiesis. In mice, dual COX inhibition enhances HPC recovery (85), while selective 5’-LOX inhibitors decrease CFU-GM and blast colony-forming cells (CFU-BL) (82). The addition of LTB4 to UCB cells cultured with growth factors enhances HPC proliferation with concomitant reduction in total CD34+ cells, while the selective LTB4 receptor antagonist CP105696 enhances production of CD34+ cells and blocks HPC proliferation (89). Overall, the available data suggest that LTB4 signaling decreases HSC self-renewal and increases differentiation, while blocking LTB4 receptor signaling increases HSC self-renewal and blocks their differentiation. This is in contrast to PGE2 which enhances self-renewal (68), and blockade of PGE2 biosynthesis enhances HPC production (90). Whether enhanced HPC production observed upon inhibition of PGE2 synthesis in vivo is a direct consequence of lack of PGE2-mediated HSC self-renewal remains to be determined. In any case, the use of PGE2 or a leukotriene receptor antagonist or LOX inhibitor in the post-transplant setting may favor self-renewal. In addition, since blockade of COX enzymes makes more arachidonic acid available to the LOX pathway (84, 91), the use of a COX inhibitor post-transplant could promote HSC differentiation via direct inhibition of PGE2 synthesis and/or increased synthesis of leukotrienes.
It is clear that prostaglandins, cannabinoids and leukotrienes have important roles in hematopoietic homeostasis, and evaluating their responses is critical to understanding eicosanoid function and development of eicosanoid-based therapeutic strategies for improvements in hematopoietic transplantation. The remainder of this article will focus on experimental systems that highlight the effects of eicosanoids on HSC and HPC in the context of how they can be used to affect trafficking to bone marrow, i.e. homing, in order to improve HSC delivery, and directed egress from bone marrow, i.e. mobilization, in order to obtain more HSC and HPC for transplant.
Prostaglandin E2 Increases Hematopoietic Homing
Homing is a description of the ability of HSC and HPC to traffic from the peripheral blood, where they are injected intravenously for hematopoietic transplantation, to the bone marrow hematopoietic niche, where they can lodge, self-renew and differentiate to successfully repopulate the host’s blood forming system. Homing is a rapid process, which is measured in hours (or at most 1–2 days) (92), and should be separated from the concept of “engraftment”, which is more a description of the culmination of events pre- and post-homing of HSC. Homing of HSC to bone marrow appears to be regulated by the same processes responsible for HSC retention within the bone marrow niche, many of which are discussed in this issue of Leukemia. Adhesion molecules aid in trafficking, leukocyte rolling, transendothelial migration, and ultimate tethering in the marrow, and several adhesion molecules have been implicated as contributing to HSC and HPC tethering in the bone marrow hematopoietic niche; notably the integrins α4β1 – very late antigen-4 (VLA-4) (93–96), α5β1 – very late antigen-5 (VLA-5) (93, 95–97), α4β7 – lymphocyte Peyer’s patch adhesion molecule-1 (LPAM-1) (98), the alpha 6 integrins (Laminins) (99, 100), CD44 (96, 101), E-selectins (102–104), and others, and are essential for proper bone marrow homing of HSC (95, 97–100, 105, 106). Similarly, the CXCR4/SDF-1α axis is a critical component of HSC homing to the bone marrow (107), and increases in CXCR4 receptor expression on HSC either with growth factors (105), or by gene overexpression (108, 109) significantly increases HSC homing and engraftment.
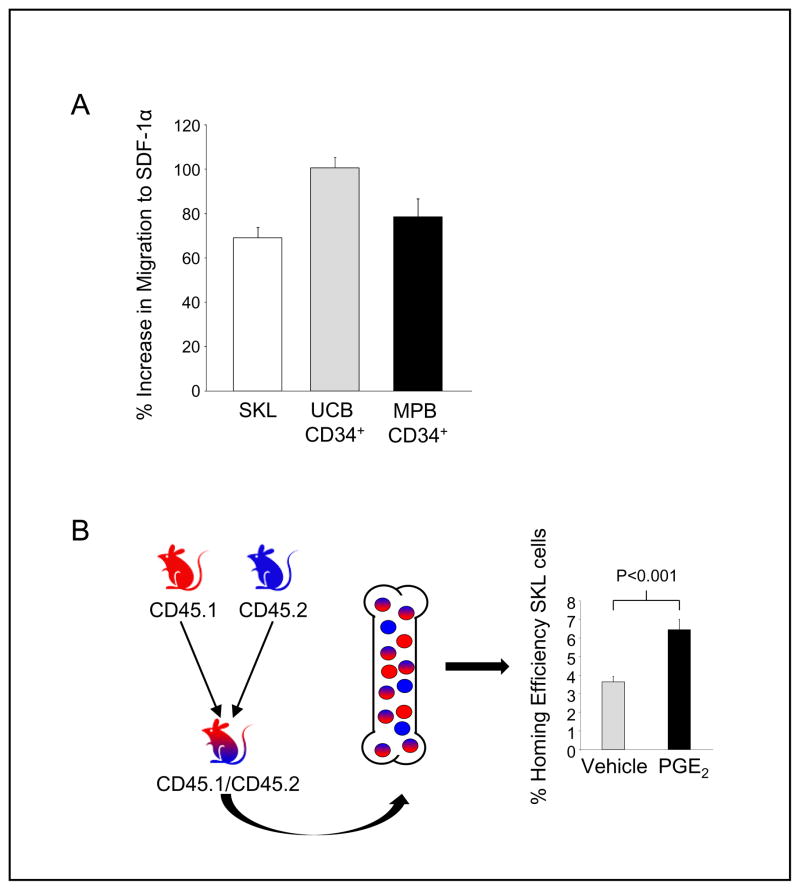
PGE2 increases CXCR4 receptor expression on murine (68) and human (68, 110) HSC and HPC. In light of this increase in CXCR4 mediated by PGE2, we evaluated the ability of a short in vitro treatment with PGE2 to increase the migration of HSC and HPC to SDF-1α. Mouse bone marrow cells were FACS sorted for the SKL population that is enriched for HSC, and CD34+ cells from human UCB and G-CSF MPB samples were acquired by magnetic antibody isolation. After short-term treatment with PGE2 in vitro, the ability of SKL, UCB CD34+ and MPB CD34+ cells to migrate to SDF-1α was evaluated using an in vitro transwell assay. Treatment with PGE2 significantly increased transwell migration of each of these hematopoietic cell populations (Figure 2A). To further evaluate if increased chemotaxis observed with PGE2- treated cells led to a functional increase in HSC bone marrow homing, we utilized a novel head- to-head homing assay, where FACS purified SKL cells from C57Bl/6 mice (CD45.2) and B6.SJL-PtrcAPep3B/BoyJ (BOYJ) (CD45.1) mice were treated with either PGE2 or vehicle and transplanted competitively into lethally irradiated hybrid C57Bl/6 / BOYJ hybrid mice (CD45.1/CD45.2). Analysis of homed SKL cells in recipient bone marrow demonstrated that PGE2 treatment enhanced SKL cell homing compared to cells treated with vehicle (Figure 2B).
Figure 2. PGE2 increases hematopoietic migration and homing.
A.) Mouse SKL cells or human CD34+ cells were treated on ice with 1 μM 16,16-dimethyl PGE2 (dmPGE2) or vehicle control for 2 hours, washed, and then re-suspended in media with 10% heat inactivated fetal bovine serum and cultured at 37 °C for 16 hours. After incubation, cells were washed, re-suspended in RPMI/0.5% BSA, and allowed to migrate to 100 ng/ml recombinant mouse or human SDF-1α through a two-chamber, 6.5 mm diameter, 5 μm pore transwell. Cells migrating to the bottom chamber were enumerated by flow cytometry, and the % increase in migration over vehicle control determined.
B.) SKL cells from CD45.1 and CD45.2 mice were isolated by FACS sorting and treated with either dmPGE2 or vehicle. Treated SKL cells were then transplanted competitively, head-to-head into lethally irradiated (1100 cGys) CD45.1/CD45.2 hybrid mice, allowing for distinction of vehicle treated, dmPGE2 treated and recipient SKL cells within the same animal. Sixteen hours post-transplant, hind limb bones were isolated and homed SKL cells determined by flow cytometry. Mean ± SEM, N=10 mice, each assayed individually, are shown.
Cannabinoids Increase Hematopoietic Progenitor Cell Mobilization
Recent evidence suggests that cannabinoids, signaling through the peripheral CB2 receptor, inhibit SDF-1α induced and CXCR4 mediated chemotaxis of Jurkat T-cells (111) and activated human peripheral T-cells (112). In addition, hematopoietic cell lines transfected with cannabinoid receptor migrate in response to the endogenous cannabinoid, 2-AG (113), and 2-AG can act as a chemoattractant for dendritic cells (114). Stimulation of cannabinoid receptors reduces adhesion molecule expression (115, 116), transendothelial migration of monocytes (116), and regulates myeloid progenitor trafficking by alterating chemokines and chemokine receptors (116, 117). Cannabinoids have also been reported to affect matrix metalloproteinase-9 (MMP-9) production (118) in a number of cell lines and can modulate neutrophil function (119, 120). Taken together, these data suggest that cannabinoids could play a role in hematopoietic mobilization, through interference in the SDF-1α/CXCR4 axis, reduction in adhesion and/or release of MMP-9, which can degrade adhesion molecule interactions between HSC and HPC with the bone marrow niche (121), or through other undefined mechanisms.
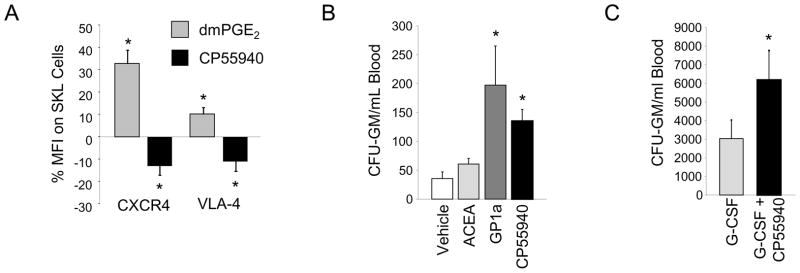
While many of the above effects attributed to cannabinoids have been defined in mature blood cells, little research has explored their function on HSC and HPC. Using a number of different antibodies and flow cytometry, we were able to clearly detect CB receptors on immunophenotypically defined mouse and human HSC (not shown). We utilized the dual CB1 and CB2 agonist CP55940 (122) and examined its effects on expression of CXCR4 and the adhesion molecule VLA-4 on SKL cells in vitro, compared to cells treated with dmPGE2. As we previously reported, short term in vitro treatment with dmPGE2 increased expression of CXCR4 on treated SKL cells (Figure 3A). In addition, dmPGE2 also increased SKL VLA-4 expression. In contrast, SKL cells treated with cannabinoids demonstrated significant reduction in both CXCR4 and VLA-4, suggesting that cannabinoids may be able to facilitate release of hematopoietic stem and progenitor cells from bone marrow niches.
Figure 3. Cannabinoid agonism rapidly and synergistically mobilizes HPC.
A.) Mouse Lineageneg bone marrow was treated with dmPGE2, the cannabinoid agonist CP55940, or vehicle control. Sixteen hours post-treatment, cells were stained for CXCR4 receptor or VLA-4, and SKL phenotypic markers, and % mean fluorescence intensity (MFI) compared to control treated cells determined. Data are Mean ± SEM, N=5 mice, each assayed individually. * P<0.05 compared to vehicle control.
B.) CFU-GM mobilization 2 hours post single administration of 10 mg/kg ACEA, 5 mg/kg GP1a or 10 mg/kg CP55940. Data are expressed as Mean ± SEM, N=3 mice per group, each assayed individually. * P<0.05 compared to vehicle control.
C.) Mobilization by G-CSF (50 μg/kg, bid X 4 days), or G-CSF for 4 days followed by a single dose of CP55940 on day 5. Data are expressed as Mean ± SEM, N=3 mice per group, each assayed individually. * P<0.05 compared to G-CSF.
In order to directly evaluate the effects of cannabinoid receptor agonists on HPC mobilization, BALB/c mice were treated with single injections of the CB1 selective agonist ACEA (123), the CB2 selective agonist GP1a (124), or the dual CB1 and CB2 agonist CP55940 and CFU-GM in peripheral blood were quantitated after 2 hours. Single administration of the CB2 selective agonist GP1a and the dual CB1/CB2 agonist CP55940 mobilized CFU-GM to peripheral blood to a significant degree (Figure 3B); whereas, the CB1 selective agonist ACEA had only marginal mobilizing activity. In separate groups of mice, we also evaluated combination mobilization with G-CSF and the dual CB1/CB2 agonist CP55940. Mice were mobilized with a standard 4-day regimen of G-CSF alone (50 μg/kg, bid X 4 days) or the G-CSF regimen plus a single dose of CP55940 administered on day 5, 16 hours after the last dose of G-CSF and 2 hours prior to sacrifice. The addition of a single dose of CP55940 to the G-CSF regimen significantly increased CFU-GM mobilization compared to G-CSF alone (Figure 3C). These data suggest that CB receptor ligation, particularly CB2, rapidly mobilizes CFU-GM, and that CB receptor activation enhances mobilization by G-CSF, likely by an effect on inhibition of CXCR4 signaling, or reductions in integrin adherence, since the kinetics of mobilization are consistent with the kinetics of mobilization by the CXCR4 antagonist AMD3100 and/or VLA-4 inhibitors. It should be noted that these effects of cannabinoids on CFU-GM mobilization are in contrast to a previously published study that showed that the CB2 receptor mediates retention of immature B cells in bone marrow sinusoids (125), perhaps suggesting cell-type specific mobilization responses. While these studies clearly indicate mobilization of HPC, further analysis of the full repertoire of HPC and HSC mobilized by cannabinoids, will aid in the potential future development of cannabinoid/cannabinoid receptor based mobilization strategies.
A Role for Leukotrienes in Hematopoietic Trafficking?
Leukotrienes are known mediators of allergic inflammation and asthma, however there is little information on their role in hematopoietic stem and progenitor trafficking. LTB4 has been shown to increase the migratory capacity of dendritic cells (126, 127), and T-cell subsets (126). It is also a potent chemoattractant for polymorphonuclear leukocytes and regulates transendothelial migration (128, 129), which is at least partially mediated by regulation of adhesion molecules. While LTB4 can induce migration of mature blood cells, only the cysteinyl leukotriene LTD4 can induce chemotaxis and transendothelial migration of CD34+ progenitors (130), which is mediated in part by regulation of VLA-4 and VLA-5 adhesion (131). However, in vivo treatment with a LTD4 receptor antagonist does not result in mobilization of CD34+ cells (131) possibly due to low baseline levels of LTD4 in steady state bone marrow. Evaluation of the use of leukotriene antagonism in combination with other mobilizing agents has not yet been explored. It remains to be determined if modulation of leukotriene activity either in vivo or ex vivo will have any efficacy in enhancing hematopoietic transplantation.
The Yin and Yang of Eicosanoid Regulation
In many physiological systems, prostaglandins, leukotrienes and endocannabinoids exhibit compensatory or opposing roles (reviewed in Table 1). The action of many NSAIDs, which block the production of PGE2, may also act by increasing signaling via endocannabinoids (132). PGE2-glycerol has been shown to mobilize calcium, activate signal transduction pathways (133) and have neurological effects opposite of those induced by 2-AG (134). Furthermore, prostaglandins and leukotrienes have been shown to have numerous opposing roles in pulmonary fibrosis (135) and in other systems they may act in a coordinate fashion (136). It is particularly noteworthy that cannabinoid ligands block CXCR4 signaling (111, 112) and neutrophil migration (120, 137), which one would expect to negatively affect homing, but enhance spontaneous release from marrow facilitating mobilization as we have demonstrated. In contrast, PGE2 enhances CXCR4 expression and signaling, which facilitates hematopoietic homing, but may result in dampening of mobilization responses. Future studies evaluating combination eicosanoid therapies, which take into account these opposing functions, are likely to lead to novel approaches to regulate hematopoietic trafficking and improve transplantation protocols. The current availability of numerous FDA approved pharmaceuticals that specifically regulate biosynthesis and signaling of prostaglandins, leukotrienes and cannabinoids will facilitate rapid translation of eicosanoid based therapeutic research, both at the level of graft isolation or measured in terms of graft performance.
Table 1.
Opposing roles of cannabinoids, leukotrienes and prostaglandins.
| System Affected | Cannabinoids | Leukotrienes | Prostaglandin E2 |
|---|---|---|---|
| CXCR4/CXCL12 | ↓(111, 112) | ---------- | ↑(68, 138, 139) |
| cAMP | ↓(140, 141) | ↓(142) | ↑↓(143) |
| Neutrophil Migration | ↓(120, 137) | ↑(128, 136) | ↑(128, 144, 145) |
| Inflammation | ↓(72, 140, 146) | ↑(31, 147) | ↑(16, 148, 149) ↓(150) |
| Myleopoiesis | ↑(74, 76, 77) | ↑(151–153) | ↓(25, 26, 62) |
| Erythropoiesis | ↓(75) | ↓(88) | ↑(86, 87, 154, 155) |
Summary of several physiological processes relevant to hematopoiesis and hematopoietic cell trafficking that are regulated by the eicosanoid system. In most cases, cannabinoids act in an opposing role to prostaglandins, while leukotrienes regulate similar or opposing functions to prostaglandin. The dual prostaglandin effects shown for some physiological processes are the result of EP receptor subtype differences.
Acknowledgments
Supported by NIH grants HL069669, HL079654, and HL096305 (to LMP). JH is supported by training grant DK07519. Flow cytometry was performed in the Flow Cytometry Resource Facility of the Indiana University Simon Cancer Center (NCI P30 CA082709).
References
- 1.Bhattacharya D, Czechowicz A, Ooi AG, Rossi DJ, Bryder D, Weissman IL. Niche recycling through division-independent egress of hematopoietic stem cells. J Exp Med. 2009;206:2837–50. doi: 10.1084/jem.20090778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–6. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 3.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102:1249–53. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 4.Nibley WE, Spangrude GJ. Primitive stem cells alone mediate rapid marrow recovery and multilineage engraftment after transplantation. Bone Marrow Transplant. 1998;21:345–54. doi: 10.1038/sj.bmt.1701097. [DOI] [PubMed] [Google Scholar]
- 5.Lanzkron SM, Collector MI, Sharkis SJ. Homing of long-term and short-term engrafting cells in vivo. Ann N Y Acad Sci. 1999;872:48–54. doi: 10.1111/j.1749-6632.1999.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 6.Vose JM, Ho AD, Coiffier B, Corradini P, Khouri I, Sureda A, et al. Advances in mobilization for the optimization of autologous stem cell transplantation. Leuk Lymphoma. 2009;50:1412–21. doi: 10.1080/10428190903096701. [DOI] [PubMed] [Google Scholar]
- 7.Moog R. Management strategies for poor peripheral blood stem cell mobilization. Transfus Apher Sci. 2008;38:229–36. doi: 10.1016/j.transci.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Broxmeyer HE. Umbilical cord transplantation: epilogue. Semin Hematol. 2010;47:97–103. doi: 10.1053/j.seminhematol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall KM, Horvath TL, Abonour R, Cornetta K, Srour EF. Decreased homing of retrovirally transduced human bone marrow CD34+ cells in the NOD/SCID mouse model. Exp Hematol. 2006;34:433–42. doi: 10.1016/j.exphem.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Broxmeyer HE. Essentials of Stem Cell Biology. Elsevier, Inc; 2006. Cord Blood Hematopoietic Stem and Progenitor Cells; pp. 133–7. [Google Scholar]
- 11.Porecha NK, English K, Hangoc G, Broxmeyer HE, Christopherson KW. Enhanced functional response to CXCL12/SDF-1 through retroviral overexpression of CXCR4 on M07e cells: implications for hematopoietic stem cell transplantation. Stem Cells Dev. 2006;15:325–33. doi: 10.1089/scd.2006.15.325. [DOI] [PubMed] [Google Scholar]
- 12.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 13.Legler DF, Bruckner M, Uetz-von AE, Krause P. Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. Int J Biochem Cell Biol. 2010;42 :198–201. doi: 10.1016/j.biocel.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–93. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- 16.Murakami M, Kudo I. Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr Pharm Des. 2006;12:943–54. doi: 10.2174/138161206776055912. [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229–40. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JA, Warner TD. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov. 2006;5:75–86. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- 19.Strand V. Are COX-2 inhibitors preferable to non-selective non-steroidal anti-inflammatory drugs in patients with risk of cardiovascular events taking low-dose aspirin? Lancet. 2007;370:2138–51. doi: 10.1016/S0140-6736(07)61909-6. [DOI] [PubMed] [Google Scholar]
- 20.Coruzzi G, Venturi N, Spaggiari S. Gastrointestinal safety of novel nonsteroidal antiinflammatory drugs: selective COX-2 inhibitors and beyond. Acta Biomed. 2007;78:96–110. [PubMed] [Google Scholar]
- 21.Tsuboi K, Sugimoto Y, Ichikawa A. Prostanoid receptor subtypes. Prostaglandins Other Lipid Mediat. 2002;68–69:535–56. doi: 10.1016/s0090-6980(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 22.Miyaura C, Inada M, Matsumoto C, Ohshiba T, Uozumi N, Shimizu T, et al. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J Exp Med. 2003;197:1303–10. doi: 10.1084/jem.20030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen QR, Miyaura C, Higashi S, Murakami M, Kudo I, Saito S, et al. Activation of cytosolic phospholipase A2 by platelet-derived growth factor is essential for cyclooxygenase-2-dependent prostaglandin E2 synthesis in mouse osteoblasts cultured with interleukin-1. J Biol Chem. 1997;272:5952–8. doi: 10.1074/jbc.272.9.5952. [DOI] [PubMed] [Google Scholar]
- 24.Raisz LG, Vanderhoek JY, Simmons HA, Kream BE, Nicolaou KC. Prostaglandin synthesis by fetal rat bone in vitro: evidence for a role of prostacyclin. Prostaglandins. 1979;17:905–14. doi: 10.1016/0090-6980(79)90061-3. [DOI] [PubMed] [Google Scholar]
- 25.Pelus LM, Broxmeyer HE, Kurland JI, Moore MA. Regulation of macrophage and granulocyte proliferation. Specificities of prostaglandin E and lactoferrin. J Exp Med. 1979;150:277–92. doi: 10.1084/jem.150.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelus LM, Broxmeyer HE, Moore MA. Regulation of human myelopoiesis by prostaglandin E and lactoferrin. Cell Tissue Kinet. 1981;14:515–26. doi: 10.1111/j.1365-2184.1981.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurland JI, Pelus LM, Ralph P, Bockman RS, Moore MA. Induction of prostaglandin E synthesis in normal and neoplastic macrophages: role for colony-stimulating factor(s) distinct from effects on myeloid progenitor cell proliferation. Proc Natl Acad Sci U S A. 1979;76:2326–30. doi: 10.1073/pnas.76.5.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinhilber D. 5-Lipoxygenase: a target for antiinflammatory drugs revisited. Curr Med Chem. 1999;6:71–85. [PubMed] [Google Scholar]
- 29.Serhan CN, Sheppard KA. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest. 1990;85:772–80. doi: 10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 31.Kim N, Luster AD. Regulation of immune cells by eicosanoid receptors. ScientificWorldJournal. 2007;7:1307–28. doi: 10.1100/tsw.2007.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ligresti A, Cascio MG, Di MV. Endocannabinoid metabolic pathways and enzymes. Curr Drug Targets CNS Neurol Disord. 2005;4:615–23. doi: 10.2174/156800705774933104. [DOI] [PubMed] [Google Scholar]
- 33.Malcher-Lopes R, Franco A, Tasker JG. Glucocorticoids shift arachidonic acid metabolism toward endocannabinoid synthesis: a non-genomic anti-inflammatory switch. Eur J Pharmacol. 2008;583:322–39. doi: 10.1016/j.ejphar.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto Y, Wang J, Morishita J, Ueda N. Biosynthetic pathways of the endocannabinoid anandamide. Chem Biodivers. 2007;4:1842–57. doi: 10.1002/cbdv.200790155. [DOI] [PubMed] [Google Scholar]
- 35.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–85. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 36.Weber A, Ni J, Ling KH, Acheampong A, Tang-Liu DD, Burk R, et al. Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. J Lipid Res. 2004;45:757–63. doi: 10.1194/jlr.M300475-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–6. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 38.Kozak KR, Crews BC, Ray JL, Tai HH, Morrow JD, Marnett LJ. Metabolism of prostaglandin glycerol esters and prostaglandin ethanolamides in vitro and in vivo. J Biol Chem. 2001;276:36993–8. doi: 10.1074/jbc.M105854200. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Ueda N. Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat. 2009;89:112–9. doi: 10.1016/j.prostaglandins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Placzek EA, Okamoto Y, Ueda N, Barker EL. Mechanisms for recycling and biosynthesis of endogenous cannabinoids anandamide and 2-arachidonylglycerol. J Neurochem. 2008;107:987–1000. doi: 10.1111/j.1471-4159.2008.05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell MD, Sato TA, Wang A, Keelan JA, Ponnampalam AP, Glass M. Cannabinoids stimulate prostaglandin production by human gestational tissues through a tissue- and CB1-receptor-specific mechanism. Am J Physiol Endocrinol Metab. 2008;294:E352–E356. doi: 10.1152/ajpendo.00495.2007. [DOI] [PubMed] [Google Scholar]
- 42.Konger RL, Brouxhon S, Partillo S, VanBuskirk J, Pentland AP. The EP3 receptor stimulates ceramide and diacylglycerol release and inhibits growth of primary keratinocytes. Exp Dermatol. 2005;14:914–22. doi: 10.1111/j.1600-0625.2005.00381.x. [DOI] [PubMed] [Google Scholar]
- 43.Aglietta M, Piacibello W, Gavosto F. Insensitivity of chronic myeloid leukemia cells to inhibition of growth by prostaglandin E1. Cancer Res. 1980;40:2507–11. [PubMed] [Google Scholar]
- 44.Pelus LM, Broxmeyer HE, Clarkson BD, Moore MA. Abnormal responsiveness of granulocyte-macrophage committed colony-forming cells from patients with chronic myeloid leukemia to inhibition by prostaglandin E1. Cancer Res. 1980;40:2512–5. [PubMed] [Google Scholar]
- 45.Taetle R, Guittard JP, Mendelsohn JM. Abnormal modulation of granulocyte/macrophage progenitor proliferation by prostaglandin E in chronic myeloproliferative disorders. Exp Hematol. 1980;8:1190–201. [PubMed] [Google Scholar]
- 46.Taetle R, Koessler A. Effects of cyclic nucleotides and prostaglandins on normal and abnormal human myeloid progenitor proliferation. Cancer Res. 1980;40:1223–9. [PubMed] [Google Scholar]
- 47.Kincade PW, Lee G, Fernandes G, Moore MA, Williams N, Good RA. Abnormalities in clonable B lymphocytes and myeloid progenitors in autoimmune NZB mice. Proc Natl Acad Sci U S A. 1979;76:3464–8. doi: 10.1073/pnas.76.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelus LM, Gold E, Saletan S, Coleman M. Restoration of responsiveness of chronic myeloid leukemia granulocyte-macrophage colony-forming cells to growth regulation in vitro following preincubation with prostaglandin E. Blood. 1983;62:158–65. [PubMed] [Google Scholar]
- 49.Moore MA, Mertelsmann R, Pelus LM. Phenotypic evaluation of chronic myeloid leukemia. Blood Cells. 1981;7:217–36. [PubMed] [Google Scholar]
- 50.Gold EJ, Conjalka M, Pelus LM, Jhanwar SC, Broxmeyer H, Middleton AB, et al. Marrow cytogenetic and cell-culture analyses of the myelodysplastic syndromes: insights to pathophysiology and prognosis. J Clin Oncol. 1983;1:627–34. doi: 10.1200/JCO.1983.1.10.627. [DOI] [PubMed] [Google Scholar]
- 51.Leitner SP, Bosl GJ, Pelus LM. Abnormal colony formation and prostaglandin E responsiveness of myeloid progenitor cells in patients cured of germ cell neoplasms after combination chemotherapy. Cancer. 1987;60:312–7. doi: 10.1002/1097-0142(19870801)60:3<312::aid-cncr2820600307>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 52.Pelus LM, Vadhan-Raj S. Modulation of responsiveness of chronic myelogenous leukemia granulocyte-macrophage colony-forming cells to growth regulation following in vivo treatment with recombinant gamma-interferon. Am J Hematol. 1988;28:21–6. doi: 10.1002/ajh.2830280105. [DOI] [PubMed] [Google Scholar]
- 53.Vadhan-Raj S, Al Katib A, Bhalla R, Pelus L, Nathan CF, Sherwin SA, et al. Phase I trial of recombinant interferon gamma in cancer patients. J Clin Oncol. 1986;4:137–46. doi: 10.1200/JCO.1986.4.2.137. [DOI] [PubMed] [Google Scholar]
- 54.Feher I, Gidali J. Prostaglandin E2 as stimulator of haemopoietic stem cell proliferation. Nature. 1974;247:550–1. doi: 10.1038/247550a0. [DOI] [PubMed] [Google Scholar]
- 55.Verma DS, Spitzer G, Zander AR, McCredie KB, Dicke KA. Prostaglandin E1-mediated augmentation of human granulocyte-macrophage progenitor cell growth in vitro. Leuk Res. 1981;5:65–71. doi: 10.1016/0145-2126(81)90097-7. [DOI] [PubMed] [Google Scholar]
- 56.Gidali J, Feher I. The effect of E type prostaglandins on the proliferation of haemopoietic stem cells in vivo. Cell Tissue Kinet. 1977;10:365–73. [PubMed] [Google Scholar]
- 57.Gentile PS, Byer D, Pelus LM. In vivo modulation of murine myelopoiesis following intravenous administration of prostaglandin E2. Blood. 1983;62:1100–7. [PubMed] [Google Scholar]
- 58.Gentile PS, Pelus LM. In vivo modulation of myelopoiesis by prostaglandin E2. II. Inhibition of granulocyte-monocyte progenitor cell (CFU-GM) cell-cycle rate. Exp Hematol. 1987;15:119–26. [PubMed] [Google Scholar]
- 59.Gentile PS, Pelus LM. In vivo modulation of myelopoiesis by prostaglandin E2. IV. Prostaglandin E2 induction of myelopoietic inhibitory activity. J Immunol. 1988;141:2714–20. [PubMed] [Google Scholar]
- 60.Pelus LM, Gentile PS. In vivo modulation of myelopoiesis by prostaglandin E2. III. Induction of suppressor cells in marrow and spleen capable of mediating inhibition of CFU-GM proliferation. Blood. 1988;71:1633–40. [PubMed] [Google Scholar]
- 61.Pelus LM. CFU-GM expression of Ia-like, HLA-DR, antigen: An association with the humoral control of human granulocyte and macrophage production. Exp Hematol. 1982;10 :219–31. [Google Scholar]
- 62.Pelus LM. Association between colony forming units-granulocyte macrophage expression of Ia-like (HLA-DR) antigen and control of granulocyte and macrophage production. A new role for prostaglandin E. J Clin Invest. 1982;70:568–78. doi: 10.1172/JCI110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malhotra S, Kincade PW. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell. 2009;4:27–36. doi: 10.1016/j.stem.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–53. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 66.Wang D, Mann JR, DuBois RN. WNT and cyclooxygenase-2 cross-talk accelerates adenoma growth. Cell Cycle. 2004;3:1512–5. doi: 10.4161/cc.3.12.1288. [DOI] [PubMed] [Google Scholar]
- 67.Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–47. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–55. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukuda S, Foster RG, Porter SB, Pelus LM. The antiapoptosis protein survivin is associated with cell cycle entry of normal cord blood CD34(+) cells and modulates cell cycle and proliferation of mouse hematopoietic progenitor cells. Blood. 2002;100:2463–71. doi: 10.1182/blood.V100.7.2463. [DOI] [PubMed] [Google Scholar]
- 70.Fukuda S, Pelus LM. Elevation of Survivin levels by hematopoietic growth factors occurs in quiescent CD34+ hematopoietic stem and progenitor cells before cell cycle entry. Cell Cycle. 2002;1:322–6. [PubMed] [Google Scholar]
- 71.Frisch BJ, Porter RL, Gigliotti BJ, Olm-Shipman AJ, Weber JM, O'Keefe RJ, et al. In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood. 2009;114:4054–63. doi: 10.1182/blood-2009-03-205823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166:3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 73.Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- 74.Valk PJ, Verbakel S, Vankan Y, Hol S, Mancham S, Ploemacher R, et al. Anandamide, a natural ligand for the peripheral cannabinoid receptor is a novel synergistic growth factor for hematopoietic cells. Blood. 1997;90:1448–57. [PubMed] [Google Scholar]
- 75.Bentzen PJ, Lang F. Effect of anandamide on erythrocyte survival. Cell Physiol Biochem. 2007;20:1033–42. doi: 10.1159/000110714. [DOI] [PubMed] [Google Scholar]
- 76.Valk PJ, Delwel R. The peripheral cannabinoid receptor, Cb2, in retrovirally-induced leukemic transformation and normal hematopoiesis. Leuk Lymphoma. 1998;32:29–43. doi: 10.3109/10428199809059244. [DOI] [PubMed] [Google Scholar]
- 77.Patinkin D, Milman G, Breuer A, Fride E, Mechoulam R. Endocannabinoids as positive or negative factors in hematopoietic cell migration and differentiation. Eur J Pharmacol. 2008;595:1–6. doi: 10.1016/j.ejphar.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Jiang S, Fu Y, Williams J, Wood J, Pandarinathan L, Avraham S, et al. Expression and function of cannabinoid receptors CB1 and CB2 and their cognate cannabinoid ligands in murine embryonic stem cells. PLoS One. 2007;2:e641. doi: 10.1371/journal.pone.0000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rayman N, Lam KH, Van LJ, Mulder AH, Budel LM, Lowenberg B, et al. The expression of the peripheral cannabinoid receptor on cells of the immune system and non-Hodgkin's lymphomas. Leuk Lymphoma. 2007;48:1389–99. doi: 10.1080/10428190701377030. [DOI] [PubMed] [Google Scholar]
- 80.Lindgren JA, Stenke L, Mansour M, Edenius C, Lauren L, Nasman-Glaser B, et al. Formation and effects of leukotrienes and lipoxins in human bone marrow. J Lipid Mediat. 1993;6:313–20. [PubMed] [Google Scholar]
- 81.Bautz F, Denzlinger C, Kanz L, Mohle R. Chemotaxis and transendothelial migration of CD34(+) hematopoietic progenitor cells induced by the inflammatory mediator leukotriene D4 are mediated by the 7-transmembrane receptor CysLT1. Blood. 2001;97:3433–40. doi: 10.1182/blood.v97.11.3433. [DOI] [PubMed] [Google Scholar]
- 82.Vore SJ, Eling TE, Danilowicz M, Tucker AN, Luster MI. Regulation of murine hematopoiesis by arachidonic acid metabolites. Int J Immunopharmacol. 1989;11:435–42. doi: 10.1016/0192-0561(89)90171-9. [DOI] [PubMed] [Google Scholar]
- 83.Braccioni F, Dorman SC, O'byrne PM, Inman MD, Denburg JA, Parameswaran K, et al. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J Allergy Clin Immunol. 2002;110:96–101. doi: 10.1067/mai.2002.125000. [DOI] [PubMed] [Google Scholar]
- 84.Elsas PX, Queto T, Mendonca-Sales SC, Elsas MI, Kanaoka Y, Lam BK. Cysteinyl leukotrienes mediate the enhancing effects of indomethacin and aspirin on eosinophil production in murine bone marrow cultures. Br J Pharmacol. 2008;153:528–35. doi: 10.1038/sj.bjp.0707586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kozubik A, Hofmanova J, Pospisil M, Netikova J, Hola J, Lojek A. Effects of drugs inhibiting prostaglandin or leukotriene biosynthesis on postirradiation haematopoiesis in mouse. Int J Radiat Biol. 1994;65:369–77. doi: 10.1080/09553009414550431. [DOI] [PubMed] [Google Scholar]
- 86.Lu L, Pelus LM, Piacibello W, Moore MA, Hu W, Broxmeyer HE. Prostaglandin E acts at two levels to enhance colony formation in vitro by erythroid (BFU-E) progenitor cells. Exp Hematol. 1987;15:765–71. [PubMed] [Google Scholar]
- 87.Rossi GB, Migliaccio AR, Migliaccio G, Lettieri F, Di RM, Peschle C, et al. In vitro interactions of PGE and cAMP with murine and human erythroid precursors. Blood. 1980;56 :74–9. [PubMed] [Google Scholar]
- 88.Estrov Z, Halperin DS, Coceani F, Freedman MH. Modulation of human marrow haematopoiesis by leucotrienes in vitro. Br J Haematol. 1988;69:321–7. doi: 10.1111/j.1365-2141.1988.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 89.Chung JW, Kim GY, Mun YC, Ahn JY, Seong CM, Kim JH. Leukotriene B4 pathway regulates the fate of the hematopoietic stem cells. Exp Mol Med. 2005;37:45–50. doi: 10.1038/emm.2005.6. [DOI] [PubMed] [Google Scholar]
- 90.Pelus LM. Blockade of prostaglandin biosynthesis in intact mice dramatically augments the expansion of committed myeloid progenitor cells (colony-forming units-granulocyte, macrophage) after acute administration of recombinant human IL-1 alpha. J Immunol. 1989;143:4171–9. [PubMed] [Google Scholar]
- 91.Bertolini A, Ottani A, Sandrini M. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarks. Curr Med Chem. 2002;9:1033–43. doi: 10.2174/0929867024606650. [DOI] [PubMed] [Google Scholar]
- 92.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 93.Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med. 1995;181:1805–15. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98:2403–11. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 95.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–96. [PubMed] [Google Scholar]
- 96.Vermeulen M, Le PF, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 97.van der Loo JC, Xiao X, McMillin D, Hashino K, Kato I, Williams DA. VLA-5 is expressed by mouse and human long-term repopulating hematopoietic cells and mediates adhesion to extracellular matrix protein fibronectin. J Clin Invest. 1998;102:1051–61. doi: 10.1172/JCI3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Katayama Y, Hidalgo A, Peired A, Frenette PS. Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104:2020–6. doi: 10.1182/blood-2003-12-4157. [DOI] [PubMed] [Google Scholar]
- 99.Qian H, Georges-Labouesse E, Nystrom A, Domogatskaya A, Tryggvason K, Jacobsen SE, et al. Distinct roles of integrins alpha6 and alpha4 in homing of fetal liver hematopoietic stem and progenitor cells. Blood. 2007;110:2399–407. doi: 10.1182/blood-2006-10-051276. [DOI] [PubMed] [Google Scholar]
- 100.Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–10. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 101.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–33. [PubMed] [Google Scholar]
- 102.Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Invest Dermatol. 2004;122:1061–9. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 103.Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102:2060–7. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 104.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci U S A. 1998;95:14423–8. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, et al. Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–91. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 106.Matsuzaki Y, Kinjo K, Mulligan RC, Okano H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 2004;20:87–93. doi: 10.1016/s1074-7613(03)00354-6. [DOI] [PubMed] [Google Scholar]
- 107.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 108.Brenner S, Whiting-Theobald N, Kawai T, Linton GF, Rudikoff AG, Choi U, et al. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22:1128–33. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- 109.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–9. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 110.Goichberg P, Kalinkovich A, Borodovsky N, Tesio M, Petit I, Nagler A, et al. cAMP-induced PKCzeta activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood. 2006;107:870–9. doi: 10.1182/blood-2005-03-0941. [DOI] [PubMed] [Google Scholar]
- 111.Ghosh S, Preet A, Groopman JE, Ganju RK. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. 2006;43:2169–79. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 112.Coopman K, Smith LD, Wright KL, Ward SG. Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int Immunopharmacol. 2007;7:360–71. doi: 10.1016/j.intimp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Jorda MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, et al. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–93. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- 114.Maestroni GJ. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004;18:1914–6. doi: 10.1096/fj.04-2190fje. [DOI] [PubMed] [Google Scholar]
- 115.Mestre L, Correa F, Docagne F, Clemente D, Guaza C. The synthetic cannabinoid WIN 55,212–2 increases COX-2 expression and PGE2 release in murine brain-derived endothelial cells following Theiler's virus infection. Biochem Pharmacol. 2006;72:869–80. doi: 10.1016/j.bcp.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 116.Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Huffman JW, et al. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2007;293:H2210–H2218. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palazuelos J, Davoust N, Julien B, Hatterer E, Aguado T, Mechoulam R, et al. The CB(2) cannabinoid receptor controls myeloid progenitor trafficking: involvement in the pathogenesis of an animal model of multiple sclerosis. J Biol Chem. 2008;283:13320–9. doi: 10.1074/jbc.M707960200. [DOI] [PubMed] [Google Scholar]
- 118.Rosch S, Ramer R, Brune K, Hinz B. R(+)-methanandamide and other cannabinoids induce the expression of cyclooxygenase-2 and matrix metalloproteinases in human nonpigmented ciliary epithelial cells. J Pharmacol Exp Ther. 2006;316:1219–28. doi: 10.1124/jpet.105.092858. [DOI] [PubMed] [Google Scholar]
- 119.Alberich JM, Rayman N, Tas M, Verbakel SE, Battista N, van LK, et al. The peripheral cannabinoid receptor Cb2, frequently expressed on AML blasts, either induces a neutrophilic differentiation block or confers abnormal migration properties in a ligand-dependent manner. Blood. 2004;104:526–34. doi: 10.1182/blood-2003-12-4357. [DOI] [PubMed] [Google Scholar]
- 120.Kurihara R, Tohyama Y, Matsusaka S, Naruse H, Kinoshita E, Tsujioka T, et al. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J Biol Chem. 2006;281:12908–18. doi: 10.1074/jbc.M510871200. [DOI] [PubMed] [Google Scholar]
- 121.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–8. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 122.Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–92. [PubMed] [Google Scholar]
- 123.Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–64. [PubMed] [Google Scholar]
- 124.Murineddu G, Lazzari P, Ruiu S, Sanna A, Loriga G, Manca I, et al. Tricyclic pyrazoles. 4. Synthesis and biological evaluation of analogues of the robust and selective CB2 cannabinoid ligand 1-(2',4'-dichlorophenyl)-6-methyl-N-piperidin-1-yl-1,4-dihydroindeno[1,2-c ]pyrazole-3-carboxamide. J Med Chem. 2006;49:7502–12. doi: 10.1021/jm060920d. [DOI] [PubMed] [Google Scholar]
- 125.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10:403–11. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Del PA, Shao WH, Mitola S, Santoro G, Sozzani S, Haribabu B. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood. 2007;109:626–31. doi: 10.1182/blood-2006-02-003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin EH, Lee HY, Bae YS. Leukotriene B4 stimulates human monocyte-derived dendritic cell chemotaxis. Biochem Biophys Res Commun. 2006;348:606–11. doi: 10.1016/j.bbrc.2006.07.084. [DOI] [PubMed] [Google Scholar]
- 128.Moreno JJ. Differential effects of arachidonic and eicosapentaenoic Acid-derived eicosanoids on polymorphonuclear transmigration across endothelial cell cultures. J Pharmacol Exp Ther. 2009;331:1111–7. doi: 10.1124/jpet.109.157891. [DOI] [PubMed] [Google Scholar]
- 129.Belanger C, Elimam H, Lefebvre J, Borgeat P, Marleau S. Involvement of endogenous leukotriene B4 and platelet-activating factor in polymorphonuclear leucocyte recruitment to dermal inflammatory sites in rats. Immunology. 2008;124:295–303. doi: 10.1111/j.1365-2567.2007.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bautz F, Denzlinger C, Kanz L, Mohle R. Chemotaxis and transendothelial migration of CD34(+) hematopoietic progenitor cells induced by the inflammatory mediator leukotriene D4 are mediated by the 7-transmembrane receptor CysLT1. Blood. 2001;97:3433–40. doi: 10.1182/blood.v97.11.3433. [DOI] [PubMed] [Google Scholar]
- 131.Boehmler AM, Drost A, Jaggy L, Seitz G, Wiesner T, Denzlinger C, et al. The CysLT1 ligand leukotriene D4 supports alpha4beta1- and alpha5beta1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. J Immunol. 2009;182:6789–98. doi: 10.4049/jimmunol.0801525. [DOI] [PubMed] [Google Scholar]
- 132.Fowler CJ. Possible involvement of the endocannabinoid system in the actions of three clinically used drugs. Trends Pharmacol Sci. 2004;25:59–61. doi: 10.1016/j.tips.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 133.Nirodi CS, Crews BC, Kozak KR, Morrow JD, Marnett LJ. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW264. 7 cells. Proc Natl Acad Sci U S A. 2004;101:1840–5. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. J Neurochem. 2007;102:1966–77. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- 135.Huang SK, Peters-Golden M. Eicosanoid lipid mediators in fibrotic lung diseases: ready for prime time? Chest. 2008;133:1442–50. doi: 10.1378/chest.08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guerrero AT, Verri WA, Jr, Cunha TM, Silva TA, Schivo IR, Dal-Secco D, et al. Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2. J Leukoc Biol. 2008;83:122–30. doi: 10.1189/jlb.0207123. [DOI] [PubMed] [Google Scholar]
- 137.Nilsson O, Fowler CJ, Jacobsson SO. The cannabinoid agonist WIN 55,212-2 inhibits TNF-alpha-induced neutrophil transmigration across ECV304 cells. Eur J Pharmacol. 2006;547:165–73. doi: 10.1016/j.ejphar.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 138.Goichberg P, Kalinkovich A, Borodovsky N, Tesio M, Petit I, Nagler A, et al. cAMP-induced PKCzeta activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood. 2006;107:870–9. doi: 10.1182/blood-2005-03-0941. [DOI] [PubMed] [Google Scholar]
- 139.Salcedo R, Zhang X, Young HA, Michael N, Wasserman K, Ma WH, et al. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood. 2003;102:1966–77. doi: 10.1182/blood-2002-11-3400. [DOI] [PubMed] [Google Scholar]
- 140.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–96. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 141.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;47 (Suppl 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Izumi T, Yokomizo T, Obinata H, Ogasawara H, Shimizu T. Leukotriene receptors: classification, gene expression, and signal transduction. J Biochem. 2002;132:1–6. doi: 10.1093/oxfordjournals.jbchem.a003185. [DOI] [PubMed] [Google Scholar]
- 143.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 144.Desouza IA, Franco-Penteado CF, Camargo EA, Lima CS, Teixeira SA, Muscara MN, et al. Inflammatory mechanisms underlying the rat pulmonary neutrophil influx induced by airway exposure to staphylococcal enterotoxin type A. Br J Pharmacol. 2005;146:781–91. doi: 10.1038/sj.bjp.0706393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lemos HP, Grespan R, Vieira SM, Cunha TM, Verri WA, Jr, Fernandes KS, et al. Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNgamma production. Proc Natl Acad Sci U S A. 2009;106:5954–9. doi: 10.1073/pnas.0812782106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burstein SH, Zurier RB. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J. 2009;11:109–19. doi: 10.1208/s12248-009-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fourie AM. Modulation of inflammatory disease by inhibitors of leukotriene A4 hydrolase. Curr Opin Investig Drugs. 2009;10:1173–82. [PubMed] [Google Scholar]
- 148.Lazarus M. The differential role of prostaglandin E2 receptors EP3 and EP4 in regulation of fever. Mol Nutr Food Res. 2006;50:451–5. doi: 10.1002/mnfr.200500207. [DOI] [PubMed] [Google Scholar]
- 149.Narumiya S. Prostanoids in immunity: roles revealed by mice deficient in their receptors. Life Sci. 2003;74:391–5. doi: 10.1016/j.lfs.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 150.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009;57:703–8. doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 151.Stenke L, Mansour M, Edenius C, Reizenstein P, Lindgren JA. Formation and proliferative effects of lipoxins in human bone marrow. Biochem Biophys Res Commun. 1991;180:255–61. doi: 10.1016/s0006-291x(05)81285-9. [DOI] [PubMed] [Google Scholar]
- 152.Stenke L, Mansour M, Reizenstein P, Lindgren JA. Stimulation of human myelopoiesis by leukotrienes B4 and C4: interactions with granulocyte-macrophage colony-stimulating factor. Blood. 1993;81:352–6. [PubMed] [Google Scholar]
- 153.Claesson HE, Dahlberg N, Gahrton G. Stimulation of human myelopoiesis by leukotriene B4. Biochem Biophys Res Commun. 1985;131:579–85. doi: 10.1016/0006-291x(85)91276-8. [DOI] [PubMed] [Google Scholar]
- 154.Lu L, Pelus LM, Broxmeyer HE. Modulation of the expression of HLA-DR (Ia) antigens and the proliferation of human erythroid (BFU-E) and multipotential (CFU-GEMM) progenitor cells by prostaglandin E. Exp Hematol. 1984;12:741–8. [PubMed] [Google Scholar]
- 155.Lu L, Pelus LM, Broxmeyer HE, Moore MA, Wachter M, Walker D, et al. Enhancement of the proliferation of human marrow erythroid (BFU-E) progenitor cells by prostaglandin E requires the participation of OKT8-positive T lymphocytes and is associated with the density expression of major histocompatibility complex class II antigens on BFU-E. Blood. 1986;68:126–33. [PubMed] [Google Scholar]