Abstract
Collateral circulation in intracranial atherosclerosis has never been systematically characterized. We investigated collaterals in a multicenter trial of symptomatic intracranial atherosclerotic disease. Baseline angiography was reviewed for information on collaterals in stenoses of the internal carotid, middle cerebral, vertebral, and basilar arteries. A battery of angiographic scales was utilized to evaluate lesion site, arterial patency, antegrade flow, downstream territorial perfusion, and collateral circulation, blinded to all other data. Collateral circulation was adequately available for analysis in 287/569 (50%) subjects with proximal arterial stenoses ranging from 50% to 99%. Extent of collaterals was absent or none in 69%, slow or minimal in 10%, more rapid, yet incomplete perfusion of territory in 7%, complete but delayed perfusion in 11%, and rapid, complete collateral perfusion in 4%. Extent of collateral flow correlated with percentage of stenosis (P<0.0001), with more severe stenoses exhibiting greater compensation via collaterals. Overall, collateral grade increased with diminished antegrade flow across the lesion (thrombolysis in myocardial ischemia) and resultant downstream perfusion (thrombolysis in cerebral infarction) (both P<0.001). Our findings provide the initial detailed description of collaterals across a variety of stenoses, suggesting that collateral perfusion is a pivotal component in pathophysiology of intracranial atherosclerosis and implicating the need for further evaluation in ongoing studies.
Keywords: angiography, cerebral ischemia, collaterals, intracranial atherosclerosis, stenosis
Introduction
Collateral circulation in the brain is one of the most influential factors in mediating the potentially devastating effects of cerebral ischemia (Liebeskind, 2003). Collaterals sustain perfusion downstream from arterial occlusions in acute stroke, determining the hemodynamic features of the ischemic penumbra, pace of infarct evolution, and propensity for hemorrhagic transformation. Collateral perfusion may effectively maintain nutritive needs of the neurovascular unit across large expanses of brain tissue for long durations after complete arterial occlusion. In fact, ischemic symptoms manifest only when collaterals fail. From a therapeutic standpoint, robust collaterals are a potent predictor of arterial recanalization, preserved tissue fate, and good clinical outcomes in acute stroke. Although much emphasis has been placed on specific arterial collateral routes that bypass flow around occlusions, the capacity of other elements such as the venous system to maintain cerebral blood volume remains essential (Tomita et al, 1980). Even when seemingly equivalent cases of proximal middle cerebral artery (MCA) occlusion are compared, the underlying etiology or mechanism such as atherosclerotic plaque or thromboembolism culminating in occlusion has a striking effect on ischemic severity (Kim et al, 2009).
Arterial occlusion due to progressive atherosclerotic stenosis of an intracranial segment may allow robust collaterals to develop over time, unlike the scenario accompanying cardioembolic or abrupt thrombotic occlusion. In contrast to the mounting knowledge on collaterals that circumvent occlusion in acute stroke, little is known about the process of collateral development or arteriogenesis that accompanies progressive intracranial stenosis (Choi et al, 2010). Many studies have explored the pattern and influence of collaterals in extracranial carotid artery stenosis. In extensive intracranial stenoocclusive disorders such as moyamoya syndrome, the presence of coexisting occlusion and exuberant proliferation of collaterals may literally cloud detailed study of the interplay between a discrete proximal arterial stenosis and distal collateral formation. A dearth of knowledge remains regarding the role of collaterals associated with intracranial stenosis.
Potential collateral routes have been cataloged with pathology from the most basic types such as the communicating arteries at the circle of Willis to more esoteric configurations that capitalize on vascular anomalies incorporating the leptomeningeal anastomoses (Brozici et al, 2003). The functional consequences of all collateral routes, however, remained undisclosed until the advent of angiography. More recent imaging advances have facilitated the noninvasive characterization of collateral flow with ultrasonography, multimodal computed tomography or magnetic resonance imaging, and positron emission tomography (Liebeskind, 2005, 2009). Each imaging modality accentuates particular features of collaterals that may vary by anatomical location, including structural details, flow capacity, territorial perfusion, or metabolic consequences. Clinical considerations alter the choice of collateral imaging acquired in routine care, including a preference for noninvasive approaches when possible to minimize risk of conventional angiography. Nevertheless, the exquisite spatial and temporal resolution of conventional angiography remains the gold standard for evaluation of collateral circulation. Angiographic descriptions of collaterals in the setting of intracranial stenosis are predominantly anecdotal, falling short of systematic characterization. The Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) trial, focused primarily on defining optimal antithrombotic regimens, required systematic angiographic definition of the degree of luminal stenosis thereby providing a opportunity to investigate associated collateral flow (Chimowitz et al, 2005). The required conventional angiography and rigorous measurements of arterial stenosis for all subjects recruited in the trial avoids the selection bias that may influence angiographic characterization in routine clinical practice (Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators, 2003). As the role of angiographic collaterals in disease course had not been previously established, such observations were not prespecified and image acquisitions were not necessarily ideally tailored for this purpose but many unaddressed questions can now be answered.
A litany of questions may now be addressed with the WASID data set regarding collaterals that compensate for progressive intracranial stenosis of an artery in the brain. The presence and patterns of collaterals with specific stenotic lesions can be described. Atherosclerotic lesion sites and the relative contribution of Willisian versus leptomeningeal collaterals may be detailed. The angiographic features of a stenosis associated with collateral flow can be assessed, identifying if there is a threshold for degree of stenosis that causes hemodynamic impairment and collateral compensation. Collateral flow should theoretically be inconsequential or nonexistent if stenosis is not hemodynamically significant (luminal stenoses below 60% to 70%), raising the question of whether there are lesion factors beyond the degree of stenosis leading to a hemodynamic effect. Consideration of antegrade or forward flow beyond the lesion scored by angiography may also be compared with the vigor of collateral flow to downstream regions. Beyond qualitative descriptions of angiographic appearances, the degree of collateral flow can be quantified. Clinical correlations may discern which patients develop collaterals in response to stenosis, based on demographics, comorbidities, time course of ischemia, or other factors. Cases enrolled in the WASID trial were symptomatic with respect to a particular arterial lesion, but the role of multifocal disease may provide an opportunity to correlate collaterals with concomitant asymptomatic lesions, as well (Nahab et al, 2008). Perhaps most importantly, an initial description on the status of collaterals in intracranial stenosis may provide the framework for predictive modeling of future recurrent ischemic events and the relative impact of emerging therapies from medical regimens to interventional strategies with angioplasty and stenting (Qureshi et al, 2009).
We therefore conducted a retrospective analysis of the baseline angiography acquired in the WASID trial to provide the first comprehensive evaluation of collaterals in the setting of symptomatic intracranial atherosclerosis. These angiographic correlations with numerous aspects of the clinical data set build upon the limited anecdotal reports of collaterals to date. Particular strengths of this systematic evaluation of collateral flow in the brain exploit the design of this seminal multicenter randomized, controlled trial including maximal avoidance of selection bias before acquisition of the conventional angiography gold standard and addition of rigorous collateral assessments to the power of the clean trial data set.
Materials and methods
Subjects and Study Design
The WASID trial was a prospective multicenter, double-blind, randomized clinical trial conducted from 1999 to 2003 at 59 sites with institutional review board approval for the study (Chimowitz et al, 2005). The trial was designed to compare antithrombotic strategies to prevent stroke or vascular death in patients with transient ischemic attack or nondisabling ischemic stroke attributable to 50% to 99% atherosclerotic stenosis of a major intracranial carotid artery (ICA), MCA, vertebral artery (VA), or basilar artery (BA) verified by conventional angiography. The complete details of the WASID trial design and principal results have been previously published (Chimowitz et al, 2005).
Angiography
All patients enrolled in the WASID trial underwent conventional angiography to confirm a symptomatic intracranial atherosclerotic stenosis (50% to 99%) of the ICA, MCA, VA, or BA. Patients who did not undergo angiography as part of routine care gave written informed consent for single vessel angiography as part of the study protocol. Angiography was performed within 18 days (median 7, range −142 to 91 days) of the index cerebral ischemic event. All conventional angiograms in the study were centrally adjudicated for the degree of luminal arterial stenosis based on caliper measurements of selected images (Samuels et al, 2000).
All cases with available baseline central angiography data were evaluated in this post hoc analysis. One investigator with extensive experience in central angiography adjudication reviewed all baseline angiograms to determine availability of data on collateral circulation corresponding to the anatomic location of the symptomatic intracranial atherosclerotic lesion. For inclusion in our analyses, adequate information on potential collateral routes had to be available for each case. Cases without angiography data that detailed either the spatial or the temporal features of potential collateral routes were excluded.
Measures of arterial stenosis at the intracranial atherosclerotic lesion were obtained from the prospective central angiography review process conducted at the time of subject enrollment. A battery of angiographic scales was utilized in our analyses to evaluate lesion site, arterial patency, antegrade flow, downstream territorial perfusion, and collateral circulation, blinded to all other data for each subject in the trial.
Antegrade or forward flow beyond the arterial stenosis was measured with the thrombolysis in myocardial ischemia (TIMI) and thrombolysis in cerebral infarction (TICI) scales (Higashida et al, 2003). As numerous variations of the TIMI scale have been used by prior investigators, we specifically implemented a scoring system of 0=no flow; 1=some penetration past the occlusion, but no flow distal to the occlusion; 2=distal perfusion but delayed filling in distal vessels; 3=distal perfusion with adequate perfusion of distal vessels. The TICI scale was applied according to the exact definitions used in the original description (Higashida et al, 2003).
Collaterals were assessed with the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) Collateral Flow Grading System, using the specific definitions in the original description (Higashida et al, 2003). These categories include 0=no collaterals visible to the ischemic site; 1=slow collaterals to the periphery of the ischemic site with persistence of some of the defect; 2=rapid collaterals to periphery of ischemic site with persistence of some of the defect and to only a portion of the ischemic territory; 3=collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase; 4=complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion. Other angiography collateral scales were applied to subsets of the entire cohort based on the arterial lesion site. For instance, the PROACT II collateral scale was used to discriminate none, partial, or full collaterals in proximal MCA lesions (Roberts et al, 2002). Similarly, collateral flow beyond posterior circulation lesions in the vertebral or basilar arteries were categorized as none, minimal, moderate, or maximal based on a previously published scale (Brandt et al, 1996). Collateral flow was also incorporated in our angiographic review as a component of the Qureshi scale that was applied to all cases in this study (Qureshi, 2002). For the main analyses of collateral flow with respect to other clinical and angiographic variables, the ASITN/SIR scale served as the principal measure of collateral circulation.
Clinical Variables
Clinical variables used in our analyses utilized demographics, medical history items, timeline for enrollment, blood pressure measurements, assessment of neurologic deficits, functional status, and other items obtained from the main trial data set. These variables were analyzed with respect to angiographic features as described above.
Statistical Analysis
Descriptive methods were used to characterize baseline angiographic features, including distributions across categories for each angiographic scale. Frequencies and percentages were calculated based on the denominator or subset of relevant cases for each parameter. When applicable, percentages with 95% confidence intervals were determined using exact binomial methods. Comparisons of group characteristics were made using the independent groups t-test and one-way analysis of variance (for means) and χ2 test (for percentages). A P value of 0.05 was considered statistically significant. P values are reported without adjustment for multiple testing. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA). Continuous data are presented as mean±s.d.
Results
Extent of Collateral Circulation
Collateral circulation was assessed on 287/569 (50%) angiograms performed at study entry demonstrating proximal arterial stenoses ranging from 50% to 99%. The site of symptomatic intracranial atherosclerotic lesions included 39 ICA, 84 MCA, 69 VA, 71 BA, and 24 combined stenoses. The distribution in percentage of luminal stenoses spanned from 50% to 99% and the demographics of this subset was similar to the main trial population. The extent of collaterals was absent or none in 69%, slow or minimal in 10%, more rapid, yet incomplete perfusion of territory in 7%, complete but delayed perfusion in 11%, and rapid, complete collateral perfusion in 4% (Figure 1). Collateral assessment of all lesion types with the scale utilized in PROACT II for the MCA yielded less dispersion of scores given the limited categories of angiographic collaterals, including none in 197/287 (69%), partial in 65/287 (23%), and full in 25/287 (9%) (Roberts et al, 2002). Evaluation of posterior circulation collateral flow with the scale proposed by Brandt et al (1996) demonstrated no collaterals in 111/157 (71%), 19/157 (12%) minimal, 14/157 (9%) moderate, and 13/157 (8%) maximal.
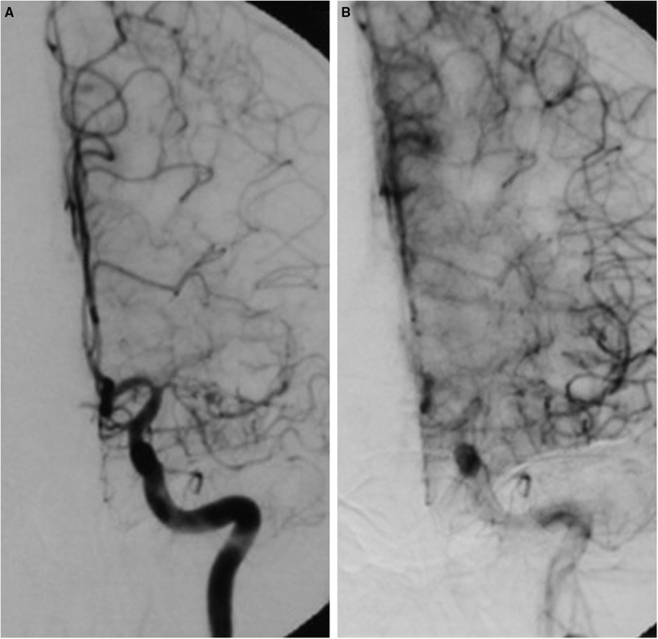
Figure 1.
Severe atherosclerotic stenosis of the proximal left middle cerebral artery (MCA) (A) with associated rapid and complete perfusion via leptomeningeal collaterals (B).
Collateral Flow Patterns
Although subtle variations were noted in the patterns, characteristic collateral flow routes were demonstrated for each particular site of atherosclerotic stenosis (Figure 2). Internal carotid artery lesions commonly recruited Willisian collaterals, whereas leptomeningeal anastomoses were seen more commonly in severe stenoses with limited Willisian circuits. Middle cerebral artery stenoses recruited varying degrees of leptomeningeal collateral flow akin to acute MCA occlusion. Vertebral artery stenoses showed considerable variability in associated collaterals, most commonly differentiated based on the presence and caliber of the contralateral VA. For BA lesions, a combination of collateral patterns were noted including anastomoses across the cerebellar hemispheres as previously described in acute occlusion and recruitment of posterior communicating arteries in only the most severe stenoses. There was no difference in the collateral flow from vessel to vessel (Table 1, P=0.91).
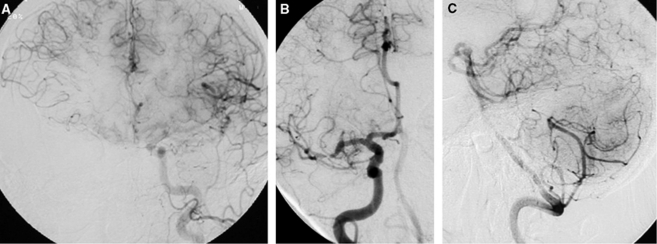
Figure 2.
Specific patterns of collateral circulation associated with anatomical sites of intracranial stenosis. Leptomeningeal collaterals provide blood flow to the right middle cerebral artery (MCA) territory from the contralateral hemisphere in the setting of severe right internal carotid artery (ICA) stenosis and limited Willisian supply (A). A combination of antegrade and retrograde collateral perfusion feeds downstream tissue beyond an MCA stenosis (B). Cerebellar anastomoses between posterior inferior and superior cerebellar arteries distribute blood to offset midbasilar atherosclerosis (C).
Table 1. ASITN/SIR collateral grade by vessela.
| Vessel | No collaterals | Partial collaterals | Complete collaterals |
|---|---|---|---|
| ICA (n=39) | 29 (74%) | 5 (13%) | 5 (13%) |
| MCA (n=84) | 55 (65%) | 17 (20%) | 12 (14%) |
| VA (n=69) | 50 (72%) | 9 (13%) | 10 (14%) |
| BA (n=71) | 49 (69%) | 12 (17%) | 10 (14%) |
| Combined (n=24) | 14 (58%) | 5 (21%) | 5 (21%) |
| Overall (n=287) | 197 (68%) | 48 (17%) | 42 (21%) |
ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; VA, vertebral artery.
Data presented are sample size (percent). χ2 P-value=0.9108.
Collaterals and Degree of Luminal Stenosis
The extent of collateral flow for all proximal arterial lesions correlated with the percentage of stenosis (Table 2, P<0.0001), with more severe stenoses exhibiting greater degrees of compensatory collateral flow. Collateral flow categorized using ASITN/SIR grade included no collaterals (n=197, mean stenosis 63%±9%), partial (n=48, mean stenosis 71%±9%), and complete (n=42, mean stenosis 81%±12%) across all lesions. Similar patterns were seen within each type of lesion (Table 2).
Table 2. Percent stenosis categorized by ASITN/SIR collateral grade by vessela.
| Vessel | No collaterals | Partial collaterals | Complete collaterals | P-value |
|---|---|---|---|---|
| ICA | 65 (8) | 76 (5) | 76 (16) | 0.0038b |
| MCA | 63 (8) | 68 (11) | 86 (15) | 0.0001c |
| VA | 63 (9) | 72 (6) | 81 (11) | 0.0001b |
| BA | 64 (9) | 75 (7) | 82 (8) | 0.0001b |
| Combined | 64 (12) | 70 (6) | 76 (10) | 0.0808 |
| Overall | 63 (9) | 71 (9) | 81 (12) | 0.0001d |
ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; VA, vertebral artery.
Data presented are mean (s.d.). Sample sizes are given in Table 1.
Tukey's post hoc no<partial, complete.
No, partial<complete.
No<partial<complete.
Although more robust collaterals were associated with increasing degree of stenosis, the full range of collateral grades was evident for both moderate and severe stenoses. Interestingly, 11% of subjects with only moderate intracranial stenosis of 50% to 69% exhibited impaired downstream perfusion and conversely, 69% of severe stenosis between 70% and 99% had rapid and complete antegrade perfusion. Collateral compensation was evident in 14% of moderate stenoses and 44% of severe stenoses had no collaterals.
Collateral Compensation for Limited Antegrade Flow Beyond Stenosis
The presence of compensatory collateral flow may be directly balanced by the amount or vigor of antegrade flow past the arterial lesion. Thrombolysis in myocardial ischemia 0 or complete occlusion was not observed in any of the cases in our analyses. Partial antegrade flow at the lesion site was determined as TIMI 1 or 2, including some flow in 6/287 (2%), with distal delayed flow past the lesion in 48/287 (17%), and distal adequate flow in 233/287 (81%). Antegrade flow across the stenosis by TIMI grade decreased with decreasing luminal patency (P<0.0001), including adequate flow at mean 65%±10%, delayed distal flow at mean 75%±12%, and only some residual flow at mean 91%±9%.
Thrombolysis in cerebral infarction measures of territorial perfusion included 6/287 (2%) with minimal perfusion, only partial filling in 11/287 (4%), complete but delayed filling in 38/287 (13%) and complete, rapid perfusion of the territory in 232/287 (81%). Downstream antegrade perfusion (TICI) generally decreased with increasing stenosis (P<0.01), including complete perfusion at mean 65%±10%, complete yet delayed perfusion at mean 74%±11%, only partial filling at mean 77%±15%, and minimal perfusion at mean 88%±12%. Similar patterns were also seen within each of the lesion types (Table 3), with significant findings within ICA, MCA, VA, and BA. As the maximal degree or percentage of luminal stenosis may be only one parameter influencing distal flow, we also attempted to analyze lesion length with antegrade perfusion yet only relative measurements were available (Figure 3). Overall, collateral grade increased with diminished antegrade flow across the lesion (TIMI) and resultant downstream perfusion (TICI) (both P<0.001).
Table 3. Percent stenosis categorized by TICI by vessel.
| Vessel |
Partial flow |
Complete flow |
P-value | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | s.d. | n | Mean | s.d. | ||
| ICA | 6 | 80 | 10 | 33 | 65 | 8 | 0.0002 |
| MCA | 19 | 74 | 14 | 65 | 65 | 11 | 0.0039 |
| VA | 12 | 77 | 13 | 57 | 65 | 9 | 0.0002 |
| BA | 11 | 81 | 9 | 60 | 66 | 10 | 0.0001 |
| Combined | 6 | 70 | 13 | 18 | 67 | 11 | 0.6063 |
BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; TICI, thrombolysis in cerebral infarction; VA, vertebral artery.
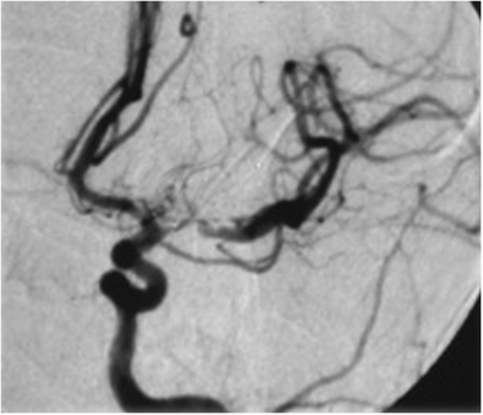
Figure 3.
Antegrade flow is largely preserved in an elongated stenosis of the left middle cerebral artery (MCA) despite considerable luminal irregularity and vessel tortuosity.
Specific Features
Specific features of collaterals with particular sites of stenosis were investigated, attempting to define cut points or thresholds for the degree of stenosis when specific routes become apparent. Although collaterals became apparent at certain degrees of stenosis and antegrade flow impairment as described above, considerable variation existed likely implicating different roles of luminal stenosis and features that may reflect hemodynamics in particular routes. These questions included what degree of luminal stenosis in the basilar artery is necessary to recruit leptomeningeal cerebellar anastomoses between the posterior inferior and superior cerebellar arteries. What degree of luminal stenosis invokes posterior communicating artery flow, or alternatively, at what stage does retrograde leptomeningeal arterial flow compensate for anterior circulation hypoperfusion (Figure 4)?
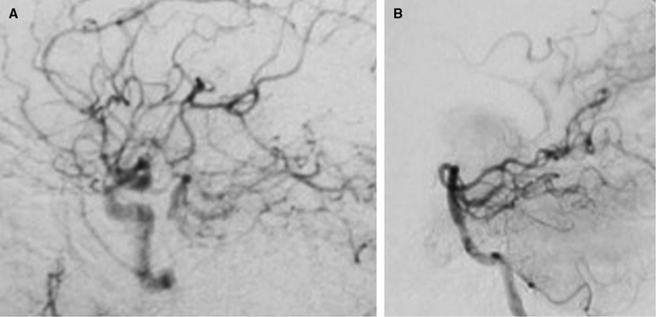
Figure 4.
Posterior communicating artery flow maintains poststenotic regions of the basilar distribution (A). In another case, posterior cerebral artery anastomoses supply distal reaches of the anterior and middle cerebral arteries in an internal carotid artery (ICA) stenosis (B).
Clinical Correlates of Collaterals
Collaterals are beneficial in compensating for arterial obstruction, yet serve as a marker of underlying disease. The association of collaterals with neurologic deficits may therefore be complex. Our analyses revealed no correlation between collateral status and severity of baseline neurologic deficit or functional status.
Recruitment of collaterals is also a dynamic process that evolves over time and in response to arterial obstruction. The potential for increased collateral flow may be greater as time lapses from the initial symptomatic event. Our analyses of collateral flow demonstrated no differences in the time from qualifying neurologic event or ischemic symptoms and the extent of collaterals in either moderate or severe stenoses (P=NS).
Multifocal atherosclerotic disease was noted on angiographic review, including the presence of asymptomatic lesions with evidence of robust collateral compensation.
As the extent of collateral flow has been inversely correlated with blood pressure in acute ischemic stroke, we analyzed blood pressure correlates in this cohort. No relationship was noted, however, between collateral grade and systolic, diastolic, or mean arterial pressures in subjects with either moderate or severe stenoses (P=NS).
Discussion
Collaterals and Intracranial Atherosclerosis
The primary focus of efforts in intracranial atherosclerosis to date has centered on stenosis or luminal compromise due to plaque with relatively little consideration of downstream flow. Although the mechanisms of ischemic stroke in intracranial atherosclerosis remain unknown, diminished flow beyond a stenosis may cause stroke due to hypoperfusion or impaired washout of thromboemboli (Caplan and Hennerici, 1998). Collateral circulation, however, may offset this potentially detrimental effect of stenosis. The results of our systematic evaluation of collaterals on angiography in WASID provide the first detailed description of collateral circulation across a variety of stenotic lesions. Our findings reveal that collaterals are often observed with intracranial atherosclerosis at varying extents. Collateral compensation should therefore be considered in the pathophysiology of intracranial atherosclerosis. Patterns of collateral flow in this chronic disorder of stenosis parallel the alternative flow routes, including Willisian and leptomeningeal anastomoses, visualized with complete arterial occlusion in acute stroke (Liebeskind, 2003).
Measures of Collaterals
Collateral flow may be quantitatively assessed using several angiographic scales initially employed with complete occlusion in acute stroke; however, the extent of collaterals may be less overt with stenosis. Although several scales exist for collateral flow, the ASITN/SIR scale effectively differentiates five degrees of collaterals incorporating both spatial and temporal features across a wide range of stenotic lesion sites. In contrast, other scales may provide less discrimination or may be restricted to particular anatomic lesions. Feasibility demonstrated in this study supports future systematic, quantitative evaluation of collaterals in other settings.
Hemodynamic Impairment and Collaterals
The effect of atherosclerotic plaque in the brain on downstream flow at angiography has not been previously quantified. Our analyses parallel related findings of distal pressure drops beyond extracranial plaque, providing strikingly similar results (Derdeyn et al, 1999; Deweese et al, 1970). Above 50% luminal stenosis, some individuals demonstrate hemodynamic impairment in antegrade flow with corresponding collateral recruitment. Hemodynamic impairment in intracranial atherosclerosis measured by TIMI and TICI grade occurs at the same threshold predicted both mathematically and at the level that pressure drops become ubiquitous in extracranial plaque (Deweese et al, 1970). This relationship was evident in our results that demonstrated decreasing TIMI and TICI grade with increasing stenosis. Flow across the intracranial stenosis (TIMI) and into the downstream territory (TICI) showed consistent decrements with respect to degree of stenosis at various lesion sites. Isolated severe cases without collaterals are likely compromised and at risk. Imperfect estimates of luminal radius alone and presence of collaterals in some cases of moderate stenosis suggest that other factors may be influencing hemodynamics.
Collaterals as Biomarker
Collateral status in intracranial atherosclerosis appears to differentiate cases beyond currently employed variables, providing an opportunity as a novel biomarker for this disorder (Chalela, 2009). Clinical correlates of collaterals were not evident in our study, suggesting the inherently complex nature of collateral flow. Collaterals mark the presence of underlying disease, yet their role is beneficial in compensating for potential hemodynamic impairment. Individuals with robust collaterals may be at risk of stroke, yet at lesser risk than those without. Perhaps those with moderate stenoses and robust collaterals are at greater risk similar to those with severe stenosis and no collateral perfusion. Modeling risk based on collaterals as a biomarker may be a promising strategy with a logical or mechanistic pathophysiological basis. Collaterals and functional demonstration of flow impairment may be more informative than isolated anatomic measures of maximal stenosis or length.
Limitations
Limitations affect our retrospective analyses in this multicenter study, where angiography was not tailored for optimal evaluation of collateral flow. A subset of only 50% of WASID subjects had collaterals evaluated. Biases may have influenced the selection of cases and our observations on collaterals, yet angiography remains the optimal modality for such pathophysiology. Angiographic analysis based on hard film copies may degrade temporal resolution and scale limitations may also be influential. Adaptation of scales for occlusion to stenosis from the acute to chronic setting may also impart difficulty. Compensatory collateral flow may also be influenced by the reduced demand for blood flow in downstream brain tissue beyond an atherosclerotic stenosis due to prior ischemic injury. Our measures of maximal degree of stenosis are only rudimentary measures of plaque architecture that influence hemodynamic parameters. Lesion length, luminal irregularity, vessel tortuosity, and other factors beyond these parameters may cause hemodynamic impairment or thromboembolic potential. Our observations included collaterals in asymptomatic lesions but our focus on symptomatic stenoses may not be as informative as investigation of collaterals in an asymptomatic cohort. Logistic difficulties may arise, however, due to the invasive nature of angiography. Clinical correlations may be hampered by the beneficial effect of collaterals in this chronic disorder. For instance, analyses of collaterals with neurologic deficits or functional status may be limited due to the relatively high functional status preserved by collaterals throughout this cohort. Finally, the time course of collateral recruitment remains difficult to study due to the nature of collaterals that may directly dictate timing of symptoms and subsequent course.
Next Steps
These initial observations on collaterals in intracranial atherosclerosis open an avenue for several lines of investigation. Collaterals should be studied in prospective manner with tailored acquisitions. Systematic evaluation may elucidate key pathophysiology and address recurrent stroke risk. Such work can be applied in trial or registry settings to discern effect of collaterals on clinical events and interaction with currently routine angiographic measures focused on stenotic plaque. Factors beyond static angiographic measures of maximal stenosis or length extending to geometry-based computational fluid dynamics may be able to accurately replicate hemodynamic effects of intracranial plaque (Schirmer and Malek, 2007). The use of angiography with potential endovascular therapies for intracranial atherosclerosis provides critical data to study both spatial and temporal features of collaterals. Such data would complement the increasing ability of clinical investigators to probe hemodynamic status with computed tomography, magnetic resonance, or positron emission tomography perfusion imaging techniques. Understanding the process of collateralization or arteriogenesis and potential collateral therapeutic strategies are predicated on delineating such baseline collateral physiology (Liebeskind, 2004).
Acknowledgments
The authors thank the extensive efforts of the Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) Investigators.
The authors declare no conflict of interest.
Footnotes
This study was supported by the National Institutes of Health (K23 NS054084 and P50 NS044378 to DSL). The WASID trial was funded by a research grant (1R01 NS36643, Principal Investigator: Dr Chimowitz) from the US Public Health Service, NINDS. In addition, the following General Clinical Research centers, funded by the NIH, provided local support for the evaluation of patients in the trial: Emory University (M01 RR00039), Case Western University, Metro Health Medical Center (5M01 RR00080), San Francisco General Hospital (M01 RR00083-42), Johns Hopkins University School of Medicine (M01 RR000052), Indiana University School of Medicine (5M01 RR000750-32), Cedars-Sinai Hospital (M01 RR00425), and the University of Maryland (M01 RR165001).
References
- Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke. 1996;27:875–881. doi: 10.1161/01.str.27.5.875. [DOI] [PubMed] [Google Scholar]
- Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003;34:2750–2762. doi: 10.1161/01.STR.0000095791.85737.65. [DOI] [PubMed] [Google Scholar]
- Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- Chalela JA. Evaluating the carotid plaque: going beyond stenosis. Cerebrovasc Dis. 2009;27 (Suppl 1:19–24. doi: 10.1159/000200438. [DOI] [PubMed] [Google Scholar]
- Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- Choi JW, Kim JK, Choi BS, Lim HK, Kim SJ, Kim JS, Suh DC. Angiographic pattern of symptomatic severe M1 stenosis: comparison with presenting symptoms, infarct patterns, perfusion status, and outcome after recanalization. Cerebrovasc Dis. 2010;29:297–303. doi: 10.1159/000275508. [DOI] [PubMed] [Google Scholar]
- Derdeyn CP, Grubb RL, Jr, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999;53:251–259. doi: 10.1212/wnl.53.2.251. [DOI] [PubMed] [Google Scholar]
- Deweese JA, May AG, Lipchik EO, Rob CG. Anatomic and hemodynamic correlations in carotid artery stenosis. Stroke. 1970;1:149–157. doi: 10.1161/01.str.1.3.149. [DOI] [PubMed] [Google Scholar]
- Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Seok JM, Bang OY, Kim GM, Kim KH, Jeon P, Chung CS, Lee KH, Alger JR, Liebeskind DS. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. 2009;29:1138–1145. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS. Collateral therapeutics for cerebral ischemia. Expert Rev Neurother. 2004;4:255–265. doi: 10.1586/14737175.4.2.255. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS.2005Collaterals in acute stroke: beyond the clot Neuroimaging Clin N Am 15553–573.x [DOI] [PubMed] [Google Scholar]
- Liebeskind DS. Imaging the future of stroke: I. Ischemia. Ann Neurol. 2009;66:574–590. doi: 10.1002/ana.21787. [DOI] [PubMed] [Google Scholar]
- Nahab F, Cotsonis G, Lynn M, Feldmann E, Chaturvedi S, Hemphill JC, Zweifler R, Johnston K, Bonovich D, Kasner S, Chimowitz M. Prevalence and prognosis of coexistent asymptomatic intracranial stenosis. Stroke. 2008;39:1039–1041. doi: 10.1161/STROKEAHA.107.499475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI.2002New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke Neurosurgery 501405–1414.discussion 14–15 [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Feldmann E, Gomez CR, Johnston SC, Kasner SE, Quick DC, Rasmussen PA, Suri MF, Taylor RA, Zaidat OO. Intracranial atherosclerotic disease: an update. Ann Neurol. 2009;66:730–738. doi: 10.1002/ana.21768. [DOI] [PubMed] [Google Scholar]
- Roberts HC, Dillon WP, Furlan AJ, Wechsler LR, Rowley HA, Fischbein NJ, Higashida RT, Kase C, Schulz GA, Lu Y, Firszt CM. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002;33:1557–1565. doi: 10.1161/01.str.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- Schirmer CM, Malek AM.2007Prediction of complex flow patterns in intracranial atherosclerotic disease using computational fluid dynamics Neurosurgery 61842–851.discussion 52 [DOI] [PubMed] [Google Scholar]
- Tomita M, Gotoh F, Amano T, Tanahashi N, Tanaka K. ‘Low perfusion hyperemia' following middle cerebral arterial occlusion in cats of different age groups. Stroke. 1980;11:629–636. doi: 10.1161/01.str.11.6.629. [DOI] [PubMed] [Google Scholar]
- Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003;22:106–117. doi: 10.1159/000068744. [DOI] [PubMed] [Google Scholar]