Abstract
Calcium-sensitive potassium (KCa) channels have been shown to modulate the diameter of cerebral pial arteries; however, little is known regarding their roles in controlling cerebral parenchymal arterioles (PAs). We explored the function and cellular distribution of small-conductance (SKCa) and intermediate-conductance (IKCa) KCa channels and large-conductance KCa (BKCa) channels in endothelial cells (ECs) and smooth muscle cells (SMCs) of PAs. Both SKCa and IKCa channels conducted the outward current in isolated PA ECs (current densities, ∼20 pA/pF and ∼28 pA/pF at +40 mV, respectively), but these currents were not detected in PA SMCs. In contrast, BKCa currents were prominent in PA SMCs (∼154 pA/pF), but were undetectable in PA ECs. Pressurized PAs constricted to inhibition of SKCa (∼16%) and IKCa (∼16%) channels, but were only modestly affected by inhibition of BKCa channels (∼5%). Blockade of SKCa and IKCa channels decreased resting cortical cerebral blood flow (CBF) by ∼15%. NS309 (6,7-dichloro-1H-indole-2,3-dione3-oxime), a SKCa/IKCa channel opener, hyperpolarized PA SMCs by ∼27 mV, maximally dilated pressurized PAs, and increased CBF by ∼40%. In conclusion, these data show that SKCa and IKCa channels in ECs profoundly modulate PA tone and CBF, whereas BKCa channels in SMCs only modestly influence PA diameter.
Keywords: BKCa, calcium-activated potassium channel, IKCa, parenchymal arterioles, SKCa
Introduction
Arterioles within the brain parenchyma are critical for regulating and maintaining brain perfusion, and are a major determinant of cerebrovascular resistance (Faraci and Heistad, 1990). These parenchymal arterioles (PAs) are composed of an endothelial cell (EC) layer and a single smooth muscle cell (SMC) layer. However, unlike arterioles in other vascular beds, PAs lack extrinsic innervation (Cipolla et al, 2004) and are encased by astrocytic projections (endfeet) (Iadecola and Nedergaard, 2007). The properties of PAs are poorly understood; however, they are known to constrict at lower intravascular pressures than the arteries on the brain surface (pial arteries) (Golding et al, 1998; Cipolla et al, 2004; Cipolla and Bullinger, 2008), consistent with an adaptive response to the prevailing intravascular pressures experienced by these vessels in vivo. This observation suggests a fundamental difference in the relationship between pressure and smooth muscle tone in PAs.
Three types of calcium-sensitive potassium (KCa) channels in the vascular wall can potentially act to oppose pressure-induced vasoconstriction: large-conductance, voltage-dependent KCa (BKCa) channels, intermediate-conductance KCa (IKCa) channels, and small-conductance KCa (SKCa) channels (Ledoux et al, 2006). The BKCa channels have a major role in opposing pressure-induced constriction (myogenic tone) in the pial arteries (Brayden and Nelson, 1992), where their activity is dynamically regulated by ryanodine receptor-mediated calcium spark activity (Nelson et al, 1995). In these same arteries, endothelial IKCa and SKCa channels do not seem to have a tonic role in opposing myogenic constriction (Cipolla et al, 2009), but can be engaged by endothelial agonists (Andresen et al, 2006), with the IKCa channel having a much more prominent role (Marrelli et al, 2003). However, in contrast to the pial arteries, recent evidence suggests that SKCa and IKCa channels tonically oppose pressure-induced constriction of PAs (Cipolla et al, 2009). This is consistent with results obtained in other vascular beds indicating that the role of the endothelial-derived hyperpolarizing factor (EDHF), a collection of dilatory influences defined by their strict dependence on endothelial IKCa and SKCa channel activity (Andresen et al, 2006), is much more prominent in small-diameter arteries and arterioles (<100 μm) than in large-diameter arteries (Andresen et al, 2006; Cipolla et al, 2009). The contribution of the BKCa channel to myogenic constriction of PAs is not clear.
The BKCa channel, composed of a pore-forming α-subunit (KCa1.1, encoded by KCNMA1) and a regulatory β-subunit (β1 in the smooth muscle), is activated by both membrane-potential depolarization and intracellular calcium (Ca2+); the associated β1 subunit (encoded by KCNMB1) increases the apparent voltage and Ca2+ sensitivity of the α-subunit (Brenner et al, 2000). The IKCa channels (KCa3.1, encoded by KCNN4) and SKCa channels, of which there are three isoforms (KCa2.1-3, encoded by KCNN1-3) are activated by Ca2+ binding to constitutively associated calmodulin on the carboxy-terminus of the channels (Ledoux et al, 2006). Both IKCa and SKCa channels are not intrinsically voltage dependent, but are blocked in a voltage-dependent manner by intracellular Ca2+ and magnesium (Mg2+) (Ledoux et al, 2008a). The major SKCa isoform in ECs appears to be SKCa3 (KCa2.3), and suppression of its expression increases vascular tone and blood pressure in mice (Taylor et al, 2003). Similarly, disruption of the IKCa channel gene leads to an increase in blood pressure (Si et al, 2006). Although BKCa channels are expressed in virtually all types of the vascular smooth muscle, whether these channels are also expressed in ECs is a matter of some controversy (Sandow and Grayson, 2009). Both SKCa and IKCa channels are prominently expressed in the vascular endothelium, and it has been suggested that these channel types may also be present in the smooth muscle (Edwards et al, 2010).
Very little is known about the physiologic roles of BKCa, SKCa, and IKCa channels in PAs. Even the basic features of these channels, such as their distribution in ECs and SMCs of PAs, have not been established. Critically, direct electrophysiological measurements of IKCa and SKCa channel currents in any type of arterioles are lacking, and only a limited number of measurements are available from larger arteries (Table 1). The channel functionality of BKCa, IKCa, and SKCa can be isolated pharmacologically using various combinations of the following synthetic and naturally occurring channel drugs and toxins: paxilline and iberiotoxin, which selectively block BKCa channels, charybdotoxin (ChTx), which blocks BKCa and IKCa channels, but not SKCa channels, TRAM-34 (1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole), which selectively blocks IKCa channels, and apamin, which blocks the three isoforms of SKCa channels, but not IKCa or BKCa channels (Ledoux et al, 2006). In addition, both SKCa and IKCa channels can be selectively opened by the synthetic compound NS309 (6,7-dichloro-1H-indole-2,3-dione3-oxime), which does not activate BKCa channels (Strøbaek et al, 2004).
Table 1. IKCa and SKCa current densities in freshly dissociated endothelial cells.
| Source | IKCa current density at 0 mV | SKCa current density at 0 mV | IKCa to SKCa current ratio | Reference |
|---|---|---|---|---|
| Mouse carotid artery | 12 pA/pF | 16 pA/pF (3- to 4-fold SKCa3 overexpressor) | 0.75 | Brähler et al (2009) |
| Mouse aortic artery | 4 pA/pF | 2.5 pA/pF | 1.6 | Ledoux et al (2008a) |
| Mouse aortic artery | 12 pA/pF (WT littermates) | 10 pA/pF | 1.2 | Si et al (2006) |
| Mouse aortic artery | Not reported | 16 pA/pF (3- to 4-fold SKCa3 overexpressor) | Taylor et al (2003) | |
| Rat carotid artery | 9.8 pA/pF | 12.7 pA/pF | 0.8 | Kohler et al (2001) (estimate) |
| Rat brain parenchymal arteriole | 27 pA/pF | 16 pA/pF | 1.7 | Figure 2 |
IKCa, intermediate-conductance calcium-sensitive potassium channel; SKCa, small-conductance calcium-sensitive potassium channel; WT, wild type.
Intracellular Ca2+, 3 μmol/L.
In this study, we exploit the unique electrophysiological and pharmacological fingerprints of SKCa and IKCa channels to obtain the first recordings of the corresponding currents in freshly isolated ECs from PAs, and provide evidence for the specific cellular localization of BKCa channels (in SMCs) and SKCa and IKCa channels (in ECs). We also elucidate the contributions of BKCa, SKCa, and IKCa channels to the regulation of tone in isolated, pressurized PAs, and assess the effect of SKCa and IKCa channel activity on cortical cerebral blood flow (CBF) in vivo. Our results support the concept that the relative contribution of KCa channels to myogenic tone is shifted such that the BKCa channel component is strikingly downregulated in PAs compared with the pial arteries. The diminished role of BKCa in PAs is only partially offset by an elevated endothelial SKCa and IKCa channel component, resulting in an overall increase in myogenic tone in these arterioles. Our results also indicate that endothelial SKCa and IKCa channels are tonically active in the cerebral microcirculation in vivo, and contribute to the regulation of CBF.
Materials and methods
Animals and Dissections
Animal procedures used in this study were performed in accordance with institutional guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Vermont. Male Sprague–Dawley rats (aged 3 to 4 months) were killed by intraperitoneal injection of sodium pentobarbital (150 mg/kg), followed by rapid decapitation. The brain was removed and placed in cold MOPS (3-(N-morpholino)propanesulfonic acid)-buffered saline. A section of the brain that included the M1 region of the middle cerebral artery (MCA), as it bifurcates from the Circle of Willis and travels along the temporal lobe, was removed. Parenchymal arterioles were dissected by loosening the brain tissue using forceps and by gently pulling on the arachnoid layer around the MCA to remove the arterioles from the brain tissue while still attached to the MCA (Straub et al, 2009). From these arterioles (∼2 to 4 mm in length), a nonbranching segment originating at least 0.5 mm beyond the point of origin of the MCA and with little associated brain tissue was selected.
Pressurized Parenchymal Arterioles
Segments of PAs ∼40 to 80 μm in diameter were mounted on similar-sized glass cannulas in an arteriograph system (Living Systems Instrumentation, Burlington, VT, USA) and pressurized to 40 mm Hg in prewarmed (35°C to 37°C) artificial cerebral spinal fluid equilibrated with 95% O2 and 5% CO2 for at least 45 minutes. Only viable arterioles, defined as those that developed pressure-induced myogenic tone, were used in subsequent experiments. Vessel diameter was continuously monitored using a CCD camera and edge-detection software (IonOptix, Milton, MA, USA). All compounds were applied to the perfusate (artificial cerebral spinal fluid), which was constantly refreshed through the arteriograph chamber. Arteriole viability was tested again at the conclusion of the experiment by depolarizing with 60 mmol/L potassium (K+) to induce constriction, after which arterioles were exposed to a Ca2+-free solution containing 100 μmol/L papaverine (a phosphodiesterase inhibitor) to induce maximal dilation. Where indicated, arterioles were denuded of the endothelium by passing a large air bubble through the lumen of the vessel; elimination of endothelial function was verified by the lack of a response to bradykinin. Changes in parenchymal arteriolar diameter were calculated as percentage change in diameter from the previous diameter.
Arteriolar Smooth Muscle Membrane-Potential Recordings
Parenchymal arterioles were dissected, mounted, and pressurized on an arteriograph chamber as described above; the Ca2+-channel blocker nimodipine (200 nmol/L) was included in the bath solution to minimize arterial wall movement. Microelectrodes were filled with a 0.5 mol/L KCl solution to produce electrode resistances of 200 to 300 MΩ, and membrane potential was measured using a WPI Intra 767 amplifier (Sarasota, FL, USA) (Brayden and Nelson, 1992) at a sample-recording rate of 5 kHz. Acceptable membrane-potential recordings were those that showed an abrupt change in potential when the cell was impaled, a stable membrane potential for 30 seconds, and unchanged tip resistance before and after impalement (tip potentials of <2 mV upon exit from the cell).
Endothelial Cell and Smooth Muscle Isolation and Electrophysiology
The isolation procedures used were modifications of our previously described methods for isolating ECs (Taylor et al, 2003; Ledoux et al, 2008a) and SMCs (Straub et al, 2009). Digestion protocols were optimized to produce a greater number of the desired cell type. For EC isolation, PAs were digested in dissociation solution containing Worthington neutral protease (dispase; 1 mg/mL) and elastase (1 mg/mL) for 60 minutes at 37°C; 0.5 mg/mL of a 70%/30% mixture of type F and type H collagenase (Sigma, St Louis, MO, USA) was included for the final minute. For isolation of SMCs, PAs were digested in dissociation solution containing papain (0.3 mg/mL) and dithioerythritol (1 mg/mL) for 20 minutes at 37°C, and then with 1.0 mg/mL of a 70%/30% mixture of type F and type H collagenase (Sigma) for 5 minutes at 37°C. Endothelial cell isolation yielded small sheets of ECs and isolated ECs, which had a characteristic round and rough shape. Smooth muscle cell isolation yielded primarily single SMCs, which were identified by their spindle-like shape and contractile behavior. Membrane currents were recorded from freshly isolated cells using the conventional whole-cell configuration of the patch-clamp technique (dialyzed with 3 μmol/L free-Ca2+ solution) and a voltage-ramp protocol (−125 to +45 mV, 200 milliseconds). Currents were sampled at 20 kHz, acquired in a voltage-clamp mode using an Axopatch 200A (MDS Analytical Technologies, Sunnyvale, CA, USA), and analyzed using pClamp Suite software (Axon Instruments, Foster City, CA, USA). The patch-clamp pipette series resistance was ∼5 to 8 MΩ. The series resistance error was negligible because of the small amplitude of the recorded currents; therefore, no compensation was applied. The capacitance error was also negligible under our conditions, with a time constant (τ) of <100 microseconds.
In Vivo Cerebral Blood Flow Measurements
Cerebral blood flow responses were assessed using a cranial window preparation, as described previously (Niwa et al, 2000). Male C57Bl/6 mice were anesthetized with an intraperitoneal injection of urethane (750 mg/kg) and α-chloralose (50 mg/kg), and a femoral arterial catheter was inserted for blood gas determination and arterial pressure measurement. Animals were mechanically ventilated for the duration of the experiment, and arterial blood gases were maintained at 32 to 35 mm Hg pCO2 and 100 to 110 mm Hg pO2 (Capstar-100, CWE, Ardmore, PA, USA). A 2 × 2 mm2 section of the parietal bone and the underlying dura in the region of the somatosensory cortex were removed. The surface of the cerebral cortex within the cranial window was continuously superfused with artificial cerebral spinal fluid (at 37°C, pH 7.4), with or without pharmacological agents. Cortical CBF was measured using a laser-Doppler probe (Perimed, Järfalla, Sweden) positioned just above the cortex and away from the pial arteries.
Immunofluorescence
The MCA and attached PAs, isolated as described above, were fixed in a 4% paraformaldehyde/phosphate-buffered saline (0.01 mol/L phosphate buffer, 0.15 mol/L NaCl) solution for 2 hours, and then permeabilized with 0.02% Triton X and blocked with 3% donkey serum or 2% gelatin for 2 hours at room temperature to prevent nonspecific binding. Middle cerebral artery/PA preparations were then incubated overnight at 4°C with primary antibodies against AQP4 (aquaporin 4) (goat anti-AQP4; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 2.0 μg/mL) or rabbit anti-UCH-L1 (ubiquitin carboxyl-terminal hydrolase L1; Zymed Laboratories, Carlsbad, CA, USA; 2.5 μg/mL diluted in 0.2% gelatin/0.1% Triton/phosphate-buffered saline). After washing with phosphate-buffered saline, Cy5-donkey-anti-goat IgG and Alexa Fluor-555-donkey-anti-rabbit IgG secondary antibodies (Invitrogen, Carlsbad, CA, USA; diluted 1:500 in 0.2% gelatin–0.1% Triton–phosphate-buffered saline) were added, and the MCA/PA preparation was incubated for 2 hours at room temperature. Nuclei were stained with a 0.5 μg/mL solution of the fluorescent nucleic acid dye DAPI (4′,6-diamidino-2-phenylindole; Invitrogen). Segments of the MCA containing at least one attached PA were mounted onto glass slides using Aqua Polymount mounting medium (Polysciences Inc., Warrington, PA, USA) and examined at × 10, × 20, and × 40 magnification using an Olympus BX50 Light Microscope (Olympus America Inc., Lake Success, NY, USA) equipped with a QImaging Retiga 2000R digital camera (QImaging, Surry, BC, Canada), and at × 40 and × 63 magnification using a Zeiss LSM 510 META Confocal Microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA). Nonspecific staining was determined in images collected from MCA/PA preparations incubated with secondary antibodies alone, and a threshold was applied to each individual channel to exclude pixels that corresponded to nonspecific staining.
Solutions
The extracellular solution for isolated cell experiments consisted of 134 mmol/L NaCl, 6 mmol/L KCl, 10 mmol/L HEPES (pH 7.4), 1 mmol/L MgCl2, 2 mmol/L CaCl2, and 10 mmol/L glucose. Low-Ca2+ dissociation solution contained 55 mmol/L NaCl, 80 mmol/L Na-glutamate, 5.9 mmol/L KCl, 2 mmol/L MgCl2, 0.1 mmol/L CaCl2, 5 mmol/L glucose, and 10 mmol/L HEPES (pH 7.3). The composition of the pipette solution used for whole-cell experiments was 134 mmol/L KCl, 5.53 mmol/L MgCl2, 0.207 mmol/L CaCl2, 5 mmol/L hydroxy-EDTA (ethylenediaminetetraacetic acid), and 10 mmol/L HEPES, adjusted to pH 7.2 with 17.8 mmol/L KOH; the final K+ concentration was 151.8 mmol/L and free-Ca2+ and free-Mg2+ concentrations were 3 μmol/L and 1 mmol/L, respectively. A MOPS-buffered saline solution (3 mmol/L MOPS pH 7.4 at 25°C, 145 mmol/L NaCl, 3 mmol/L KCl, 1.2 mmol/L NaH2PO4, 1.17 mmol/L MgSO4, 2 mmol/L CaCl2, 5 mmol/L glucose, 10 mg/mL bovine serum albumin) was used for dissection of PAs. Prewarmed artificial cerebral spinal fluid (125 mmol/L NaCl, 3 mmol/L KCl, 26 mmol/L NaHCO3, 1.25 mmol/L NaH2PO4, 1 mmol/L MgCl2, 2 mmol/L CaCl2, 5 mmol/L glucose; 35°C to 37°C) was used for pressurized arteriole and in vivo experiments.
Reagents
Apamin, ChTx, paxilline, and TRAM-34 were purchased from BioMol International (Farmingdale, NY, USA). NS309 was kindly provided by Dr Søren-Peter Olesen (Neurosearch A/S, Ballerup, Denmark). All other compounds used were purchased from Sigma.
Statistics
All data are presented as mean±s.e.m. Data were analyzed using ANOVA (analysis of variance), followed by Tukey's multiple comparison tests, or paired or unpaired Student's t-test, as appropriate. Differences with P-values <0.05 were considered statistically significant.
Results
Isolated Parenchymal Arterioles Lack Extrinsic Innervation and are Encased by Astrocytic Processes
Parenchymal arterioles that branched from the MCA were studied (Figure 1). In 25 PAs evaluated from 6 MCAs, the unpressurized diameter ranged from 8 to 53 μm (mean, 25±2.8 μm; median, 26 μm). The nerves were identified by staining for the pan-neuronal marker UCH-L1 (previously known as neuron cytoplasmic protein 9.5), and astrocytes were identified by staining for AQP4, which is uniquely expressed in astrocytes and is concentrated in astrocytic endfeet (Iadecola and Nedergaard, 2007). Aquaporin4 staining was not detected in the MCA, but intense staining was observed in PAs beginning at a point shortly after branching off the MCA (Figure 1). The MCA exhibited prominent UCH-L1 staining, consistent with extensive innervation. In contrast, little or no innervation was observed on PAs, and any detectable nerve staining was lost after a short distance (<50 μm) from the branch point with the MCA, consistent with previous reports (Cipolla et al, 2004).
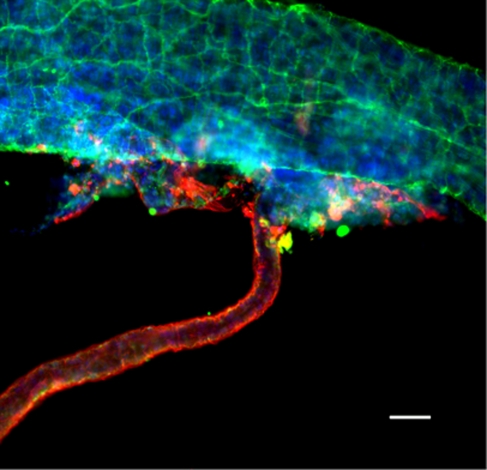
Figure 1.
Isolated MCAs with attached parenchymal arterioles. Representative photomicrograph of an isolated MCA and single parenchymal arteriole stained with UCH-L1 (green), aquaporin 4 (red), and DAPI (blue) to detect nerves, astrocytes, and nuclei, respectively, showing that parenchymal arterioles are encased by astrocytic processes and lack extrinsic nerves. There was no detectable astrocyte staining on isolated MCAs and little or no detectable nerve staining on parenchymal arterioles. The image is a two-dimensional representation of a compressed Z-stack series. Scale bar, 50 μm. DAPI, 4′,6-diamidino-2-phenylindole; MCA, middle cerebral artery; UCH-L1, ubiquitin carboxyl-terminal hydrolase L1.
Functional KCa Channels Exhibit a Cell Type-Specific Distribution in Endothelial and Smooth Muscle Cells of Parenchymal Arterioles
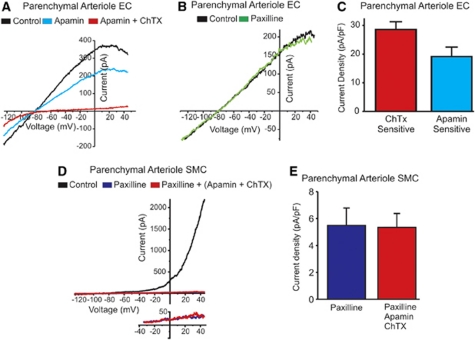
The electrophysiological properties of parenchymal arteriolar ECs have not been characterized previously. To determine whether ECs in PAs have functional SKCa and IKCa channels, we measured whole-cell currents in freshly dissociated ECs using the patch-clamp technique. Cells were dialyzed with a 3 μmol/L free-Ca2+ solution to maximally stimulate SKCa and IKCa channels, and current–voltage relationships of whole-cell currents were recorded using a voltage-ramp protocol (−125 to +45 mV, 200 milliseconds). Endothelial cells from PAs had a mean capacitance of 8.0±0.7 pF, consistent with previous reports from mouse aortic ECs (10.6±0.8 pF (Ledoux et al, 2008a)). Positive to the K+ equilibrium potential (EK), membrane current increased and then decreased (inward rectification) with progressive membrane-potential depolarization (Figures 2A and 2B), similar to I–V relationships reported in other types of ECs (Ledoux et al, 2008a). The selective SKCa channel blocker apamin reduced the K+ current by 37.5%±5.6% at +40 mV, and the remainder of the current was inhibited in the presence of the IKCa channel blocker ChTx. The SKCa and IKCa current densities were 19.9±3.5 pA/pF and 28.4±2.8 pA/pF, respectively, at +40 mV (Figure 2C), and reversed near EK (−80.1 and −81.7 mV for SKCa and IKCa, respectively; EK=−83.0 mV). The selective BKCa channel inhibitor paxilline (Imlach et al, 2008) had no effect (Figure 2B), indicating that BKCa channels did not contribute to the observed current.
Figure 2.
SKCa and IKCa currents in ECs, and BKCa currents in SMCs from parenchymal arterioles. (A, B) Current–voltage (I–V) relationships of whole-cell currents recorded from an isolated parenchymal EC using a voltage-ramp protocol (−125 to +45 mV, 200 milliseconds) and dialyzed with a 3 μmol/L free-Ca2+ and 1 mmol/L free-Mg2+ solution. The characteristic inward rectification must be noted. EC currents were insensitive to the BKCa channel blocker paxilline (500 nmol/L) (panel B), but were almost completely eliminated by sequential addition of the SKCa blocker apamin (300 nmol/L) and the IKCa blocker ChTX (100 nmol/L) (P<0.05) (panel A). (C) Mean IKCa current density (ChTX sensitive, n=5 cells) and SKCa current density (apamin sensitive, n=3 cells) measured at +40 mV. (D) Whole-cell current–voltage relationship for a SMC from a parenchymal arteriole recorded under the conditions described above. The typical outward rectification caused by the activation of BKCa channels, and the much larger currents at positive membrane potentials compared with EC cells must be noted (∼7-fold larger at +40 mV; red trace in panel A). Paxilline almost completely abolished the K+ current in SMCs isolated from parenchymal arterioles (P<0.05). The lower I–V curve illustrates the lack of an effect of both apamin and ChTx on membrane currents in the presence of paxilline. (E) The remaining SMC K+ current at +40 mV was not altered by both SKCa and IKCa channel blockers (n=5). BKCa, large-conductance, voltage-dependent calcium-sensitive potassium channel; ChTX, charybdotoxin; EC, endothelial cell; IKCa, intermediate-conductance calcium-sensitive potassium channel; SKCa, small-conductance calcium-sensitive potassium channel; SMC, smooth muscle cell.
Parenchymal arteriolar SMCs exhibited a markedly different current–voltage relationship with the same solutions, and showed very prominent outward rectification (Figure 2D). The outward K+ current was greatly reduced (91.5%±3.3% at +40 mV) by the BKCa channel inhibitor paxilline. In the presence of paxilline, application of the IKCa and SKCa blockers ChTx and apamin did not affect outward currents (Figures 2D (inset) and 2E). The BKCa current density in SMCs was quite substantial (154.4±72.4 pA/pF at +40 mV), particularly in comparison with EC SKCa and IKCa current densities. These results indicate that expression of functional KCa channels is cell-type specific, with BKCa channels in SMCs of PAs, and SKCa and IKCa channels in ECs.
Blockade of Endothelial Cell SKCa and IKCa Channels Constricts Parenchymal Arterioles, whereas Inhibition of BKCa Channels has a Minimal Effect
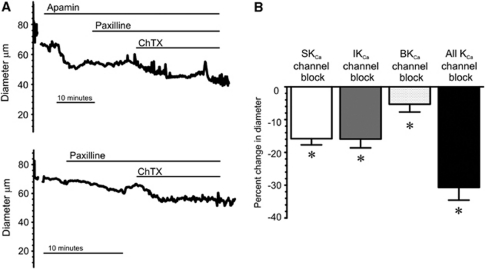
To elucidate the roles of EC SKCa and IKCa channels and SMC BKCa channels in the regulation of arteriolar function, we determined the effects of SKCa, IKCa, and BKCa blockers on parenchymal arteriolar diameter. Increase in intravascular pressure to physiologic levels (40 mm Hg) constricted PAs by 26.0%±1.2% (n=38), consistent with previous observations (Cipolla and Bullinger, 2008). Combinations of KCa channel blockers were used to isolate the relative role of each channel type in the regulation of PA tone, as shown by representative diameter traces in Figure 3. Application of the SKCa channel blocker apamin constricted pressurized arterioles by 15.8%±1.9% (Figures 3A and 3B). Paxilline, a BKCa channel blocker, caused a small constriction (5.3%±2.4%); a similar constriction to paxilline was observed in the presence apamin (Figures 3A and 3B). As paxilline (500 nmol/L) did not affect IKCa or SKCa currents in isolated ECs, but essentially eliminated BKCa currents in SMCs (see Figure 2), it was always applied before the IKCa/BKCa blocker ChTx to isolate IKCa channel effects on parenchymal diameter. In the presence of paxilline, addition of ChTx to block IKCa channels constricted PAs by 15.9%±2.7%, similar to the constriction caused by apamin alone (Figure 3). The synthetic IKCa channel inhibitor, TRAM-34 (1 μmol/L), constricted PAs by 21%±5% (n=3), consistent with a previous report (Cipolla et al, 2009). When applied together, SKCa, IKCa, and BKCa channel blockers constricted pressurized (40 mm Hg) PAs by 30.7%±3.9%.
Figure 3.
Blockade of SKCa and IKCa channels causes a greater constriction of pressurized parenchymal arterioles than that of BKCa channels. (A) Representative diameter traces of parenchymal arterioles pressurized to 40 mm Hg. The upper trace illustrates the effects of sequential abluminal application of apamin (300 nmol/L), paxilline (500 nmol/L), and ChTx (100 nmol/L). The lower trace illustrates the effect of paxilline (500 nmol/L) and ChTx (100 nmol/L) in the absence of apamin. (B) Summary data illustrating mean constrictions to apamin alone (‘SKCa channel block'), to ChTx in the presence of paxilline to block BKCa channels (‘IKCa channel block'), to paxilline alone (BKCa channel block'), and to the combination of apamin, paxilline, and ChTx (‘all KCa channel blockers'). Individual inhibition of SKCa, IKCa, and BKCa channels significantly constricted arterioles with myogenic tone (*P<0.05, n=15, repeated-measures ANOVA, Tukey's test). ANOVA, analysis of variance; BKCa, large-conductance, voltage-dependent calcium-sensitive potassium channel; ChTX, charybdotoxin; IKCa, intermediate-conductance calcium-sensitive potassium channel; SKCa, small-conductance calcium-sensitive potassium channel.
Activation of Endothelial Cell IKCa Channels Dilates and Hyperpolarizes Parenchymal Arterioles
Blockade of EC SKCa and IKCa channels caused significant vasoconstriction. Therefore, opening SKCa and/or IKCa channels should produce vasodilation. To test this hypothesis, we determined the effect of NS309, a selective opener of SKCa and IKCa channels (Strøbaek et al, 2004), on pressurized arterioles (Figure 4). NS309 (1 μmol/L) caused a large increase in both SKCa and IKCa channel currents in isolated parenchymal arteriolar ECs (Supplementary Figure 1), consistent with the results of the study by Strøbaek et al (2004). NS309 (1 μmol/L) caused maximal dilation of pressurized (40 mm Hg) PAs (n=11; Figures 4A and 4C). The dilatory effect of NS309 was prevented by preincubation with a combination of apamin and ChTx, or by removing the endothelium (Figure 4B), consistent with NS309 activation of endothelial SKCa and IKCa channels.
Figure 4.
The SKCa and IKCa channel opener NS309 maximally dilates pressurized (40 mm Hg) parenchymal arterioles. (A) Representative diameter trace illustrating dilation to NS309 (1 μmol/L) and reversal by apamin (300 nmol/L) and/or ChTX (100 nmol/L). (B) Representative diameter trace illustrating the lack of an effect of NS309 on endothelium-denuded parenchymal arterioles. (C) Summary data showing the effects of NS309 (1 μmol/L) alone (control), and in the presence of L-NNA (50 μmol/L)+indomethacin (10 μM), BaCl2 (30 μmol/L), paxilline (500 nmol/L), apamin (300 nmol/L)+ChTx (100 nmol/L), and without endothelium. The dilatory response to NS309 was significantly blunted in the presence of SKCa and IKCa channel blockers and in endothelium-denuded arterioles (*P<0.01, ANOVA and Tukey's post hoc analysis; n=3 to 4). (D) Summary data showing the effects of 500 nmol/L NS309 on pressurized parenchymal arterioles; NS309-induced dilation was prevented with IKCa channel block (paxilline was used in combination with ChTX to isolate the IKCa channel effects), but not with SKCa channel block (*P<0.01, paired t-test; n=4 to 5). ANOVA, analysis of variance; ChTX, charybdotoxin; EC, endothelial cell; IKCa, intermediate-conductance calcium-sensitive potassium channel; L-NNA, L-NG-nitroarginine; NS309, 6,7-dichloro-1H-indole-2,3-dione3-oxime; SKCa, small-conductance calcium-sensitive potassium channel.
It is possible that activation of EC SKCa and IKCa channels could indirectly affect endothelial nitric oxide (NO) synthase (NOS) and cyclooxygenase activity by increasing EC global Ca2+ (Ledoux et al, 2008a; Sheng et al, 2009). The combination of L-NG-nitroarginine (L-NNA) (50 μmol/L) and indomethacin (10 μmol/L) constricted PAs by 26.0%±3.9% (n=14), which is similar to the previously reported response to NOS inhibition alone (Cipolla et al, 2009). Inhibition of NOS and cyclooxygenase with L-NNA (50 μmol/L) and indomethacin (10 μmol/L) had no effect on NS309-induced dilation of PAs; the Kir channel inhibitor BaCl2 (30 μmol/L) and the BKCa channel inhibitor paxilline (500 nmol/L) were similarly ineffective in blocking the dilation to NS309 (Figure 4C). Interestingly, inhibition of IKCa channels with ChTx (in the presence of paxilline), prevented the dilation to NS309, whereas inhibition of SKCa channels did not (Figure 4D). Collectively, these results indicate that NS309 dilates PAs primarily by activation of EC IKCa channels, independent of NOS or cyclooxygenase activity.
A likely mechanism for this EC IKCa channel-dependent relaxation of the smooth muscle is through SMC hyperpolarization. To test this possibility, we measured the membrane potential of SMCs in pressurized (40 mm Hg) PAs using microelectrodes. The resting membrane potential was −38.6±0.8 mV (n=10), which is 10 to 15 mV more depolarized than that of the pial arteries at the same pressure (Knot and Nelson, 1998; Albarwani et al, 2003). NS309 (1 μmol/L) caused a 27 mV hyperpolarization of SMCs to −65.6±3.6 mV. Blocking BKCa or SKCa channels in the presence of NS309 did not significantly affect membrane-potential hyperpolarization to NS309 (Figures 5A and 5B). However, blocking IKCa channels with ChTx reversed the membrane-potential hyperpolarization to NS309 (Figures 5A and 5B). These results indicate that activation of endothelial IKCa channels by NS309 dilates PAs through hyperpolarization of parenchymal arteriolar SMCs.
Figure 5.
NS309 acts through IKCa channels to hyperpolarize the smooth muscle membrane potential in pressurized (40 mm Hg) parenchymal arterioles. (A) Illustration of smooth muscle membrane potential (resting −35.5 mV) hyperpolarization to NS309 (1 μmol/L) (−66.4 mV), the lack of an effect of apamin (300 nmol/L) and paxilline (500 nmol/L), and reversal by ChTx (100 nmol/L) (−36.9 mV). (B) Summary data showing changes in membrane potential in response to 1 μmol/L NS309. The hyperpolarization to NS309 was not altered by blocking SKCa or BKCa channels, yet IKCa channel block repolarized parenchymal arterioles (*P<0.01 compared with membrane potential in the presence of NS309; n=4 arterioles). BKCa, large-conductance, voltage-dependent calcium-sensitive potassium channel; ChTX, charybdotoxin; IKCa, intermediate-conductance calcium-sensitive potassium channel; NS309, 6,7-dichloro-1H-indole-2,3-dione3-oxime; SKCa, small-conductance calcium-sensitive potassium channel.
Contribution of SKCa and IKCa Channels to Resting Cortical Cerebral Blood Flow In Vivo
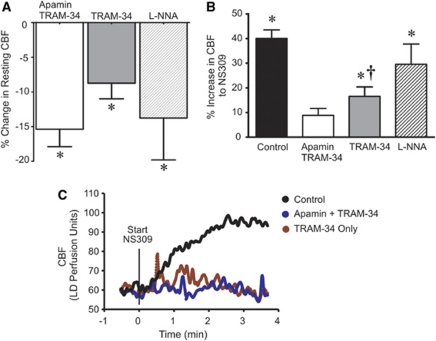
The results obtained in isolated arterioles indicate a tonic dilatory influence of EC SKCa and IKCa channel activity on PA tone. To determine whether this is the case in vivo, we assessed the effects of EC SKCa and IKCa channel modulation on cortical CBF using laser-Doppler flowmetry in a cranial window preparation. Combined inhibition of SKCa and IKCa channels by superfusing the cortical surface of the brain with apamin (300 nmol/L) and TRAM-34 (10 μmol/L), a specific inhibitor of IKCa channels, decreased resting cortical CBF by 15%±2% (Figure 6A). The reduction in resting cortical CBF with combined SKCa and IKCa blockade was similar to that observed with NOS inhibition (14%±6%). Inhibition of IKCa channels reduced resting cortical CBF by 9%±2%. We have previously shown that BKCa channel inhibition with paxilline does not affect resting cortical CBF (Girouard et al, 2010). These results indicate that EC SKCa and IKCa channels contribute to the determination of resting cortical CBF.
Figure 6.
Effects of SKCa and IKCa channel modulation on cortical CBF. (A) Effect on resting cortical CBF. Combined SKCa and IKCa block (300 nmol/L apamin+10 μmol/L TRAM-34) reduced resting CBF by 15%±2% (*P<0.05, n=11), compared with 14%±6% with 1 mmol/L L-NNA (*P<0.05, n=5). Inhibition of IKCa only (TRAM-34) accounted for about half of the combined effect of apamin+TRAM-34 (9%±2%, n=5, P<0.05 compared with resting CBF). (B) Effect of the SKCa/IKCa activator NS309 on cortical CBF under control conditions and in the presence of various pharmacological inhibitors. Cranial superfusion of NS309 (10 μmol/L) increased cortical CBF by 40%±3% (*P<0.05 versus baseline CBF, n=15). Combined SKCa and IKCa inhibition (apamin+TRAM-34) and inhibition of IKCa only significantly attenuated the hyperemic response to NS309 (†P<0.05 versus control; n=7 and n=6, respectively), whereas NOS blockade with L-NNA had no effect on the NS309 response (n=5). (C) Representative laser-Doppler traces of cortical CBF responses to cranial window superfusion of the SKCa and IKCa channel opener NS309. Cortical superfusion of NS309 began at 0 minutes. Pharmacological inhibitors were administered by cortical superfusion 30 minutes before the addition of NS309. CBF, cerebral blood flow; IKCa, intermediate-conductance calcium-sensitive potassium channel; L-NNA, L-NG-nitroarginine; NOS, nitric oxide synthase; NS309, 6,7-dichloro-1H-indole-2,3-dione3-oxime; SKCa, small-conductance calcium-sensitive potassium channel; TRAM-34, 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole.
Activation of SKCa and/or IKCa channels by cortical superfusion of NS309 (10 μmol/L) produced a robust 40%±3% increase in cortical CBF that exhibited a rapid onset (<1 minute after administration) (Figures 6B and 6C, Control). This NS309-induced cortical hyperemia was largely prevented by apamin and TRAM-34 (9%±3% in CBF to NS309). In agreement with its effects on isolated PAs (see Figure 4), IKCa channel block attenuated the NS309-induced increase in CBF to a similar extent as combined SKCa and IKCa block (Figure 6). Inhibition of NOS (1 mmol/L L-NNA) had no effect on the CBF response to NS309, suggesting that activation of SKCa/IKCa channels with NS309 does not produce cortical hyperemia through increased NO synthesis. These results suggest that the CBF response to NS309 is primarily IKCa mediated, which is consistent with observations in isolated PAs.
Discussion
The Unique Environment of Parenchymal Arterioles
Previous studies have shown that cortical arterioles are encased in astrocytic processes and lack extrinsic nerves (Iadecola and Nedergaard, 2007). This environment is unique compared with that of other vascular beds. We showed that isolated PAs lack extrinsic innervation, consistent with previously published data (Cipolla et al, 2004). In addition, AQP4 staining surrounded isolated PAs, indicating that astrocytic processes tightly adhere to the isolated arterioles. These data also indicate that the loss of innervation and association with astrocytes occur very near (<50 μm) the point at which a PA branches from a pial artery on the brain surface.
KCa Channels in the Vascular Wall
The BKCa channels have been identified in virtually all types of the smooth muscle (Ledoux et al, 2006). In this study, we show that SMCs, but not ECs, from PAs also have functional BKCa channels (Figure 2D). Although there are conflicting reports regarding the expression of BKCa channels in ECs (see Sandow and Grayson (2009) and references therein), our data from parenchymal arteriolar ECs support the consensus view that functional BKCa channels are absent in the healthy, native endothelium (Gauthier et al, 2002; Sandow and Grayson, 2009).
The responses of arteries to SKCa and IKCa channel blockers suggest that these channels are likely present in the endothelium of most vascular beds. However, there are relatively few direct measurements of SKCa and IKCa channel currents in freshly dissociated ECs, and to date, all such measurements have been obtained using ECs from larger caliber arteries (Table 1) (Kohler et al, 2001; Burnham et al, 2002; Bychkov et al, 2002; Taylor et al, 2003; Si et al, 2006; Ledoux et al, 2008a; Brähler et al, 2009). In this study, we provide the first measurements of SKCa and IKCa channel currents from arteriolar ECs (Figure 2). The most notable difference between SKCa and IKCa currents in parenchymal arteriolar ECs and those measured in ECs from larger peripheral arteries is the current density. The SKCa and IKCa current densities in parenchymal arteriolar ECs are ∼1.6- to 6.7-fold higher than those measured in ECs from other vascular beds (Table 1), suggesting a greater influence of these channels on adjacent SMCs in these arterioles. To our knowledge, functional SKCa and IKCa channels (i.e., current measurements) have not been detected in vascular SMCs from healthy arteries, although immunostaining for IKCa channels has been observed in pial artery SMCs (McNeish et al, 2006), and vascular injury has been shown to induce IKCa channel expression in proliferating SMCs (Grgic et al, 2009).
The BKCa channel current density recorded in SMCs (dialyzed with 3 μmol/L intracellular Ca2+) from PAs was 154 pA/pF at +40 mV and accounted for 92% of the outward current, values similar to those reported previously (Gerzanich et al, 2001). The remaining current in SMCs was insensitive to ChTx and apamin, and likely represents current through voltage-dependent K+ channels (KV1.2/1.5) (Straub et al, 2009).
Regulation of Parenchymal Arteriole Tone by Endothelial SKCa and IKCa Channels
In the pial and peripheral arteries, endothelium-dependent hyperpolarization to endothelial agonists—the EDHF response—is prevented by blockers of SKCa and IKCa channels. Although in these arterial preparations, SKCa and IKCa blockers have little effect on arterial tone and SMC membrane potential in the absence of agonists, the defining feature of this EDHF phenomenon is its dependence on SKCa and IKCa channels (Andresen et al, 2006; Edwards et al, 2010). Our results indicate that EC SKCa and IKCa channels, in the absence of exogenous agonists, have a significant tonic role in opposing pressure-induced constriction in PAs, independent of NOS or cyclooxygenase activity. Thus, in PAs, apparently unlike many vascular beds, EDHF has a role in regulating myogenic tone, possibly through direct electrical coupling of the single endothelial and smooth muscle layers in these vessels.
NS309, a potent opener of SKCa and IKCa channels (Strøbaek et al, 2004), caused a profound arteriolar smooth muscle hyperpolarization, vasodilation, and an increase in cortical CBF (Figures 4, 5, and 6). Interestingly, blockers of IKCa channels, but not of SKCa channels, largely prevented NS309-induced hyperpolarization, dilation, and hyperemia. This observation is perplexing in light of the clear effects of both SKCa and IKCa channel blockers on membrane potential, myogenic tone, and CBF under basal (unstimulated) conditions, and the similarity in the potency of NS309 towards exogenously expressed SKCa (EC50=30 nmol/L) and IKCa (EC50=10 nmol/L) channels in transfected HEK 293 cells (Strøbaek et al, 2004). Consistent with the data obtained from the study by Strøbaek et al (2004), we found that NS309 activates both SKCa and IKCa currents in isolated ECs. Similar to the effects of NS309, Marrelli et al (2003) and You et al (1999) have reported that agonist-induced EDHF responses in the pial arteries and PAs depend on IKCa channels, and not on SKCa channels. This may reflect clustering of IKCa channels in endothelial projections through the internal elastic lamina that abut the smooth muscle (Sandow et al, 2006; Dora et al, 2008; Ledoux et al, 2008b). Given the differential spatial organization of SKCa and IKCa channels, it is possible that SKCa channels are maximally activated by local Ca2+ but IKCa channels are not. Therefore, blockade of either channel would cause constriction, but activation by NS309 would primarily act through IKCa channels. The resolution of this intriguing observation, although beyond the scope of current study, may provide insights into the unique and functionally divergent roles of SKCa and IKCa channels in ECs.
NS309 caused a profound increase in cortical CBF, which was largely prevented by IKCa channel inhibition (Figure 6), consistent with the effects on isolated, pressurized PAs (Figure 4) and the prominent role of IKCa channels in EDHF-mediated dilations of cerebral arteries (Marrelli et al, 2003). It is likely that this hyperemic response reflects activation of endothelial IKCa channels because current evidence suggests that IKCa channels are not expressed in central nervous system neurons or astrocytes (Pedarzani and Stocker, 2008). The SKCa channels in neurons could potentially be affected by cortical superfusion of apamin (SKCa channel blocker) or NS309 (SKCa/IKCa channel activator), and indirectly alter CBF by modulating neuronal activity. However, inhibition of SK channel activity in neurons would be predicted to increase neuronal excitability by depolarizing the membrane potential, producing an increase in CBF (neurovascular coupling). Yet, with cortical superfusion of the SKCa and IKCa blockers, apamin and TRAM-34, we observed a reduction in CBF. Conversely, activation of neuronal SKCa channels with NS309 would be expected to hyperpolarize neurons and reduce neuronal metabolism, which would (if anything) lead to a reduction in CBF. In stark contrast to this predicted outcome, we found that cortical superfusion of NS309 produced robust hyperemia. Thus, it is unlikely that modulation of SKCa channel activity in neurons contributes to the observed effects of cortical superfusion of apamin and TRAM-34, or NS309, on CBF.
Role of the BKCa Channel in Parenchymal Arterioles
In contrast to the effects of SKCa and IKCa channel blockers on PA tone and CBF, BKCa channel block had only a modest effect on parenchymal arteriolar tone in our studies (<5%). We have previously reported that BKCa channel block also causes only a modest constriction of PAs in brain slices and has little effect on resting cortical CBF (Girouard et al, 2010). This is in contrast with pressurized pial arteries, where blockade of BKCa channels causes a constriction of ∼30% (Brayden and Nelson, 1992; Nelson et al, 1995). It should be noted that effects of K channel blockers on myogenic tone similar to those described in this study for PAs have been reported in pial arteries (rat MCA) (Marrelli et al, 2003). However, differences in experimental methodology used in this previous study, including the use of a nonpaired experimental design and differences in methods used to calculate changes in diameter, make direct comparisons difficult. In vivo, blockers of BKCa channels have been shown to cause a small (3%), but significant, constriction of pial arterioles (Taguchi et al, 1995), and produce a larger effect only at high systemic blood pressures or in the presence of an exogenous vasoconstrictor (Paterno et al, 2000). In the pial arteries, BKCa channels are activated by micromolar intracellular Ca2+ delivered by local calcium release events through ryanodine receptors (Ca2+ sparks) in the sarcoplasmic reticulum (Nelson et al, 1995). Our results indicate that the density of BKCa currents in SMCs of PAs is substantial; thus, it is unlikely that the diminished contribution of the BKCa channel to parenchymal arteriolar tone is attributable to a reduction in functional BKCa channels. Although Ca2+ dynamics in SMCs of PAs have not been studied in pressurized PAs, it is tempting to speculate that the diminished role of BKCa channels in the regulation of arteriolar tone may reflect a lack of Ca2+ sparks in arteriolar myocytes. Alternatively, a loss of the regulatory β1 subunit of the BKCa channels, which enhances the apparent Ca2+ and voltage sensitivity of the pore-forming α-subunit (Brenner et al, 2000), might account for a muted BKCa involvement in regulating arteriolar tone. Regardless of the underlying mechanism, the loss of BKCa channel functionality in intact PAs may contribute to the enhanced pressure-induced constriction of these arterioles.
In conclusion, our data are consistent with a model in which PAs depolarize and constrict more to intravascular pressure because SMC BKCa channels do not oppose vasoconstriction, an effect that is only partially compensated by the elevated hyperpolarizing and dilating influence of EC SKCa and IKCa channels. Our results indicate that modulation of SKCa and IKCa channels in the endothelium of PAs has a profound effect on cortical blood flow. Therefore, dysfunction of EC SKCa and IKCa channels could compromise intra-CBF, whereas activation of IKCa channels could enhance CBF, and thus may represent a therapeutic target to ameliorate reduced CBF that accompanies a number of cerebrovascular disorders.
Acknowledgments
The authors are grateful to Drs David Hill-Eubanks, Rachael Baylie, Swapnil Sonkusare, Fabrice Dabertrand, and Gayathri Krishnamorthy for their insightful comments on this manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by NIH grants RO1 HL044455, RO1 HL098243, 2R37DK 053732, PO1 HL07738, P20 RR016435, and T32 HL07944, and the Totman Trust for Medical Research to MTN.
Supplementary Material
References
- Albarwani S, Nemetz L, Madden J, Tobin A, England S, Pratt P, Rusch N. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2003;551 (Part 3:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- Brähler S, Kaistha A, Schmidt V, Wölfle S, Busch C, Kaistha B, Kacik M, Hasenau A, Grgic I, Si H, Bond C, Adelman J, Wulff H, de Wit C, Hoyer J, Köhler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- Brayden J, Nelson M. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Burnham M, Bychkov R, Félétou M, Richards G, Vanhoutte P, Weston A, Edwards G. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychkov R, Burnham M, Richards G, Edwards G, Weston A, Félétou M, Vanhoutte P. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla M, Bullinger L. Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation. 2008;15:495–501. doi: 10.1080/10739680801986742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla M, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol. 2004;44:1–8. doi: 10.1097/00005344-200407000-00001. [DOI] [PubMed] [Google Scholar]
- Cipolla M, Smith J, Kohlmeyer M, Godfrey J. SKCa and IKCa channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa31 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Félétou M, Weston A. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflügers Archiv Eur J Physiol. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Faraci F, Heistad D. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Liu C, Popovic A, Albarwani S, Rusch NJ. Freshly isolated bovine coronary endothelial cells do not express the BKCa channel gene. J Physiol. 2002;545:829–836. doi: 10.1113/jphysiol.2002.029843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Zhang F, West GA, Simard JM. Chronic nicotine alters NO signaling of Ca(2+) channels in cerebral arterioles. Circ Res. 2001;88:359–365. doi: 10.1161/01.res.88.3.359. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Robertson CS, Bryan RM. Comparison of the myogenic response in rat cerebral arteries of different calibers. Brain Res. 1998;785:293–298. doi: 10.1016/s0006-8993(97)01419-4. [DOI] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses—relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Imlach W, Finch S, Dunlop J, Meredith A, Aldrich R, Dalziel J. The molecular mechanism of ‘ryegrass staggers', a neurological disorder of K+ channels. J Pharmacol Exp Ther. 2008;327:657–664. doi: 10.1124/jpet.108.143933. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508 (Part 1:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler R, Brakemeier S, Kuhn M, Behrens C, Real R, Degenhardt C, Orzechowski H-D, Pries AR, Paul M, Hoyer J. Impaired hyperpolarization in regenerated endothelium after balloon catheter injury. Circ Res. 2001;89:174–179. doi: 10.1161/hh1401.093460. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Bonev AD, Nelson MT. Ca2+-activated K+ channels in murine endothelial cells: block by intracellular calcium and magnesium. J Gen Physiol. 2008a;131:125–135. doi: 10.1085/jgp.200709875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci. 2008b;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterno R, Heistad DD, Faraci FM. Potassium channels modulate cerebral autoregulation during acute hypertension. Am J Physiol Heart Circ Physiol. 2000;278:H2003–H2007. doi: 10.1152/ajpheart.2000.278.6.H2003. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Stocker M. Molecular and cellular basis of small- and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol. 2009;297:H1–H7. doi: 10.1152/ajpheart.00042.2009. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function. J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Ella S, Davis M, Hill M, Braun A. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Heyken W-T, Wolfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Kohler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Straub SV, Girouard H, Doetsch PE, Hannah RM, Wilkerson MK, Nelson MT. Regulation of intracerebral arteriolar tone by Kv channels: effects of glucose and PKC. Am J Physiol Cell Physiol. 2009;297:C788–C796. doi: 10.1152/ajpcell.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøbaek D, Teuber L, Jørgensen T, Ahring P, Kjaer K, Hansen R, Olesen S, Christophersen P, Skaaning-Jensen B. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Taguchi H, Heistad DD, Kitazono T, Faraci FM. Dilatation of cerebral arterioles in response to activation of adenylate cyclase is dependent on activation of Ca2+-dependent K+ channels. Circ Res. 1995;76:1057–1062. doi: 10.1161/01.res.76.6.1057. [DOI] [PubMed] [Google Scholar]
- Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- You J, Johnson TD, Marrelli SP, Bryan RM., Jr Functional heterogeneity of endothelial P2 purinoceptors in the cerebrovascular tree of the rat. Am J Physiol Heart Circ Physiol. 1999;277:H893–H900. doi: 10.1152/ajpheart.1999.277.3.H893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.