Abstract
Two arabinosidases, α-l-arabinopyranosidase (no EC number) and α-l-arabinofuranosidase (EC 3.2.1.55), were purified from ginsenoside-metabolizing Bifidobacterium breve K-110, which was isolated from human intestinal microflora. α-l-Arabinopyranosidase was purified to apparent homogeneity, using a combination of ammonium sulfate fractionation, DEAE-cellulose, butyl Toyopearl, hydroxyapatite Ultrogel, QAE-cellulose, and Sephacryl S-300 HR column chromatography, with a final specific activity of 8.81 μmol/min/mg. α-l-Arabinofuranosidase was purified to apparent homogeneity, using a combination of ammonium sulfate fractionation, DEAE-cellulose, butyl Toyopearl, hydroxyapatite Ultrogel, Q-Sepharose, and Sephacryl S-300 column chromatography, with a final specific activity of 6.46 μmol/min/mg. The molecular mass of α-l-arabinopyranosidase was found to be 310 kDa by gel filtration, consisting of four identical subunits (77 kDa each, measured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]), and that of α-l-arabinofuranosidase was found to be 60 kDa by gel filtration and SDS-PAGE. α-l-Arabinopyranosidase and α-l-arabinofuranosidase showed optimal activity at pH 5.5 to 6.0 and 40°C and pH 4.5 and 45°C, respectively. Both purified enzymes were potently inhibited by Cu2+ and p-chlormercuryphenylsulfonic acid. α-l-Arabinopyranosidase acted to the greatest extent on p-nitrophenyl-α-l-arabinopyranoside, followed by ginsenoside Rb2. α-l-Arabinofuranosidase acted to the greatest extent on p-nitrophenyl-α-l-arabinofuranoside, followed by ginsenoside Rc. Neither enzyme acted on p-nitrophenyl-β-galactopyranoside or p-nitrophenyl-β-d-fucopyranoside. These findings suggest that the biochemical properties and substrate specificities of these purified enzymes are different from those of previously purified α-l-arabinosidases. This is the first reported purification of α-l-arabinopyranosidase from an anaerobic Bifidobacterium sp.
Plant polysaccharides and glycosides have composite structures containing complex mixtures of polysaccharides, such as cellulose, arabinogalactan, ginsenosides, and musenin (3, 16, 21). l-Arabinose is a common component of several polysaccharides and glycosides. Ginsenosides Rb2 and Rc are the main components of ginseng (the root of Panax ginseng C. A. Meyer, Araliaceae) and are frequently used in China, Korea, and Japan, as well as other Asian countries, as a traditional medicine. Ginsenosides Rb2 and Rc are l-arabinofuranoside- and l-arabinopyranoside-bound glycosides, respectively, in ginsenoside Rd (22). These ginsenosides are transformed to compound K, via ginsenoside Rd, by intestinal bacteria in the human intestine (4). The pharmacological actions of these ginsenosides have been explained on the basis of the biotransformation of ginsenosides by glycosidases of human intestinal bacteria (2, 4, 5, 9, 23). For example, the protopanaxadiol ginsenosides are transformed to compound K by human intestinal bacteria. Compound K exhibits antimetastatic and/or anticarcinogenic effects. These results suggest that arabinofuranoside- and arabinopyranoside-bound glycosides may be hydrolyzed to ginsenoside Rd by different arabinosidases.
α-l-Arabinofuranosidases (EC 3.2.1.55), previously isolated from many microbes, catalyze the hydrolysis of nonreducing terminal α-l-arabinofuranoside linkages in arabinofuranose-containing polysaccharides such as arabinogalactan (21). Nevertheless, l-arabinopyranoside linkage-hydrolyzing α-l-arabinopyranosidases have not been purified, although β-d-galactosidase (also called α-l-arabinopyranosidase) was recently cloned in Clostridium cellulovorans (14).
In this preliminary study, the α-l-arabinopyranose and α-l-arabinofuranose linkages of ginsenosides Rb2 and Rc were easily hydrolyzed to ginsenoside Rd by Bifidobacterium breve K-110, a human anaerobic intestinal bacterium. Therefore, we purified α-l-arabinopyranosidase and α-l-arabinofuranosidase from B. breve K-110, which is a potent ginsenoside-hydrolyzing bacterium of the human intestinal bacteria.
MATERIALS AND METHODS
Materials.
p-Nitrophenyl-α-l-arabinopyranoside (PNAp), p-nitrophenyl-α-l-arabinofuranoside (PNAf), p-nitrophenyl-β-xylopyranoside, sodium thioglycolate, tosyl-l-lysine chlormethyl ketone, iodoacetic acid, N-ethylmaleneimide, p-chlormercuryphenylsulfonic acid (PCMS), carbodiimide, paraoxon, dithiothreitol, mercaptoethanol, and ascorbic acid were purchased from Sigma Chemical Co. (St. Louis, Mo.). Arabinogalactan from larch wood was purchased from Fluka Co., Ltd. (Tokyo, Japan). The ginsenosides were isolated by means of a previously established method (5, 16). A general anaerobic medium was purchased from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). Tryptic soy broth and other media were purchased from Difco Co. (Detroit, Mich.). DEAE-cellulose, hydroxyapatite Ultrogel, and butyl Toyopearl were purchased from Sigma Chemical Co. Sephacryl S-300, molecular weight markers for gel filtration, and protein electrophoresis markers were purchased from Amersham Pharmacia Biotech (Piscataway, N.J.). The protein assay reagent was purchased from Bio-Rad Laboratories (Hercules, Calif.). All other chemicals were of analytical reagent grade.
Isolation of ginsenoside Rb2- and Rc-hydrolyzing bacteria from human intestinal microflora.
Bacterial strains, previously isolated from the fresh feces of a healthy Korean man, were cultured in general anaerobic broth and assayed for the ability to transform ginsenosides Rb2 and Rc to ginsenoside Rd or compound K. The strains identified as possessing these enzymatic activities were subsequently characterized according to the criteria described by Scardovi (18).
Assay of enzyme activity.
The reaction mixture, containing 200 μl of 2 mM PNAp (or PNAf or ginsenosides), 100 μl of the enzyme, and 300 μl of 50 mM phosphate buffer (pH 7.0), was incubated for 0.5, 1, and 5 h at 37°C. The reaction was stopped by the addition of 400 μl of 0.5 M NaOH. The absorbance of the mixture was measured at 405 nm with a UV spectrophotometer (Shimadzu UV-120-02). In the case of the ginsenosides, the reaction mixture was stopped by extraction with butanol. The butanol fraction was evaporated, and the residue was assayed by thin-layer chromatography TLC, using TLC plates, silica gel 60F254 (Merck Co.), and developing solvent CHCl3-methanol-H2O (65:35:10 [vol/vol]; lower phase). The plates were stained by spraying with methanol-H2SO4 (95:5 [vol/vol]) and then were heated. The stained plates were analyzed with a TLC scanner (Shimadzu model CS-9301PC).
One unit of enzyme activity was defined as the amount required to catalyze the formation of 1.0 μmol of p-nitrophenol (or ginsenoside Rd) per min under standard assay conditions. The specific activity was defined in terms of units per milligram of protein.
Protein measurement.
Protein was measured by the Bradford method, using bovine serum albumin as the standard (7).
Purification of α-l-arabinopyranosidase and α-l-arabinofuranosidase from B. breve K-110.
B. breve K-110 was cultured at 37°C for 20 h under anaerobic conditions in 10 liters of tryptic soy broth containing 0.1% ascorbic acid and 0.01% sodium thioglycolate and was harvested by centrifugation for 30 min at 5,000 × g. The pellets were washed twice with cold 50 mM sodium phosphate buffer, pH 7.0 (buffer A), and suspended in 150 ml of the same buffer, and the suspended cells were ultrasonicated on ice for 15 min (100 W, 60% pulse mode). The disrupted cells were centrifuged at 10,000 × g for 60 min and the supernatant was used as a crude enzyme solution. The crude enzyme was precipitated with 70% saturated ammonium sulfate and centrifuged at 10,000 × g for 60 min. The pellets were resuspended with 70 ml of 25 mM sodium phosphate buffer and dialyzed twice against 3 liters of buffer A. All purification procedures were performed at 4°C.
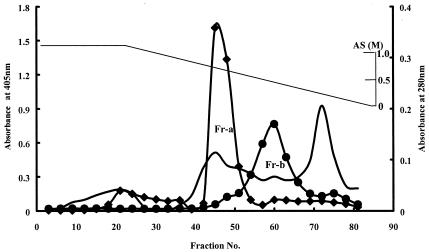
The dialysate was applied to a DEAE-cellulose column (2.8 by 34 cm) that had previously been equilibrated with buffer A. The column was washed with 250 ml of the same buffer. A linear gradient elution was performed with 300 ml of buffer A and 300 ml of the same buffer containing 1 M KCl. All of the fractions obtained were assayed for α-l-arabinopyranosidase and α-l-arabinofuranosidase activities. The active fractions were pooled and dialyzed for 18 h against an equal volume of buffer A containing 2.0 M ammonium sulfate. The combined dialysate from the DEAE-cellulose column was applied to a butyl Toyopearl column (2.8 by 4.0 cm) that was previously equilibrated with buffer A containing 1.0 M ammonium sulfate. The column was washed with 120 ml of the same buffer, and a linear gradient elution was performed with 300 ml of buffer A containing 1.0 M ammonium sulfate followed by 300 ml of buffer A (Fig. 1). All of the obtained fractions were assayed for α-l-arabinopyranosidase and α-l-arabinofuranosidase activities. Two fractions (fraction a, α-l-arabinopyranosidase; fraction b, α-l-arabinofuranosidase) were identified.
FIG. 1.
Elution profile of α-l-arabinopyranosidase and α-l-arabinofuranosidase activities from butyl Toyopearl column. Diamonds, α-l-arabinopyranosidase activity; circles, α-l-arabinopyranosidase activity; unmarked line, absorbance at 280 nm. AS, ammonium sulfate. The enzyme activities were indicated as absorbance at 405 nm. Fr-a and Fr-b, fractions a and b, respectively.
Purification of α-l-arabinopyranosidase.
Fraction a was dialyzed twice against 3 liters of a 10 mM phosphate buffer (pH 7.0) (buffer B) to purify the α-l-arabinopyranosidase further. The dialysate was applied to a hydroxyapatite Ultrogel column (2.8 by 3.0 cm) that had previously been equilibrated with buffer B. α-l-Arabinopyranosidase was eluted from the column with 240 ml of sodium phosphate buffer (linear gradient, 10 to 150 mM); the active enzyme fractions (32 ml in 8 fractions) were pooled and dialyzed twice against 3 liters of buffer A for 18 h. The dialysate was applied to a QAE-cellulose column (1.6 by 3.0 cm) that had previously been equilibrated with buffer A. The column was washed with 250 ml of the same buffer, and a linear gradient elution was performed with 200 ml of buffer A followed by 200 ml of the same buffer containing 1 M KCl. All of the obtained fractions were assayed for α-L-arabinopyranosidase activity. The active fractions were pooled and dialyzed twice against 2 liters of buffer A for 18 h. The dialysate was concentrated to approximately 1.5 ml by using the Advantec pressure filtration system at 9 lb/in2 and 4°C, with PM-10 membranes. The concentrated solution was applied to a Sephacryl S-300 HR column (1.6 by 70 cm) that had previously been equilibrated with buffer A and was then eluted (flow rate, 0.5 ml/min; fraction volume, 1.02 ml). The active fractions (6.12 ml in 6 fractions) were found to be homogeneous α-l-arabinopyranosidase by native and denatured polyacrylamide gel electrophoresis (PAGE).
Purification of α-l-arabinofuranosidase.
The α-l-arabinofuranosidase active fraction (fraction b) collected from the butyl Toyopearl column chromatography described above was dialyzed twice against 3 liters of buffer B. The dialysate was passed through a hydroxyapatite Ultrogel column (2.8 by 3.0 cm) that had previously been equilibrated with buffer B. The active enzyme fractions were pooled and applied to a Q-Sepharose column (1.0 by 1.5 cm) that had previously been equilibrated with buffer A. The column was washed with 250 ml of the same buffer, and a linear gradient elution was performed with 100 ml of buffer A followed by 100 ml of the same buffer containing 1 M KCl. All of the fractions obtained were assayed for α-l-arabinofuranosidase activity. The active fractions were pooled and dialyzed twice against 2 liters of buffer A for 18 h. The dialysate was concentrated by using the Advantec pressure filtration system, with PM-10 membranes, to approximately 2 ml. The concentrated solution was applied to a Sephacryl S-300 HR column (1.6 by 70 cm) that had previously been equilibrated with buffer A and was then eluted (flow rate, 0.5 ml/min; fraction volume, 1.02 ml). The active fractions (4.08 ml in 4 fractions) were found to be homogeneous α-l-arabinopyranosidase by native and denatured PAGE.
Characterization of α-l-arabinosidases.
Electrophoresis was performed in a discontinuous polyacrylamide gel (10% separating gel and 4% stacking gel; 1-mm thickness) under native or denatured conditions by the procedure described by Laemmli (15). The gel was treated with Coomassie brilliant blue R250 and was further stained with silver. The molecular weights of the purified enzymes were estimated by comparison with molecular weight markers. Enzyme activity staining was performed as follows. The electrophoresis gel was cut at 5-mm intervals, immersed in the enzyme reaction mixture instead of the enzyme, and incubated at 37°C for 5 h. The reaction was stopped by the addition of 500 μl of 0.25 M NaOH, and the absorbance was measured at 405 nm by use of a UV spectrophotometer. The molecular weight of the native enzyme was estimated by gel filtration using a Sephacryl S-300 column (1.6 by 70 cm) that had previously been equilibrated with a gel filtration low- and high-molecular-weight calibration kit (from Sigma and Amersham Pharmacia Biotech).
The optimum pHs for the purified enzymes were obtained with the following buffers: 50 mM acetate (pH 3.0 to 5.5), 50 mM phosphate (pH 5.5 to 8.0), and 50 mM NaOH-glycine (pH 8.0 to 10.0).
To investigate the effects of salt concentrations on enzyme activity, the enzymes were incubated with a substrate in reaction mixtures containing various concentrations of NaCl, KCl, or ammonium sulfate for 30 min at 37°C and their activities were assayed.
Kinetic constants of arabinosidases were determined by measuring the initial rates at various substrate concentrations (0.0025, 0.01, 0.025, 0.1, 0.25, 1.0, and 2.5 mM) under standard reaction conditions.
To investigate the effect of metals and chemical modifying agents on enzyme activity, the enzymes were incubated with various concentrations of metals and chemical modifying agents for 30 min at 37°C and their activities were measured.
To obtain the amino acid compositions of the purified enzyme fractions, the fractions were dialyzed twice against distilled water and then lyophilized. After acid hydrolysis in 6 N HCl at 110°C for 20 and 36 h, the compositions were analyzed in a Beckman 6300E amino acid analyzer. For internal amino acid sequence analyses of α-l-arabinopyranosidase and α-l-arabinofuranosidase, both enzymes were digested with trypsin and analyzed with an Applied Biosystem protein sequencer, model 492, at the Korea Basic Science Institute.
RESULTS
Screening of ginsenoside Rb2- and Rc-hydrolyzing human intestinal bacteria.
Preliminary assays of five samples of human feces revealed ginsenoside Rb2- and Rc-hydrolyzing activities in all instances, but at different levels (data not shown). Among the intestinal bacteria isolated from the human feces, 12 potently hydrolyzed the ginsenosides Rb2 and Rc to the ginsenoside Rd or compound K. Of these, the isolate K-110 was selected as having the highest ability to convert these substrates; in this instance, ginsenoside Rd was the main product. The K-110 isolate was identified as B. breve on the basis of criteria defined by Scardovi: it was anaerobic, gram positive, non-spore-forming, nonmotile, and fructose-6-phosphate-phosphoketolase positive and fermented d-ribose, lactose, cellobiose, melezitose, raffinose, and sorbitol, but not l-arabinose (18). The α-l-arabinopyranosidase and α-l-arabinofuranosidase activities were observed to be progressive with the growth of B. breve K-110 and reached a plateau after 18 to 22 h of cultivation. B. breve K-110 constitutively produced these enzymes (data not shown).
Purification of α-l-arabinopyranosidase and α-l-arabinofuranosidase.
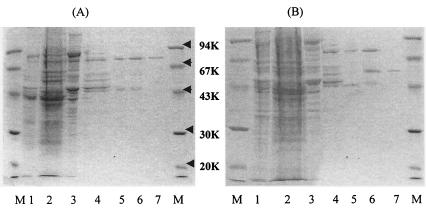
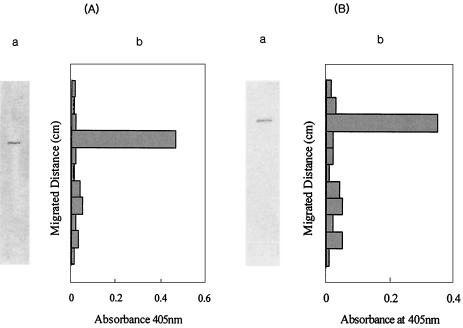
α-l-Arabinopyranosidase was purified 62-fold from the crude extract, with a yield of 4.3%, by the procedures shown in Table 1. The specific activity of the homogeneously purified α-l-arabinopyranosidase was 8.71 μmol/min/mg. Only a single band for the purified enzyme was observed in a PAGE gel with sodium dodecyl sulfate (SDS) (Fig. 2A). The Coomassie brilliant blue-stained band for the purified enzyme was at the identical location as the enzyme activity peaks seen on non-SDS-PAGE gels (Fig. 3A).
TABLE 1.
Summary of purification of α-l-arabinopyranosidase and α-l-arabinofuranosidase from B. breve K-110
| Enzyme or purification step | Total activity (μmol/min) | Total protein (mg) | Sp act (μmol/min/mg) |
|---|---|---|---|
| α-l-Arabinopyranosidase | |||
| Crude extract | 14.30 | 98.60 | 0.15 |
| Ammonium sulfate precipitation | 12.10 | 82.83 | 0.15 |
| DEAE-cellulose column chromatography | 8.94 | 40.14 | 0.22 |
| Butyl Toyopearl column chromatography | 7.20 | 4.69 | 1.54 |
| Hydroxyapatite Ultrogel column chromatography | 5.61 | 1.27 | 4.42 |
| QAE-cellulose column chromatography | 2.12 | 0.39 | 5.44 |
| Sephacryl S-300 HR column chromatography | 0.61 | 0.07 | 8.71 |
| α-l-Arabinofuranosidase | |||
| Crude extract | 10.09 | 46.10 | 0.11 |
| Ammonium sulfate precipitation | 8.45 | 37.88 | 0.22 |
| DEAE-cellulose column chromatography | 6.07 | 18.79 | 0.45 |
| Butyl Toyopearl column chromatography | 3.09 | 3.64 | 0.85 |
| Hydroxyapatite Ultrogel column chromatography | 1.85 | 1.50 | 1.23 |
| Q-Sepharose column chromatography | 1.45 | 0.41 | 3.54 |
| Sephacryl S-300 column chromatography | 0.71 | 0.11 | 6.46 |
FIG. 2.
SDS-PAGE of α-l-arabinopyranosidase (A) and α-l-arabinofuranosidase (B). (A) Lane 1, crude extract; lane 2, ammonium sulfate precipitation; lane 3, DEAE-cellulose column; lane 4, butyl Toyopearl column; lane 5, hydroxyapatite column; lane 6, QAE-cellulose column; lane 7, Sephacryl S-300 column. (B) Lane 1, crude extract; lane 2, ammonium sulfate precipitation; lane 3, DEAE-cellulose column; lane 4, butyl Toyopearl column; lane 5, hydroxyapatite column; lane 6, Q-Sepharose column; lane 7, Sephacryl S-300 column. M, molecular mass markers.
FIG. 3.
Native PAGE of α-l-arabinopyranosidase (A) and α-l-arabinofuranosidase (B). (A) Panel a, native PAGE; panel b, α-l-arabinopyranosidase activity of native PAGE fragments. (B) Panel a, native PAGE; panel b, α-l-arabinofuranosidase activity of native PAGE.
α-l-Arabinofuranosidase was purified 59-fold from the crude extract, with a yield of 0.7%, by the procedures shown in Table 1. The specific activity of homogeneously purified α-l-arabinofuranosidase was 6.46 μmol/min/mg. A single band for the purified enzyme was observed in the PAGE gel, with SDS (Fig. 2B). The Coomassie brilliant blue-stained band for the purified enzyme was at the identical location as the enzyme activity peaks on non-SDS-PAGE gels (Fig. 3B).
Characterization of α-l-arabinopyranosidase and α-l-arabinofuranosidase.
The molecular mass of α-l-arabinopyranosidase was 310 kDa by Sephacryl S-300 HR chromatography and 77 kDa by SDS-PAGE (Fig. 2). The molecular mass of α-l-arabinofuranosidase was 60 kDa by both Sephacryl S-300 chromatography and SDS-PAGE.
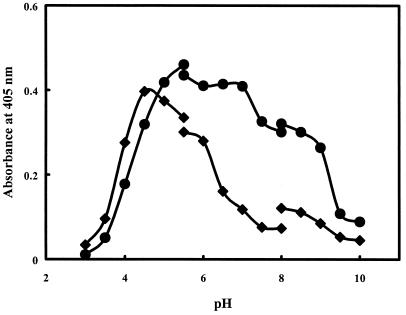
When the activities of the purified α-l-arabinopyranosidase and α-l-arabinofuranosidase were assayed at 37°C, the optimal pHs for these enzymes were found to be 5.5 and 4.5, respectively (Fig. 4). The activities of both purified enzymes were little affected by NaCl or KCl ionic strength (0 to 1 M), but α-l-arabinofuranosidase was inhibited by ammonium sulfate, with a 50% inhibitory concentration of 2.5 M (data not shown). When these enzymes were incubated at 37°C for 5 h, their residual activities were >90% of the original activities.
FIG. 4.
pH profile of α-l-arabinopyranosidase and α-l-arabinofuranosidase activities. The enzyme activities were assayed under standard conditions. Diamonds, α-l-arabinofuranosidase activity; circles, α-l-arabinopyranosidase activity.
The effects of chemical modifying agents and metal ions on purified α-l-arabinopyranosidase and α-l-arabinofuranosidase are shown in Tables 2 and 3. PCMS alone potently inhibited both enzymes. Both enzymes were inhibited by Cu2+ and Fe2+. However, most metal ions had no inhibitory effect on the enzymes.
TABLE 2.
Effects of chemical modifying agents on α-l-arabinopyranosidase and α-l-arabinofuranosidase activities
| Modifying agenta | 50% Inhibitory concentration (mM)b for:
|
|
|---|---|---|
| α-l-arabinopyranosidase | α-l-arabinofuranosidase | |
| Phenylmethylsulfonyl fluoride | 108 | 102 |
| Tosyl-l-lysine chlormethyl ketone | 115 | 122 |
| Iodoacetic acid | 101 | 110 |
| Paraoxon | 92 | 98 |
| PCMS | 41 | 22 |
| Dithiothreitol | 118 | 118 |
| Mercaptoethanol | 121 | 122 |
| 2,3-Butanediol | 97 | 91 |
| N-Ethylmaleneimide | 101 | 105 |
| Carbodiimide | 98 | 114 |
Final concentration, 0.05 mM.
For homogenously purified enzyme activity, 0.05 U was taken as 100%.
TABLE 3.
Effects of metals on α-l-arabinopyranosidase and α-l-arabinofuranosidase activities
| Metala | Residual activity (%)b
|
|
|---|---|---|
| α-l-arabinopyranosidase | α-l-arabinofuranosidase | |
| Control | 100 | 100 |
| Mg2+ | 104 | 99 |
| Ca2+ | 132 | 96 |
| Ni2+ | 105 | 86 |
| Ba2+ | 94 | 119 |
| Co2+ | 117 | 111 |
| Cu2+ | 22 | 36 |
| Pb2+ | 95 | 107 |
| Fe2+ | 46 | 45 |
| Li2+ | 105 | 86 |
| Zn2+ | 91 | 103 |
| EDTA | 96 | 91 |
Final concentration, 1 mM.
For the homogenously purified enzyme activity, 0.05 U was taken as 100%.
From the amino acid composition analysis, purified α-l-arabinopyranosidase and α-l-arabinofuranosidase were observed to contain large portions of glycine and serine (data not shown). The amino acid compositions of both enzymes were similar, but not identical. The pI values for purified α-l-arabinopyranosidase and α-l-arabinofuranosidase were 3.9 and 4.0, respectively.
The internal sequences of a peptide obtained by digestion of each enzyme with trypsin are shown in Table 4. The internal sequence of α-l-arabinopyranosidase exhibited significant homology, of 57%, to that of the recently cloned β-d-galactosidase (α-l-arabinopyranosidase) of C. cellulovorans (14). However, those of the other reported glycosidases did not exhibit significant homology. The internal sequence of α-l-arabinofuranosidase exhibited a higher level of homology, 71%, than that previously reported for α-l-arabinofuranosidase of Bifidobacterium longum (19), and poor homology, 43%, compared to that previously reported for α-l-arabinofuranosidases from Oceanobacillus iheyensis (8) and Bacillus subtilis (20). However, those of the other reported glycosidases did not exhibit significant homology (<30%) (6, 10, 24).
TABLE 4.
Internal amino acid sequences of α-l-arabinopyranosidase and α-l-arabinofuranosidase
| Enzyme | Internal amino acid sequence | Homology (%) | Reference |
|---|---|---|---|
| α-l-Arabinopyranosidase | VIYLTDA | ||
| β-d-Galactosidase (α-l-arabinopyranosidase) of C. cellulovorans | 450 TLYLTDD | 57 | 6 |
| α-l-Arabinofuranosidase | TVNRALE | ||
| α-l-Arabinofuranosidase of B. longum | 473 AVNRSLE | 71 | 11 |
| α-l-Arabinofuranosidase of B. subtilis | 285 TINLSLD | 43 | 12 |
| α-l-Arabinofuranosidase of O. iheyensis | 142 TANRQPP | 43 | 13 |
Substrate specificity.
The substrate specificities of α-l-arabinopyranosidase and α-l-arabinofuranosidase for synthetic substrates and natural glycosides were investigated (Table 5). The best substrate for α-l-arabinopyranosidase was PNAp, followed by ginsenoside Rb2. This enzyme transformed ginsenoside Rb2 to ginsenoside Rd [not compound K or 20(S)-protopanaxadiol]. PNAf, p-nitrophenyl-β-galactopyranoside, p-nitrophenyl-β-fucopyranoside, and cellobiose were not effective as substrates. The best substrate for α-l-arabinofuranosidase was PNAf, followed by ginsenoside Rc. This enzyme transformed ginsenoside Rc to ginsenoside Rd [not compound K or 20(S)-protopanaxadiol]. Purified α-l-arabinofuranosidase did not hydrolyze p-nitrophenyl-β-galactopyranoside, p-nitrophenyl-β-xylopyranoside, p-nitrophenyl-β-fucopyranoside, or cellobiose.
TABLE 5.
Substrate specificity of B. breve K-110 α-l-arabinopyranosidase and α-l-arabinofuranosidase
| Substrate | Enzyme activity (%)
|
|
|---|---|---|
| α-l-Arabino- pyranosidase | α-l-Arabino- furanosidase | |
| PNAp | 100 | 3.2 |
| PNAf | <1 | 100 |
| p-Nitrophenyl-β-galactopyranoside | 0 | 0 |
| p-Nitrophenyl-β-fucopyranoside | 0 | 0 |
| p-Nitrophenyl-α-glucopyranoside | 0 | 0 |
| p-Nitrophenyl-β-glucopyranoside | 0 | 0 |
| p-Nitrophenyl-β-xylopyranoside | 0 | 0 |
| Maltose | 0 | 0 |
| Cellobiose | 0 | 0 |
| Ginsenoside Rb2 | 1.2 | 0 |
| Ginsenoside Rc | 0 | 12.1 |
| Ginsenoside Ra1 | 0 | 0 |
| Ginsenoside Ra2 | 0 | 0 |
| Arabinogalactana | 0 | 0 |
Final concentration, 0.5 mg/ml.
Kinetic constants of α-l-arabinosidases.
Using PNAp and ginsenoside Rb2 as substrates for α-l-arabinopyranosidase, the Km and Vmax values were estimated to be 0.16 mM and 10.7 μmol/min/mg, respectively, for PNAp and 0.086 mM and 0.13 μmol/min/mg, respectively, for Rb2 (Table 6). Using PNAf and ginsenoside Rc as substrates for α-l-arabinofuranosidase, the Km and Vmax values were estimated to be 0.22 mM and 9.30 μmol/min/mg, respectively, for PNAf and 0.084 mM and 0.91 μmol/min/mg, respectively, for Rc.
TABLE 6.
Km and Vmax values for α-l-arabinopyranosidase and α-l-arabinofuranosidase
| Enzyme |
Km (mM) with substrate:
|
Vmax (μmol/min/mg of protein) with substrate:
|
||||||
|---|---|---|---|---|---|---|---|---|
| PNAp | PNAf | Rb2 | Rc | PNAp | PNAf | Rb2 | Rc | |
| α-l-Arabinopyranosidase | 0.16 | —a | 0.086 | — | 10.7 | — | 0.13 | — |
| α-l-Arabinofuranosidase | 1.01 | 0.22 | — | 0.084 | 0.31 | 9.30 | — | 0.91 |
—, not detectable.
DISCUSSION
Intestinal microflora play significant roles in the metabolism of unabsorbable dietary components, arabinogalactan, and ginsenosides and in the enterohepatic circulation of endogenous and exogenous substances (12, 13). For the purpose of investigating the biological action of the components of ginseng, the metabolism by human intestinal bacteria of ginsenosides from ginseng has been studied previously (1, 5, 11, 12).
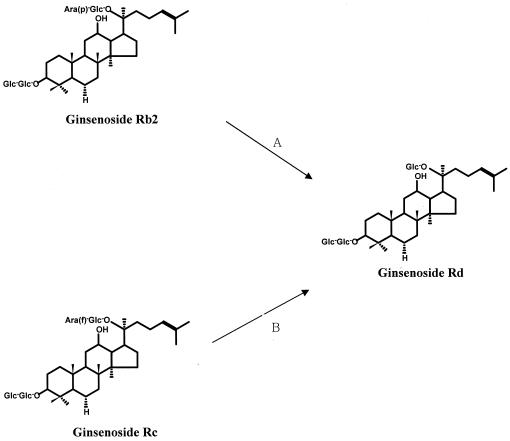
The major components of ginseng are ginsenosides and glycosides containing an aglycone with a dammarane skeleton (16, 22). To express the pharmacological actions of these ginsenosides, it is thought that ginseng saponins must be metabolized by human intestinal bacteria after they are orally administered(2, 7, 23). In studies related to the biotransformation of ginsenosides, Hasegawa et al. and Bae et al. isolated Prevotella oris, Fusobacterium K-60, and Bacteroides HJ-15 from human intestinal feces (5, 9). Park et al. purified β-glucosidase, which is involved in the metabolism of ginsenoside Rb1 from Fusobacterium K-60 (17). However, studies on purification and characterization related to the metabolism of ginsenosides by the α-l-arabinosidases of intestinal bacteria have not been reported. Therefore, we purified and characterized the ginsenoside Rb2-hydrolyzing α-l-arabinopyranosidase and the ginsenoside Rc-hydrolyzing α-l-arabinofuranosidase from B. breve K-110 (Fig. 5), which was isolated from human intestinal feces as a ginsenoside-hydrolyzing intestinal bacterium.
FIG. 5.
Proposed metabolic conversions for the ginsenosides Rb2 and Rc by α-l-arabinopyranosidase (A) and α-l-arabinofuranosidase (B) from B. breve K-110.
The molecular masses of purified α-l-arabinopyranosidase and α-l-arabinofuranosidase were determined to be 310 kDa (consisting of four identical 77-kDa subunits, as measured by SDS-PAGE) and 60 kDa (60 kDa was also measured by SDS-PAGE), respectively, by a gel filtration method using a Sephacryl S-300 HR column. These results suggest that α-l-arabinopyranosidase consists of four identical subunits and that α-l-arabinofuranosidase is a monomer. The optimal pHs for these enzymes were found to be 5.5 and 4.5, respectively. These enzymes were thermostable during incubation at 37°C. Both enzymes were inhibited by Cu2+ and Fe2+. PCMS alone of the chemical modifiers potently inhibited both enzymes. These results suggest that cysteine may be important for the catalytic activity of these enzymes. When internal sequences of two purified α-l-arabinosidases were compared with those of previously reported glycosidases, that of the α-l-arabinofuranosidase purified for the present study showed a higher level of homology, of 71%, than the recently reported α-l-arabinofuranosidase of B. longum (19). However, the internal sequence of α-l-arabinopyranosidase showed poor homology to the previously reported glycosidases. Of these, the internal sequence of the α-l-arabinopyranosidase purified for the present study showed moderate homology, of 57%, to that of the recently reported cloned β-d-galactosidase (α-l-arabinopyranosidase) from C. cellulovorans (14). When the substrate specificities of α-l-arabinopyranosidase and α-l-arabinofuranosidase for synthetic substrates and natural glycosides were investigated, α-l-arabinopyranosidase hydrolyzed PNAp and ginsenoside Rb2, but did not hydrolyze PNAf, p-nitrophenyl-β-galactopyranoside, p-nitrophenyl-β-xylopyranoside, or p-nitrophenyl-β-fucopyranoside. The purified enzyme substrate specificity for p-nitrophenyl-β-galactopyranoside was quite different from that of the recently cloned β-d-galactosidase (α-l-arabinopyranosidase) (14). α-l-Arabinofuranosidase hydrolyzed PNAf and ginsenoside Rc and weakly catalyzed PNAp. However, it did not hydrolyze PNAp, p-nitrophenyl-β-galactopyranoside or p-nitrophenyl-β-fucopyranoside. The purifiedα-l-arabinofuranosidase substrate specificity was different from that of the previously reported α-l-arabinofuranosidase from B. longum (19), even though the purified substrate specificity could not be sufficiently compared with those of previously reported enzymes. However, the purified enzyme may be genetically similar to the α-l-arabinofuranosidase from B. longum.
In conclusion, this is the first report of the purification and characterization of ginsenoside-hydrolyzing α-l-arabinosidases from human intestinal bacteria. The substrate specificities and characterization of α-l-arabinosidases are different from those previously reported for α-l-arabinosidases. The α-l-arabinopyranosidase and α-l-arabinofuranosidase produced from human intestinal bacteria could transform ginsenoside Rb2 and Rc to ginsenoside Rd in the human intestine. Finally, we suggest that the newly purified α-l-arabinopyranosidase must be classified differently from α-l-arabinofuranosidase.
Acknowledgments
This work was supported by a grant from Il-Hwa Co., Guri, Kyonggi-do,Korea.
REFERENCES
- 1.Akao, T., M. Kanaoka, and K. Kobashi. 1998. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration-measurement of compound K by enzyme immunoassay. Biol. Pharm. Bull. 21:245-249. [DOI] [PubMed] [Google Scholar]
- 2.Akao, T., H. Kida, M. Kanaoka, M. Hattori, and K. Kobashi. 1998. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from panax ginseng. J. Pharm. Pharmacol. 50:1155-1160. [DOI] [PubMed] [Google Scholar]
- 3.Aspinall, G. O. 1980. Chemistry of cell-wall polysaccharides, p. 473-500. In J. Press (ed.), The biochemistry of plants, vol. 3. Academic Press, Inc., New York, N.Y.
- 4.Bae, E.-A., S.-Y. Park, and D.-H. Kim. 2000. Constitutive β-glucosidase hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol. Pharm. Bull. 23:1481-1485. [DOI] [PubMed] [Google Scholar]
- 5.Bae, E.-A., M.-K. Choo, S.-Y. Park, H.-Y. Shin, and D.-H. Kim. 2002. Metabolism of ginsenoside Rc by human intestinal bacteria and its related antiallergic activity. Biol. Pharm. Bull. 25:743-747. [DOI] [PubMed] [Google Scholar]
- 6.Bourne, Y., and B. Henrissat. 2001. Glycoside hydrolases and glycosyltransferases: families and functional modules. Curr. Opin. Struct. Biol. 11:593-600. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Gothel, S. F., R. Schmid, A. Wipat, N. M. Carter, P. T. Emmerson, C. R. Harwood, and M. A. Marahiel. 1997. An internal FK506-binding domain is the catalytic core of the prolyl isomerase activity associated with the Bacillus subtilis trigger factor. Eur. J. Biochem. 244:59-65. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa, H., J. H. Sung, and Y. Benno. 1997. Role of human intestinal Prevotella oris in hydrolyzing ginseng saponins. Planta Med. 63:436-440. [DOI] [PubMed] [Google Scholar]
- 10.Henrissat, B. 1991. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanaoka, M., T. Akao, and K. Kobashi. 1994. Metabolism of ginseng saponins, ginsenosides, by human intestinal flora. J. Trad. Med. 11:241-245. [Google Scholar]
- 12.Karikura, M., T. Miyase, H. Tanizawa, T. Tanizawa, and Y. Takino. 1991. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VI. The decomposition products of ginsenoside Rb2 in the stomach of rats. Chem. Pharm. Bull. 39:400-404. [DOI] [PubMed] [Google Scholar]
- 13.Kobashi, K., and T. Akao. 1997. Relation of intestinal bacteria to pharmacological effects of glycosides. Bifidobacteria Microflora 16:1-7. [Google Scholar]
- 14.Kosugi, A., K. Murahima, and R. H. Doi. 2002. Characterization of two noncellulosomal subunits, ArfA and BgaA, from Clostridium cellulovorans that cooperate with the cellulosome in plant cell wall degradation. J. Bacteriol. 184:6859-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Nagai, M., S. Ando, N. Tanaka, O. Tanaka, and S. Shibata. 1972. Chemical studies on the Oriental plant drugs. XXVIII. Saponins and sapogenins of ginseng: stereochemistry of the sapogenin of ginsenoside Rb1, Rb2 and Rc. Chem. Pharm. Bull. 20:1212-1216. [Google Scholar]
- 17.Park, S.-Y., E.-A. Bae, J. H. Sung, S. K. Lee, and D.-H. Kim. 2001. Purification and characterization of ginsenoside Rb1-metabolizing β-glucosidase from Fusobacterium K-60, a human intestinal anaerobic bacterium. Biosci. Biotechnol. Biochem. 65:1163-1169. [DOI] [PubMed] [Google Scholar]
- 18.Scardovi, V. 1984. Genus Bifidobacterium, p. 1418-1434. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology. Williams and Wilkins, Baltimore, Md.
- 19.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M. Zalhlen, F. Desierre, P. Bork, M. Delley, R. D. Pridmore, and F. Arignoi. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takami, H., Y. Takaki, and I. Uchiyama. 2002. Genome sequence of Oceanobacillus iheyensis from the Iheya Ridge and its unexpected adaptive capabilities to extreme environment. Nucleic Acids Res. 30:3927-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson, J. A. 1993. Molecular biology of xylan degradation. FEMS Microbiol. 104:65-82. [DOI] [PubMed] [Google Scholar]
- 22.Trease, G. E., and W. C. Evans (ed.). 1983. Pharmacognosy, p. 485-486. Bailliere Tindall, London, United Kingdom.
- 23.Wakabayashi, C., H. Hasegawa, J. Murata, and I. Saiki. 1997. In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol. Res. 9:411-417. [PubMed] [Google Scholar]
- 24.Zverlov, V. V., W. Liebl, M. Bachleitner, and W. H. Schwarz. 1998. Nucleotide sequence of arfB of Clostridium stercoranrium and prediction of catalytic residues of α-l-arabinofuranosidases based on local similarity with several families of glycosyl hydrolases. FEMS Microbiol. Lett. 164:337-343. [DOI] [PubMed] [Google Scholar]