Abstract
Excitatory amino-acid transporters (EAATs) transport glutamate into cells under physiologic conditions. Excitatory amino-acid transporter type 3 (EAAT3) is the major neuronal EAAT and also uptakes cysteine, the rate-limiting substrate for synthesis of glutathione. Thus, we hypothesize that EAAT3 contributes to providing brain ischemic tolerance. Male 8-week-old EAAT3 knockout mice on CD-1 mouse gene background and wild-type CD-1 mice were subjected to right middle cerebral artery occlusion for 90 minutes. Their brain infarct volumes, neurologic functions, and brain levels of glutathione, nitrotyrosine, and 4-hydroxy-2-nonenal (HNE) were evaluated. The EAAT3 knockout mice had bigger brain infarct volumes and worse neurologic deficit scores and motor coordination functions than did wild-type mice, no matter whether these neurologic outcome parameters were evaluated at 24 hours or at 4 weeks after brain ischemia. The EAAT3 knockout mice contained higher levels of HNE in the ischemic penumbral cortex and in the nonischemic cerebral cortex than did wild-type mice. Glutathione levels in the ischemic and nonischemic cortices of EAAT3 knockout mice tended to be lower than those of wild-type mice. Our results suggest that EAAT3 is important in limiting ischemic brain injury after focal brain ischemia. This effect may involve attenuating brain oxidative stress.
Keywords: focal brain ischemia, glutamate transporter, ischemic tolerance, mouse, oxidative stress
Introduction
Glutamate is the major excitatory neurotransmitter. An extracellular enzyme to metabolize glutamate has not yet been found. Thus, glutamate uptake through glutamate transporters (also known as excitatory amino-acid transporters, EAATs) is the major mechanism to reduce extracellular glutamate concentrations (Danbolt, 2001). Excitatory amino-acid transporters are cell plasma membrane proteins that use the transmembrane gradients of Na+, K+, and H+ as a driving force to uptake glutamate under physiologic conditions (Billups et al, 1998; Danbolt, 2001). Thus, EAATs have an important role in regulating glutamate neurotransmission and in maintaining extracellular glutamate concentrations below toxic levels for brain cells.
Five EAATs (EAAT1 to EAAT5) have been identified so far. Whereas EAAT1 to EAAT3 are expressed in many brain regions including the cerebrum, hippocampus, and cerebellum, EAAT4 is largely restricted to the molecular cell layer of the cerebellum and EAAT5 is mainly found in the retina (Danbolt, 2001; Rothstein et al, 1994). In rodents, EAAT1 and EAAT2 are found in glial cells, EAAT3 and EAAT4 are mainly expressed in neurons, and EAAT5 is located in neurons and glial cells of the retina (Arriza et al, 1997; Lehre et al, 1995; Rothstein et al, 1994). It has been suggested that EATT2 and EAAT3 are the major glial and neuronal EAATs, respectively, based on functional and expressional data.
Glutamate excitotoxicity caused by increased extracellular glutamate concentrations has been considered as a major mechanism for ischemic brain cell death (Lipton, 1999). Owing to their functions in regulating extracellular glutamate concentrations, the role of EAATs in ischemic brain injury has been investigated. Multiple lines of evidence have suggested a neuroprotective role of EAAT2 (Chu et al, 2007; Rao et al, 2001; Rothstein et al, 1996). In contrast, downregulation of EAAT3 by antisense oligonucleotides did not significantly change neurologic outcome at 1 day after focal brain ischemia (Rao et al, 2001). However, EAAT3 also transports cysteine (Chen and Swanson, 2003). Cysteine is the rate-limiting substrate for synthesis of glutathione, the major intracellular antioxidant (Dringen et al, 1999). Excitatory amino-acid transporter type 3-deficient cells have decreased levels of glutathione and increased vulnerability to oxidants (Aoyama et al, 2006). As oxidative stress-induced cell injury/death is one of the major mechanisms for ischemic cell injury/death (Lipton, 1999), we hypothesize that EAAT3 has a role in ischemic tolerance of the brain. To test this hypothesis, we evaluated neurologic outcomes of both wild-type and EAAT3 knockout mice after focal brain ischemia. The oxidative stress status in the ischemic penumbra was also assessed.
Materials and methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 1996.
Animals
The EAAT3 knockout mice were descendants of mice used in a previous study (Peghini et al, 1997). These mice have a CD-1 mouse gene background. It was confirmed that they did not express EAAT3 proteins (Aoyama et al, 2006; Peghini et al, 1997).
Mouse Middle Cerebral Artery Occlusion
Eight-week-old male CD-1 wild-type and EAAT3 knockout mice were used in this study. Anesthesia was induced with 4% isoflurane and maintained with 1.5% isoflurane through an anesthesia mask. Body temperature was maintained at 37°C using a thermostat-controlled heating blanket. Heart rate, breathing rate, and arterial blood oxygen saturation of mice were monitored continuously and noninvasively using a MouseOX Murine Plus Oximeter System (Starr Life Sciences Corporation, Oakmont, PA, USA) during the surgery. Blood (∼20 μL) was collected from the tails of 16 CD-1 wild-type mice and 16 EAAT3 knockout mice at 5 minutes before and 5 minutes after achievement of middle cerebral artery occlusion (MCAO) to measure blood glucose levels using an ACCU-CHEK advantage meter system (Roche Diagnostics, Indianapolis, IN, USA). Middle cerebral artery occlusion was performed using the monofilament suture method. After a midline incision was made at the ventral surface of the neck skin, the right common carotid artery, external carotid artery, and internal carotid artery were isolated. An 8-0 monofilament nylon surgical suture with a rounded tip (Beijing Sunbio Biotech Co. Ltd, Beijing, China) was introduced through the right external carotid artery to the internal carotid artery until resistance was felt. Anesthesia was stopped once the right MCA was occluded. Mice were reanesthetized by isoflurane at 90 minutes after the onset of MCAO to remove the suture. This reanesthesia lasted for ∼2 minutes. After recovery from anesthesia, mice were placed back in their cages with ad libitum access to food and water.
Evaluation of Motor Coordination, Neurologic Deficit Scores, and Infarct Volumes
Neurologic deficit scores, motor coordination, and infarct volumes of animals were evaluated at 24 hours after transient MCAO in the short-observation duration study. In the long-observation duration study, neurologic deficit scores and motor coordination were evaluated at 2 and 4 weeks after MCAO. Animals were killed at 4 weeks after MCAO to assess their infarct volumes.
Neurologic deficit scores were evaluated based on an eight-point scale by an individual blinded to the group assignment. Mice were scored as follows: 0, no apparent deficits; 1, failure to extend the left forepaw fully; 2, decreased grip of the left forelimb; 3, spontaneous movement in all directions, contralateral circling only if pulled by the tail; 4, circling or walking to the left; 5, walking only if stimulated; 6, unresponsiveness to stimulation and with depressed level of consciousness; and 7, dead (Li and Zuo, 2009; Rogers et al, 1997).
Motor coordination was evaluated just before and at 24 hours, 2 weeks, or 4 weeks after transient MCAO. Mice were placed on an accelerating rotarod. The speed of the rotarod was increased from 4 to 40 r.p.m. in 5 minutes. The latency and speed of the rotarod at which a mouse fell off the rotarod were recorded. Each mouse was tested five times. The speed–latency index (latency in seconds × speed in r.p.m.) of each of the five tests was calculated, and the mean index of the five trials was used to reflect the motor coordination function of each mouse before or after MCAO. All mice were trained for two continuous days before the formal tests. They were placed on the rotarod five times each day, and this training occurred just before they were subjected to transient MCAO.
The assessment of infarct volumes for animals killed at 24 hours after MCAO was performed with 2,3,5-triphenyltetrazolium chloride staining as we described before (Li and Zuo, 2009). In brief, mice were killed by 5% isoflurane and the brains were removed rapidly. The brains were coronally cut into eight 1-mm-thick slices, except for the first and last slices that were 2 mm thick. The slices were incubated with 2% 2,3,5-triphenyltetrazolium chloride solution. The evaluation of infarct volumes at 4 weeks after transient MCAO was performed after hematoxylin and eosin staining as described previously (Li and Zuo, 2009; Zheng and Zuo, 2004), because 2,3,5-triphenyltetrazolium chloride staining will no longer adequately differentiate the infarcted area from the noninfarcted brain regions at this time point. In brief, rats were killed by isoflurane and transcardially perfused by saline and then 4% phosphate-buffered paraformaldehyde. The brains were removed and stored in the same fixative solution for 7 days. Paraffin coronal sections of 8-μm thickness were taken using a microtome at 1-mm intervals over the entire brain. The sections were stained with hematoxylin and eosin. The infarct areas were quantified using NIH Image 1.60 (NIH, Bethesda, MD, USA). The percentage of infarct volumes in ipsilateral hemisphere volumes was calculated to account for cerebral edema and differential shrinkage resulting from brain ischemia and tissue processing and to correct for the individual difference in brain volumes.
Measurement of Glutathione, Glutathione Disulfide, Nitrotyrosine-Containing Proteins, and 4-Hydroxy-2-Nonenal
Mice with or without brain ischemia were killed at 24 hours after transient MCAO by 5% isoflurane and transcardially perfused with normal saline. The contralateral and ipsilateral frontal cortex area 1 (Fr1) to the brain ischemic side was collected. Frontal cortex area 1 was chosen because this area is believed to be a penumbral region during transient MCAO (Memezawa et al, 1992). The levels of glutathione and glutathione disulfide (GSSG) in the brain tissues were measured using the Glutathione Assay kit (Cayman Chemical, Ann Arbor, MI, USA). The contents of nitrotyrosine-containing proteins and 4-hydroxy-2-nonenal (HNE) in the brain tissues were assessed using the OxiSelect Nitrotyrosine ELISA (enzyme-linked immunosorbent assay) kit (Cell Biolabs Inc., San Diego, CA, USA) and the OxiSelect HNE-His Adduct ELISA kit (Cell Biolabs Inc.), respectively, according to the manufacture's protocols. All assays were performed using samples obtained from CD-1 wild-type mice and EAAT3 knockout mice treated in parallel. The results of EAAT3 knockout mice were normalized to those of CD-1 wild-type mice.
Western Blot Analysis
Total lysates of the hippocampus (100 μg protein per lane) were subjected to western blot analysis. The primary antibodies used were the rabbit polyclonal anti-EAAT1 antibody (1:2,000 dilution; Cell Signaling Technology Inc., Danvers, MA, USA), the rabbit polyclonal anti-EAAT2 antibody (1:1,000 dilution; Cell Signaling Technology Inc.), the rabbit polyclonal anti-EAAT3 antibody (1:2,000 dilution; Alpha Diagnostic International Inc., San Antonio, TX, USA), and the rabbit polyclonal anti-actin antibody (1:4,000 dilution; Sigma Chemical Co., St Louis, MO, USA). The protein bands were visualized using enhanced chemiluminescence methods. The densities of EAAT1, EAAT2, and EAAT3 protein bands were normalized to those of actin to control for errors in protein sample loading and transferring during western blotting. The results of EAAT3 knockout mice were then normalized to those of CD-1 wild-type mice.
Gross Brain Vasculature Analysis
Mice without any treatments or brain ischemia were killed by isoflurane and immediately transcardially perfused with normal saline, followed by a solution containing 50% India ink and 5% gelatin in normal saline (Leypoldt et al, 2009). The brains were removed carefully. The circle of Willis was then examined and photographed under a dissecting microscope.
Statistical Analysis
Parametrical data are presented as means±s.d. The comparisons of the results of physiologic parameters, speed–latency index ratio, infarct volume, western blotting and baseline glutathione, GSSG, HNE, nitrotyrosine-containing proteins, and the ratio of right/left cerebral hemisphere volumes between CD-1 wild-type mice and EAAT3 knockout mice were performed by Student's t-test. Neurologic deficit scores were analyzed by the Mann–Whitney rank-sum test. The results of glutathione, GSSG, nitrotyrosine-containing proteins, and HNE in the ischemic and nonischemic Fr1 of CD-1 wild-type and EAAT3 knockout mice were analyzed by one-way analysis of variance, followed by the Student–Newman–Keuls test. The mortality rates between wild-type and EAAT3 knockout mice after MCAO were compared by z-test. P<0.05 was accepted as significant. All statistical analyses were performed using the SigmaStat program (SYSTAT Software Inc., Point Richmond, CA, USA).
Results
The physiologic data of mice used in this study are presented in Table 1. The results of heart rate, breathing rate, and arterial blood oxygen saturation presented in this table were the readings when MCAO was achieved. These parameters were continuously monitored during anesthesia. There was no difference in body weights and intrasurgery breathing rates, arterial blood oxygen saturation, and blood glucose levels between wild-type and EAAT3 knockout mice. The heart rates of EAAT3 knockout mice were slower than those of wild-type mice, although the heart rates of both wild-type and EAAT3 knockout mice were within the normal ranges of mice reported before (Kramer et al, 1993). No animals had an episode of hypoxia during surgery. To confirm our noninvasive monitoring results, a group of five mice were subjected to anesthesia and MCAO in the same manner. Arterial blood was sampled at the end of anesthesia and had the following results: pH: 7.255±0.048, PaCO2: 55.5±12.9 mm Hg, PaO2: 96±17 mm Hg, and hematocrit: 35%±4%. It is noteworthy that these five animals did not contribute data to other analyses because of the concern that arterial blood sampling and blood loss secondary to the sampling may interfere with the results.
Table 1. Physiologic results.
| Wild-type mice | EAAT3−/− mice | P-value | |
|---|---|---|---|
| Body weight (g) | 32.7±1.9 | 32.2±2.1 | 0.258 |
| Heart rate (times/min) | 453±75 | 399±55 | 0.002 |
| Respiration rate (times/min) | 80±27 | 81±23 | 0.856 |
| Arterial blood oxygen saturation (%) | 99.0±0.2 | 99.0±0.2 | 0.581 |
| Blood glucose (before MCAO, mg/dL) | 196±35 | 190±28 | 0.607 |
| Blood glucose (after MCAO, mg/dL) | 195±33 | 194±30 | 0.908 |
EAAT3, excitatory amino-acid transporter type 3; MCAO, middle cerebral artery occlusion.
Heart rate, respiration rate, and oxygen saturation were the readings when MCAO was achieved. Blood was collected at 5 minutes before and 5 minutes after MCAO for glucose measurement. Results are means±s.d. (n=16–40).
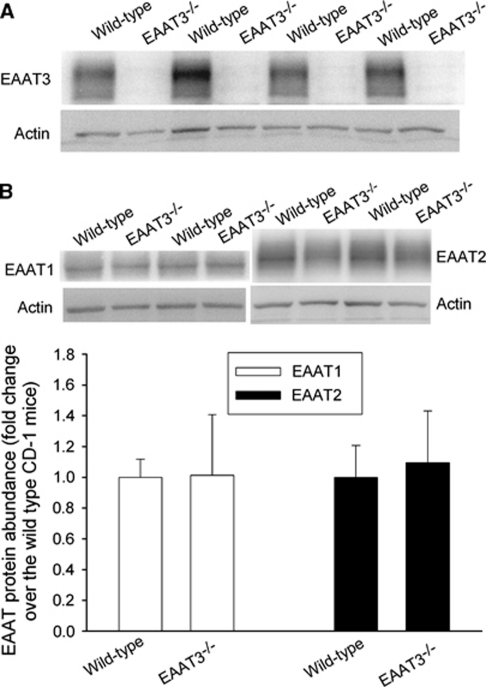
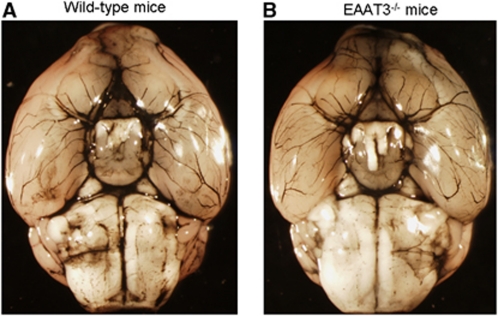
Consistent with previous studies (Aoyama et al, 2006; Peghini et al, 1997), we did not detect any EAAT3 proteins in the EAAT3 knockout mouse brains. The level of EAAT1 and EAAT2 proteins in the brains of EAAT3 knockout mice was similar to that of CD-1 wild-type mice (Figure 1) (Aoyama et al, 2006). There were no gross differences in the anterior, middle, and posterior cerebral artery anatomy between CD-1 wild-type and EAAT3 knockout mice (Figure 2).
Figure 1.
The expression of glutamate transporters (EAATs) in mouse brains. The results of EAAT3 are presented in A, and the results of EAAT1 and EAAT2 are shown in B. In panel B, a representative western blot is shown in the top panel, and the graphic presentation of EAAT protein abundance quantified by integrating the volume of autoradiograms from six CD-1 wild-type mice and six EAAT3 knockout mice is shown in the bottom panel. Values in graphs are expressed as fold change over the CD-1 wild-type mice and presented as means±s.d. EAAT, excitatory amino-acid transporter.
Figure 2.
Cerebral vascular anatomy. Representative images showing gross cerebral vasculatures stained by India ink (n=3 for wild-type (A) and EAAT3 knockout mice (B)). EAAT, excitatory amino-acid transporter.
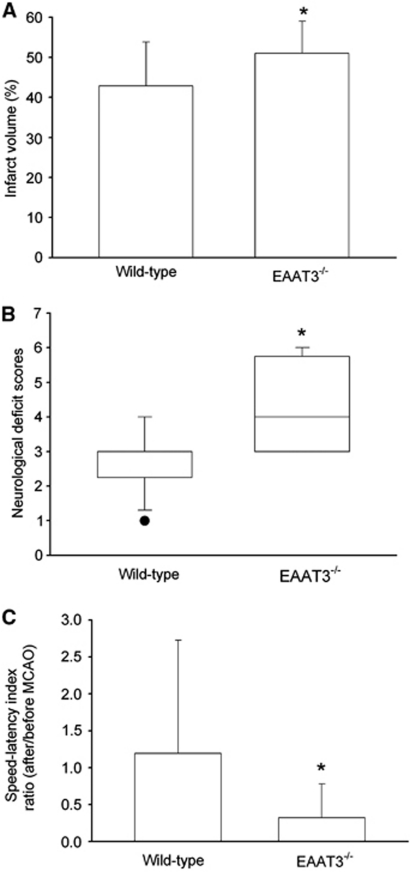
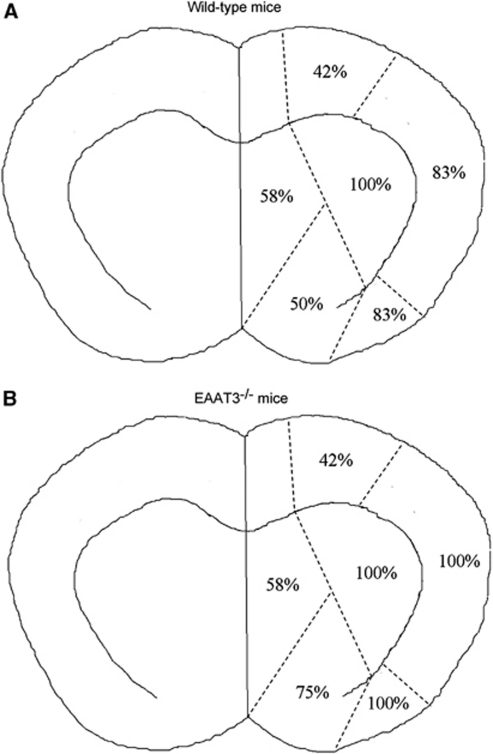
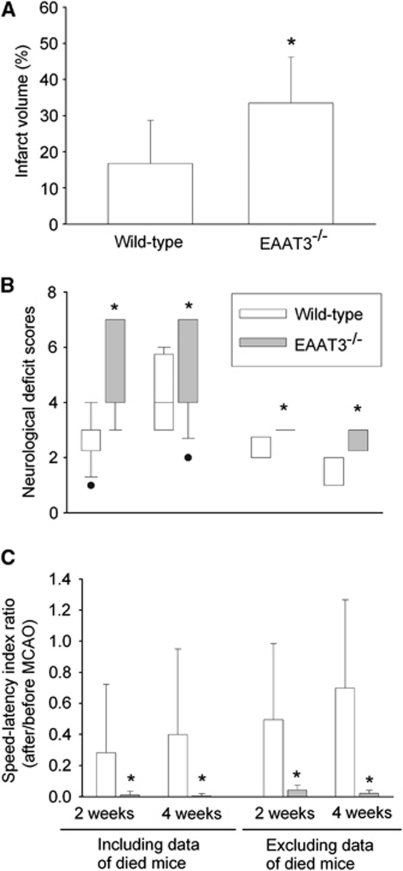
At 24 hours after transient right MCAO, the ratio of right/left cerebral hemisphere volumes were 1.10±0.05 and 1.08±0.04, respectively, for wild-type and EAAT3 knockout mice (n=12, P=0.208), indicating a similar level of edema in the ischemic hemisphere (Manley et al, 2000). However, EAAT3 knockout mice had bigger infarct brain volumes than did wild-type mice (51%±8% and 43%±11%, respectively, of the cerebral hemispheres, n=12, P=0.048) (Figure 3). This increased infarction occurred in both cerebral cortical and subcortical structures (Figure 4). Many of these structures are in regions considered to be the ischemic core after prolonged MCAO in rodents (Memezawa et al, 1992). The EAAT3 knockout mice also had worse neurologic deficit scores (75% confidence interval: 3.0 to 5.5 and 2.5 to 3.0, respectively, for knockout and wild-type mice, n=12, P=0.005) and motor coordination functions (75% confidence interval for the performance on rotarod: 0.03 to 0.51 and 0.33 to 1.34, respectively, for knockout and wild-type mice, n=12, P=0.009) after MCAO (Figure 3). Wild-type and EAAT3 knockout mice were not different in pre-MCAO neurologic deficit scores (all mice had a neurologic deficit score 0) or speed–latency index (2,890±2,941 r.p.m. seconds and 3,178±1,866 r.p.m. seconds, respectively, for wild-type and EAAT3 knockout mice, n=12, P=0.76).
Figure 3.
Neurologic outcome at 24 hours after focal brain ischemia. (A) Percentage of infarct volume in ipsilateral hemisphere volume. Results are means±s.d. (n=12). (B) Neurologic deficit scores evaluated immediately before animals were killed for the assessment of infarct sizes (data are presented in panel A). Results are presented in a box plot format (n=12). •: Lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25% to 75% interval including the median of the data. (C) Performance on rotarod. Mice were tested before and 24 hours after MCAO and the speed–latency index ratio of these two tests is presented. The speed–latency index before MCAO was 2,890±2,941 r.p.m. seconds and 3,178±1,866 r.p.m. seconds (P=0.76), respectively, for wild-type CD-1 mice and EAAT3 knockout mice. Results are means±s.d. (n=12). *P<0.05 compared with wild-type CD-1 mice. EAAT, excitatory amino-acid transporter; MCAO, middle cerebral artery occlusion.
Figure 4.
Topography of infarct brain distribution. Coronal sections at the bregma+0.14 mm from animals that were subjected to a 90-minute middle cerebral artery occlusion 24 hours before were analyzed for the presence of infarcted tissues. Data in the figure indicate percentages of animals with infarction in the respective regions in total animals (n=12 for each group). EAAT, excitatory amino-acid transporter.
In the long observation study, we subjected 14 wild-type mice and 16 EAAT3 knockout mice to a 90-minute MCAO. One wild-type mouse and four EAAT3 knockout mice died on day 1 after MCAO (the day to perform MCAO was counted as day 0). Five additional wild-type mice died within 5 days after MCAO, and eight additional EAAT3 knockout mice died within 7 days after MCAO. Eight wild-type mice and four EAAT3 knockout mice survived till the end of the 4-week observation period. The mortality rates were 42.9% and 75%, respectively, for wild-type and EAAT3 knockout mice (P=0.156). The EAAT3 knockout mice had bigger infarct brain volumes than did wild-type mice at 4 weeks after MCAO. The EAAT3 knockout mice also had a worse neurologic outcome than did wild-type mice as assessed by neurologic deficit scores and performance on rotarod, no matter whether the outcome was evaluated at 2 or 4 weeks after MCAO or whether the data of mice that died before the corresponding evaluation times (the dead mice were assigned 7 and 0, respectively, for the neurologic deficit scores and speed–latency index ratio for the performance on rotarod) were included in the analyses (Figure 5).
Figure 5.
Neurologic outcome at 4 weeks after focal brain ischemia. (A) Percentage of infarct volume in ipsilateral hemisphere volume at 4 weeks after middle cerebral artery occlusion (MCAO). Animals that died within the 4-week observation time window were not included in the analysis. Results are means±s.d. (n=4 to 8). (B) Neurologic deficit scores evaluated at 2 or 4 weeks after MCAO. Animals that died within 2 or 4 weeks after MCAO received a neurologic deficit score 7 for the corresponding times. Analyses including and excluding the data obtained from animals that died before the respective evaluation times are presented in the figure. Results are presented in a box plot format (n=4 to 16). •: Lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25% to 75% interval including the median of the data. (C) Performance on rotarod. Mice were tested before and 2 or 4 weeks after MCAO, and the speed–latency index ratio of these two tests is presented. The speed–latency index before MCAO was 1,797±1,334 r.p.m. seconds (n=14) and 1,850±1,503 r.p.m. seconds (n=16) (P=0.919) for wild-type CD-1 mice and EAAT3 knockout mice, respectively. Animals that died within 2 or 4 weeks after MCAO received 0 for the speed–latency index ratio at the corresponding times. Analyses including and excluding the data from animals that died before the respective evaluation times are presented in the figure. Results are means±s.d. (n=4 to 16). *P<0.05 compared with wild-type CD-1 mice. EAAT, excitatory amino-acid transporter.
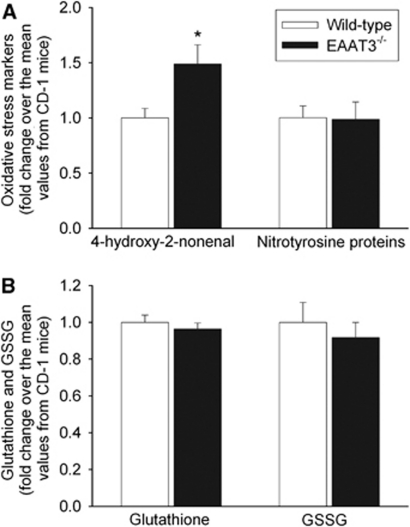
The HNE levels in the Fr1 of EAAT3 knockout mice were higher than those in wild-type mice under control conditions. However, nitrotyrosine-containing protein contents in the Fr1 were not different between these two types of mice. There was a small and nonstatistically significant decrease in baseline glutathione and GSSG contents in the Fr1 of EAAT3 knockout mice (Figure 6).
Figure 6.
Levels of oxidative stress markers, glutathione, and glutathione disulfide (GSSG) in the frontal cortex area 1 under control conditions. All animals were not subjected to brain ischemia. Results are means±s.d. (n=8). *P<0.05 compared with wild-type CD-1 mice. EAAT, excitatory amino-acid transporter.
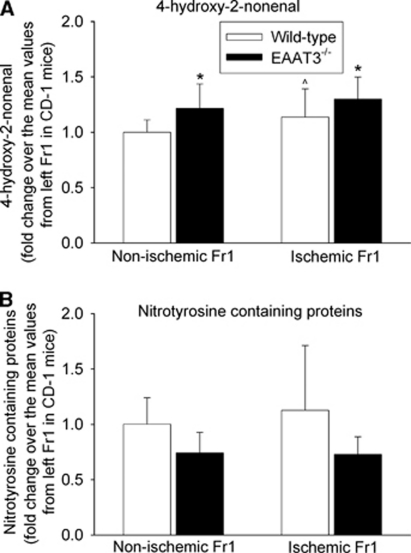
The HNE levels in the ischemic Fr1 were significantly higher than those in the nonischemic Fr1 in wild-type mice. The HNE expression in the ischemic and nonischemic Fr1 of EAAT3 knockout mice was significantly higher than that in the corresponding Fr1 of wild-type mice (Figure 7). However, there was no difference in nitrotyrosine-containing protein contents among the ischemic and nonischemic Fr1 of wild-type and EAAT3 knockout mice (Figure 7).
Figure 7.
Expression of nitrotyrosine-containing proteins and 4-hydroxy-2-nonenal (HNE). The frontal cortex area 1 (Fr1) was removed for assay of HNE and nitrotyrosine-containing proteins at 24 hours after a 90-minute right middle cerebral artery occlusion. Results are means±s.d. (n=11 to 12). *P<0.05 compared with the values from the corresponding site of wild-type CD-1 mice. ∧P<0.05 compared with the nonischemic Fr1 of wild-type CD-1 mice. EAAT, excitatory amino-acid transporter.
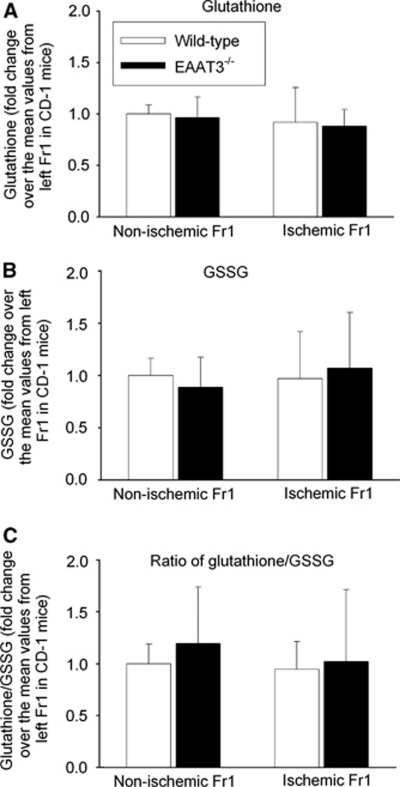
Glutathione levels in the ischemic Fr1 had a tendency to be lower than those in the nonischemic Fr1 in both wild-type and EAAT3 knockout mice. These differences were not statistically significant. There were no differences in GSSG levels and ratios of glutathione/GSSG among the right and left Fr1 of wild-type and EAAT3 knockout mice (Figure 8).
Figure 8.
The expression of glutathione and glutathione disulfide (GSSG). The frontal cortex area 1 (Fr1) was removed for assay of glutathione and GSSG at 24 hours after a 90-minute right middle cerebral artery occlusion. Results are means±s.d. (n=10). EAAT, excitatory amino-acid transporter.
Discussion
Several lines of evidence have suggested an important role of EAAT2 in modulating brain ischemic tolerance. An early study showed that antisense knockdown of EAAT2 in the normoxic rat brain increased extracellular glutamate concentrations and induced excitotoxicity (Rothstein et al, 1996). Antisense knockdown of EAAT2 also increased infarct volumes and worsened neurologic functions after transient MCAO in rats (Rao et al, 2001). Increasing the expression of EAAT2 by ceftriaxone before brain ischemia improved the neurologic outcome of rats after transient MCAO (Chu et al, 2007). These findings strongly indicate that EAAT2 increases brain tolerance to ischemia.
The role of EAAT3 in brain ischemic tolerance is not clear. An early study showed that antisense knockdown of EAAT3 did not increase extracellular glutamate levels but caused mild neurotoxicity in the normoxic rat brains (Rothstein et al, 1996). Antisense knockdown of EAAT3 expression by ∼65% appeared to cause a slight increase in brain infarct volumes at 24 hours after focal brain ischemia. However, this increase was not statistically significant and neurologic functions were not changed by EAAT3 knockdown (Rao et al, 2001). These findings suggest that EAAT3 may not have an important role in ischemic brain injury. We showed in this study that EAAT3 knockout mice had bigger brain infarct volumes and worse neurologic functions than did wild-type mice after focal brain ischemia, no matter whether the evaluation of neurologic outcome was performed at 24 hours or at 4 weeks after ischemia. The pre-MCAO neurologic functions of EAAT3 knockout and wild-type mice were not different. These two types of mice had similar gross cerebral vasculatures, and EAAT3 is not known to be expressed in any blood vessels. Thus, these results clearly show that EAAT3, like EAAT2, increases brain ischemic tolerance. This conclusion is different from the findings derived from the study using antisense oligonucleotides to downregulate EAAT3. The reason for these different conclusions may be very likely due to different degrees of reduction in EAAT3 expression in our study and in the previous study. In addition, our animals do not express EAAT3 in all brain regions, and downregulation of EAAT3 by antisense oligonucleotides may be restricted to certain brain regions.
We along with others have shown that EAAT3 can uptake cysteine as effectively as it uptakes glutamate (Lee et al, 2009; Zerangue and Kavanaugh, 1996). The uptake of cysteine through EAAT3 is much more effective than that through EAAT1 and EAAT2 (Bendahan et al, 2000; Zerangue and Kavanaugh, 1996). These findings, together with the knowledge that neurons lack a cystine-glutamate exchanger (Sato et al, 2002) through which the heteroexchange of cystine with glutamate is a way to acquire cysteine in the form of cystine in most cell types, suggest that cysteine uptake by EAAT3 may be a major mechanism for supplying the substrate for glutathione synthesis in neurons. In supporting this idea, it has been shown that EAAT3 knockout mice have a decreased glutathione level and an increased vulnerability to oxidants in the hippocampal slices (Aoyama et al, 2006). Oxidative stress has been considered as a major inducer to cause ischemic cell death (Lipton, 1999). Thus, increased oxidative stress may be a mechanism for the worse neurologic outcome of EAAT3 knockout mice after focal brain ischemia. Consistent with this possibility, the expression of HNE, a marker for oxidative stress in lipids (Aoyama et al, 2006), was significantly increased in the ischemic penumbral cerebral cortex of EAAT3 knockout mice compared with wild-type mice. Baseline HNE contents in the cerebral cortex of EAAT3 knockout mice were also higher than those in wild-type mice, suggesting that these knockout mice have increased lipid oxidative stress even under control conditions.
However, the levels of glutathione and nitrotyrosine-containing proteins, a marker for oxidative stress in proteins (Aoyama et al, 2006; Yuan et al, 2006), were not changed significantly in the ischemic penumbral cerebral cortex or in the nonischemic cerebral cortex of EAAT3 knockout mice compared with wild-type mice. The reason for there being no detectable change in the levels of glutathione and nitrotyrosine-containing proteins is not known. A dilution effect from other brain cells, such as glial cells, may underestimate the deficiency of glutathione and the possible increase of nitrotyrosine-containing proteins in the neurons of EAAT3 knockout mice. In addition, compensatory effects from other uptake systems in neurons, such as the neutral amino-acid transporter system ASCT1 that is expressed in neurons and can uptake cysteine (Shafqat et al, 1993; Yamamoto et al, 2003), may decrease the difference in glutathione levels between wild-type and EAAT3 knockout mice. Interestingly, a previous study showed that hippocampal glutathione levels of EAAT3 knockout mice were significantly lower than those in wild-type mice (Aoyama et al, 2006). This result is different from our data. Although the reasons for this discrepancy are not known, the use of different brain regions (the hippocampus versus the cerebral cortex) in the studies may contribute to different findings.
Although EAATs transport extracellular glutamate into cells under physiologic conditions, it has been recognized that EAATs can perform reversed transport of glutamate under pathophysiological conditions, such as ischemia (Rossi et al, 2000). In this case, glutamate moves through EAATs according to its gradient from intracellular compartments to extracellular space. This reversed transport increases extracellular glutamate concentrations, which was considered to contribute significantly to ischemia-induced extracellular glutamate accumulation (Phillis et al, 2000; Rossi et al, 2000). However, a more recent study showed that reversed glutamate transport through EAATs may not be a major source for increased extracellular glutamate concentrations in the ischemic penumbra in rats (Feustel et al, 2004). Similarly, the types of EAATs that perform reversed transport under ischemic conditions are also controversial. We along with others have shown that ischemia-induced extracellular glutamate accumulation can be inhibited by dihydrokainate, a relatively specific EAAT2 inhibitor (Jung et al, 2008; Seki et al, 1999; Shimamoto et al, 1998), suggesting reversed transport through EAAT2. However, dihydrokainate was unable to inhibit glutamate release and anoxic depolarization current in the hippocampal slices subjected to simulated ischemia in vitro (Roettger and Lipton, 1996; Rossi et al, 2000) and extracellular glutamate accumulation in the ischemic penumbra of the rat brain (Feustel et al, 2004). Although neuronal EAATs located in the glutamatergic presynaptic terminals were indicated to be involved in in vitro simulated ischemia-induced glutamate release from hippocampal slices (Rossi et al, 2000), EAAT3 is absent in glutamatergic presynaptic terminals (Danbolt, 2001). These controversial findings regarding the role of reversed glutamate transport through EAAT2 and EAAT3 in contributing to ischemia-induced glutamate release and the importance of reversed glutamate transport through EAATs in extracellular glutamate accumulation under ischemic conditions, along with the neuroprotective role of EAAT2 reported in the literature (Chu et al, 2007; Rao et al, 2001; Rothstein et al, 1996) and our current results on EAAT3, do not suggest an important contribution of reversed glutamate transport through EAAT2 and EAAT3 to ischemic brain injury.
The mortality rate of EAAT3 knockout mice trended to be higher than that of wild-type mice after a 90-minute MCAO. The EAAT3 knockout mice did not appear to have severer brain edema but had bigger infarct brain volumes and worse neurologic functions at 24 hours after MCAO. These results suggest that the possible increase in mortality in EAAT3 knockout mice may be caused by severer brain injury presented in these mice after MCAO.
The authors declare no other financial supports for this study, except for those grants stated on the title page from funding agencies for nonprofit. The authors also declare no conflict of interest in the content of this study.
Footnotes
This study was supported by a grant (R01 GM065211 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, USA, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), and the Department of Anesthesiology, University of Virginia.
References
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahan A, Armon A, Madani N, Kavanaugh MP, Kanner BI. Arginine 447 plays a pivotal role in substrate interactions in a neuronal glutamate transporter. J Biol Chem. 2000;275:37436–37442. doi: 10.1074/jbc.M006536200. [DOI] [PubMed] [Google Scholar]
- Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, Szatkowski M, Attwell D. Physiological and pathological operation of glutamate transporters. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cystein uptake in cortical neuron cultures. J Neurochem. 2003;84:1332–1339. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke. 2004;35:1164–1168. doi: 10.1161/01.STR.0000124127.57946.a1. [DOI] [PubMed] [Google Scholar]
- Jung HH, Lee JJ, Washington JM, Zuo Z. Inability of volatile anesthetics to inhibit oxygen-glucose deprivation-induced glutamate release via glutamate transporters and anion channels in rat corticostriatal slices. Brain Res. 2008;1227:234–239. doi: 10.1016/j.brainres.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K, van Acker SA, Voss HP, Grimbergen JA, van der Vijgh WJ, Bast A. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Methods. 1993;30:209–215. doi: 10.1016/1056-8719(93)90019-b. [DOI] [PubMed] [Google Scholar]
- Lee SA, Choi JG, Zuo Z. Volatile anesthetics attenuate oxidative stress-reduced activity of glutamate transporter type 3. Anesth Analg. 2009;109:1506–1510. doi: 10.1213/ANE.0b013e3181b6709a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypoldt F, Choe CU, Gelderblom M, von Leitner EC, Atzler D, Schwedhelm E, Gerloff C, Sydow K, Boger RH, Magnus T. Dimethylarginine dimethylaminohydrolase-1 transgenic mice are not protected from ischemic stroke. PLoS One. 2009;4:e7337. doi: 10.1371/journal.pone.0007337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Memezawa H, Minamisawa H, Smith M-L, Siesjo BK. Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp Brain Res. 1992;89:67–78. doi: 10.1007/BF00229002. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, Ren J, O'Regan MH. Transporter reversal as a mechanism of glutamate release from the ischemic rat cerebral cortex: studies with DL-threo-beta-benzyloxyaspartate. Brain Res. 2000;868:105–112. doi: 10.1016/s0006-8993(00)02303-9. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roettger V, Lipton P. Mechanism of glutamate release from rat hippocampal slices during in vitro ischemia. Neuroscience. 1996;75:677–685. doi: 10.1016/0306-4522(96)00314-4. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J Neurosci. 2002;22:8028–8033. doi: 10.1523/JNEUROSCI.22-18-08028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y, Feustel PJ, Keller RW, Jr, Tranmer BI, Kimelberg HK. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke. 1999;30:433–440. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- Shafqat S, Tamarappoo BK, Kilberg MS, Puranam RS, McNamara JO, Guadano-Ferraz A, Fremeau RT., Jr Cloning and expression of a novel Na(+)-dependent neutral amino acid transporter structurally related to mammalian Na+/glutamate cotransporters. J Biol Chem. 1993;268:15351–15355. [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nishizaki I, Furuya S, Hirabayashi Y, Takahashi K, Okuyama S, Yamamoto H. Characterization of rapid and high-affinity uptake of L-serine in neurons and astrocytes in primary culture. FEBS Lett. 2003;548:69–73. doi: 10.1016/s0014-5793(03)00742-7. [DOI] [PubMed] [Google Scholar]
- Yuan HB, Huang Y, Zheng S, Zuo Z. Hypothermic preconditioning reduces Purkinje cell death possibly by preventing the over-expression of inducible nitric oxide synthase in rat cerebellar slices after an in vitro simulated ischemia. Neuroscience. 2006;142:381–389. doi: 10.1016/j.neuroscience.2006.06.053. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol. 1996;493 (Part 2:419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of p38 mitogen-activated protein kinase. Mol Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]