Abstract
Stroke is a devastating neurovascular disease with limited therapeutic options. The pathogenesis of stroke involves complex interrelated molecular mechanisms including excitotoxicity, oxidative and nitrosative stress, cortical spreading depolarizations, inflammation, necrosis, and apoptosis. Successful development of stroke therapeutics depends on understanding these molecular mechanisms and how to counteract them to limit tissue damage during stroke. Activation of the parasympathetic nervous system (PNS) has been shown to antagonize a multiplicity of pathologic mechanisms. Elements of parasympathetic activation such as vagus nerve stimulation have already been used successfully in treating brain disorders such as epilepsy and depression. This review discusses the anatomical basis and molecular mechanisms involved in activation of the PNS, and assesses the strength of available evidence for the further development of this modality into a stroke therapy.
Keywords: parasympathetic, sphenopalatine, stroke, therapeutics, vagus
Introduction
Stroke is a devastating neurovascular disease with high mortality and a staggering economic burden on the United States, estimated at $73.7 billion for the year 2010 (Lloyd-Jones et al, 2010). With recombinant tissue plasminogen activator as the only available treatment for ischemic stroke, the need to develop new stroke therapies is imperative. Neuroprotection is an experimental approach aimed at developing therapies that counteract the molecular mechanisms of stroke, which include excitotoxicity, oxidative and nitrosative stress, cortical spreading depolarizations, inflammation, necrosis and apoptosis (for review see Moskowitz et al, 2010; Furlan et al, 2003). However, in spite of impressive neuroprotection in preclinical stroke models, many neuroprotective agents have failed in clinical trials. These failures have been blamed on poor research design, inadequate preclinical testing, and redundancy in the mechanisms of cerebral ischemia (for review see Ginsberg, 2008; Savitz, 2007). Given that the relative contributions of individual molecular mechanisms to the severity of stroke remain unknown, the successful development of stroke therapy may hinge on exploring treatment modalities with multiple mechanisms of action.
Activation of various aspects of the parasympathetic nervous system (PNS) has shown multiple therapeutic benefits in brain diseases. Examples include vagus nerve stimulation (VNS) for epilepsy (Ardesch et al, 2007) and cholinesterase inhibitors for Alzheimer's disease (López-Pousa et al, 2010). However, PNS activation has not yet been successfully developed as a therapy for stroke. Optimistically, various forms of PNS activation, namely VNS (Ay et al, 2009; Miyamoto et al, 2003), sphenopalatine ganglion (SPG) stimulation (Yarnitsky et al, 2006), cholinesterase inhibitors (Akasofu et al, 2003), and α7 nicotinic acetylcholine receptor (nAChRα7) agonists (Parada et al, 2010) have shown neuroprotection in both preclinical models of cerebral ischemia and in vitro neuronal hypoxia. The mechanisms involved in these neuroprotective effects remain unclear. This review discusses the neuroanatomical (Figure 1) and molecular basis (Figure 2) for the beneficial effects of PNS activation, and provides an assessment of the strength of current available data on its applications in ischemic stroke. Using the updated recommendations of Stroke Therapy Academic Industry Roundtable (Fisher et al, 2009) as a yardstick, we make suggestions for further studies aimed at developing this therapeutic approach.
Figure 1.
 |
 |
 |
Figure 2.
 |
 |
 |
Anatomy of the vagus nerve
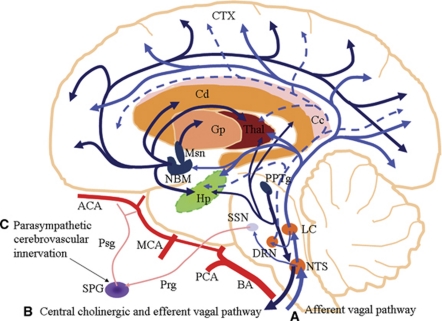
To understand the mechanisms of vagus nerve activation, it is important to know both its peripheral innervation and central projections. The vagus nerve is the main parasympathetic innervation for the thoracic and abdominopelvic viscera. The vagus nerve is composed of predominantly afferent fibers (80%), which originate from cell bodies in the superior (jugular) vagal ganglion and the larger inferior (nodose) vagal ganglion situated just below the jugular foramen. The efferent arm of the vagus nerve consists of preganglionic visceromotor fibers, originating from the dorsal motor nucleus of the vagus nerve and the nucleus ambiguus in the medulla oblongata. The preganglionic fibers synapse with postganglionic fibers located in the parasympathetic ganglia, close to the viscera they innervate (for review see Henry, 2002).
Central Projections of Afferent Vagus Nerve Fibers
Majority of the afferent vagus nerve fibers project bilaterally to the nucleus of the tractus solitarius (NTS) in the medulla oblongata. The rest of the fibers project ipsilaterally to nucleus of the spinal tract of the trigeminal nerve, medial reticular formation of the medulla, area postrema, dorsal motor nucleus of the vagus, and nucleus ambiguus (Kalia and Sullivan, 1982). The NTS projects directly to the parabrachial nuclei, the cerebellum, the raphe, the periaqueductal gray, and the locus coeruleus (LC). Through the NTS, vagus nerve also makes extensive polysynaptic projections to the thalamus, hypothalamus, the limbic system, and the cerebral cortex (Figure 1; Pathway A) (for review see Henry, 2002).
Central Vagal-Mediated Neurotransmission
The NTS is involved in the processing and integration of a wide range of visceral and somatic sensory information (Bailey et al, 2008). The neurotransmission involved in this function includes excitatory components such as glutamate and the N-methyl--aspartate receptor (Lam et al, 2010), as well as inhibitory components such as α-aminobutyric acid (Bailey et al, 2008). The NTS makes projections to the LC (Van Bockstaele et al, 1999), which is the major source of the neurotransmitter, norepinehrine, for the entire brain, including the cerebral cortex (Levitt and Moore, 1978). It has been shown electrophysiologically that VNS initially causes increased firing of the LC leading to the release of norepinephrine. The norepinephrine then stimulates α1-adrenergic receptors in the dorsal raphe nucleus, resulting in serotonin (5-hydroxytryptamine, 5-HT) release (Manta et al, 2009). This explains the finding that VNS caused the release of norepinephrine from the LC as early as 1 hour after stimulation, whereas 5-HT was released from the dorsal raphe nucleus only at day 14 of stimulation (Dorr and Debonnel, 2006). Florin-Lechner et al (1996) showed that the LC can release norepinephrine in both tonic and phasic modes and that the level of cortical norepinephrine depends on the frequency of LC stimulation. Cortical norepinephrine has been shown to either tonically suppress glutamate-evoked discharge or initially facilitate glutamate-evoked discharge followed by suppression, depending on whether it stimulates β-adrenergic receptors or α1-adrenergic receptors, respectively (Devilbiss and Waterhouse, 2000). The dorsal raphe nucleus projects serotonergic neurons extensively to many parts of the brain including the cerebral cortex (Figure 1; Pathway A) (Van Bockstaele et al, 1993).
Cholinergic system
Acetylcholine (ACh) is a neurotransmitter at the neuromuscular junction, in autonomic ganglia, and in postganglionic parasympathetic nerve-target organ junctions and some postganglionic sympathetic nerve-target junctions (Barrett et al, 2010). There is also an intercommunicating central cholinergic network involving the medial prefrontal cortex, anterior cingulate cortex, insular cortex, paraventricular nucleus, central nucleus of the amygdala, and lateral hypothalamic area. This network sends signals via the periaqueductal gray in the midbrain, through the parabrachial nuclei in the pons to medullary nuclei including NTS, nucleus ambiguus, and ventrolateral medulla. The main output of the central cholinergic system is through the vagus nerve (Figure 1; Pathway B) and the stellate ganglia (for review see Benarroch, 1993). This is in agreement with the finding of Pavlov et al (2006) that intracerebroventricular administration of a selective muscarinic agonist activated the cholinergic antiinflammatory pathway peripherally, resulting in decreased tumor necrosis factor-α production in endotoxemia. The cholinergic antiinflammatory pathway is a product of the inflammatory reflex, the afferent arm of which senses tissue damage and sends signals via the afferent vagus nerve to the brain. The efferent arm consists of efferent vagal signals that decrease cytokine expression through the binding of ACh to nAChRα7 expressed on macrophages (Borovikova et al, 2000). Microglia also express nAChRα7, activation of which has been shown to attenuate inflammatory response in the brain (Shi et al, 2009; Shytle et al, 2004). Cholinergic signaling is also present in a variety of non-neuronal cells such as endothelial cells (Heeschen et al, 2002). Cholinergic signaling in endothelial cells has been shown to inhibit expression of adhesion molecules (Saeed et al, 2005).
Parasympathetic cerebrovascular innervation
The SPG is a parasympathetic ganglion that receives preganglionic neurons from the superior salivatory nucleus (Agassandian et al, 2002). Postganglionic neurons from the SPG, which join the ethmoidal nerve, have been shown to enter the cranial cavity via ethmoidal foramina where they innervate the cerebral blood vessels (Hara and Weir, 1986; Hara et al, 1993). These postganglionic neurons contain nitric oxide synthase (Yoshida et al, 1993), ACh (Kimura et al, 1997) and vasoactive intestinal peptide (Suzuki et al, 1988). Agassandian et al also showed that the cerebrovascular part of NTS connects directly with the parasympathetic preganglionic neurons at the superior salivatory nucleus (Figure 1; Pathway C). Through this NTS-superior salivatory nucleus pathway, the baroreceptors participate in the control of cerebrovascular tone (Agassandian et al, 2002, 2003).
Neuroprotective effects of parasympathetic activation
Vagus Nerve Stimulation—Effect of Neurotransmitters
Electrical VNS has been shown to attenuate cerebral ischemic injury (Ay et al, 2009; Miyamoto et al, 2003). In a transient model of focal cerebral ischemia (2 hours of ischemia followed by reperfusion) in rats, Ay et al (2009) found that VNS significantly decreased infarct size and neurologic deficit at 24 hours after ischemia/reperfusion. In a mechanistic approach, Miyamoto et al (2003) found that VNS significantly decreased extracellular glutamate levels between 15 and 20 minutes after 5 minutes of transient global ischemia model in Mongolian gerbils. Excessive glutamate release has a role in excitotoxicity during cerebral ischemia through the activation of N-methyl--aspartate receptor (Bosel et al, 2005; Choi, 1985). The vagus nerve may also be stimulated pharmacologically to ameliorate ischemic stroke injury. We have previously shown that ghrelin treatment caused various vagus nerve-mediated antiinflammatory effects such as suppression of neutrophil infiltration and proinflammatory cytokine levels in cerebral ischemia (Cheyuo et al, 2010). Similarly, the melanocortins, an endogenous group of peptides, were shown to mediate a vagus nerve-dependent downregulation of plasma and brain tumor necrosis factor-α levels after ischemic stroke (Ottani et al, 2009). The receptors of both ghrelin (Zhang et al, 2004) and melanocortins (Wan et al, 2008) are expressed at the NTS. The mechanisms for the beneficial effects of VNS in ischemic stroke remain largely unknown.
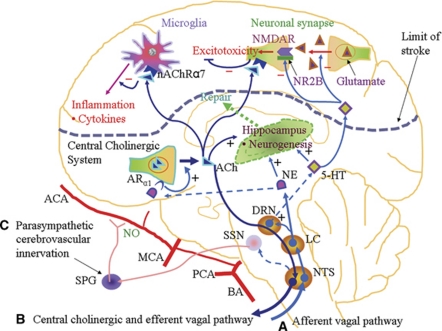
However, one may speculate on possible mechanisms of action based on the effects of neurotransmitters, which are released by VNS. It has been clearly shown that VNS leads to an early release of norepinephrine from the LC (Dorr and Debonnel, 2006), which projects to various parts of the brain including the cerebral cortex (Levitt and Moore, 1978). Various studies have shown neuroprotective effects of norepinephrine, including antiinflammatory effects via α1-adrenergic receptors (Dello et al, 2004; Kalinin et al, 2006). Moreover, lesions of the LC worsened cerebral ischemia (Blomqvist et al, 1985; Nishino et al, 1991). The norepinephrine released through VNS also stimulates the release of 5-HT from the dorsal raphe nucleus (Manta et al, 2009). 5-Hydroxytryptamine has been shown to antagonize excitotoxicity by inhibiting glutamate release via 5-HT1A receptors (Marcoli et al, 2004). In addition, 5-HT selectively downregulates the NR2B subunit of the N-methyl--aspartate receptor (Yuen et al, 2005), which is associated with excitotoxicity in contrast to the survival-promoting NR2A subunit (Chen et al, 2008). Taken together, VNS may suppress inflammation and excitotoxicity through the release of norepinephrine and 5-HT, respectively (Figure 2; Pathway A).
Cholinergic Antiinflammatory Pathway
Brain parenchymal inflammation (Dawson et al, 1996) and systemic inflammation (McColl et al, 2007) both have deleterious roles in ischemic stroke. The cholinergic antiinflammatory pathway suppresses inflammation through nAChRα7-mediated suppression of the nuclear factor-κB pathway (Borovikova et al, 2000). The cholinergic antiinflammatory pathway is driven by the central cholinergic system in the brain, whose peripheral output is through the efferent vagus nerve (Pavlov et al, 2006). The effector receptor, nAChRα7, is expressed on peripheral blood macrophages as well as residential macrophages such as microglia in the brain (Shytle et al, 2004). The LC, which is the source of norepinephrine, has a direct adrenergic connection to the forebrain basal cholinergic system (Smiley et al, 1999; Zaborszky and Cullinan, 1996), and norepinephrine has been shown to stimulate excitation of cholinergic neurons via α1-adrenergic receptors (Fort et al, 1995). Thus, VNS may activate the cholinergic antiinflammatory response within the brain by stimulating this pathway to release ACh, which then activates nAChRα7 on microglia (Figure 2; Pathway B). Stimulation of nAChRα7 on microglia has been shown to attenuate inflammatory responses in the brain (Shi et al, 2009; Shytle et al, 2004). Similarly, the nAChRα7 agonist, PNU282987, was shown to protect neuronal cells from oxidative stress by stimulating a JAK2/PI3K/Akt cascade (Parada et al, 2010). The cholinergic antiinflammatory pathway may also improve the outcome of ischemic stroke by suppressing systemic inflammation.
Stroke stimulates a robust peripheral inflammatory response (Offner et al, 2006). C-reactive protein is a marker of peripheral inflammation in stroke, and elevation of C-reactive protein has been associated with poor outcomes in stroke (Welsh et al, 2009). Other systemic inflammatory responses to cerebral ischemia that worsen the overall outcome of stroke include intestinal barrier dysfunction, bacterial translocation, and sepsis (Caso et al, 2009; Schulte-Herbruggen et al, 2009; Tascilar et al, 2010). Vagus nerve stimulation, which activates the cholinergic antiinflammatory pathway, suppresses systemic inflammation (Pavlov et al, 2006), decreases C-reactive protein levels in heart failure (Zhang et al, 2009), and also prevents increase in intestinal barrier dysfunction after brain injury (Bansal et al, 2010). Thus, VNS, through the activation of the cholinergic antiinflammatory pathway, can suppress both brain parenchymal inflammation and peripheral inflammation leading to neuroprotection in ischemic stroke. In addition, cholinergic signaling in the brain may also suppress excitotoxicity by inhibiting glutamate-induced p38 MAPK signaling (Asomugha et al, 2010) (Figure 2; Pathway B).
Parasympathetic Modulation of Cerebral Blood Flow
The cerebral blood vessels are innervated by nitroxidergic-cholinergic neurons from the SPG, a parasympathetic ganglion (Kimura et al, 1997; Nozaki et al, 1993; Suzuki et al, 1990). Stimulation of this SPG-nitroxidergic-cholinergic system has been shown to increase cerebral blood flow through vasodilation mediated by nitric oxide release (Toda et al, 2000) (Figure 2; Pathway C). Sphenopalatine ganglion stimulation in a rat model of cerebral ischemia resulted in reduction in infarct size and neurologic deficit (Yarnitsky et al, 2006). Importantly, unlike other experimental stroke therapies that exert neuroprotective effects within a narrow therapeutic time window, SPG stimulation starting at 24 hours after cerebral ischemia still significantly decreased mortality and improved long-term neurologic outcome (Solberg et al, 2008). Using imaging studies, Henninger and Fisher (2007) showed that SPG stimulation resulted in increased perfusion of the penumbra, thus correcting diffusion–perfusion mismatch, leading to reduction in infarct size. Conversely, parasympathetic denervation of cerebral blood vessels (Kano et al, 1991) and resection of nerve bundles from the SPG (Diansan et al, 2010) worsened cerebral ischemia. Interestingly, SPG stimulation has also been shown to increase blood–brain barrier (BBB) permeability (Yarnitsky et al, 2004a, 2004b). Even though the BBB permeability is transiently increased in ischemic stroke (Belayev et al, 1996), it is likely that the delivery of some therapeutic molecules could still be impaired. Thus, administration of neuroprotective agents together with SPG stimulation in cerebral ischemia could lead to synergistic beneficial effects. However, some investigators also associate deleterious effects such as hemorrhage in thrombolytic therapy to the opening of the BBB in stroke (Kastrup et al, 2008). Thus, further studies are needed to assess whether the increased BBB permeability in SPG stimulation has any independent deleterious effects in the stroke setting.
Parasympathetic activation enhances neurogenesis
Neurogenesis occurs in the adult brain in two regions, namely the subgranular zone of the hippocampus and the subventricular zone of the lateral ventricles. Under normal physiological conditions, the neural stem cells in the subgranular zone and subventricular zone produce neuroblasts, which migrate to the dentate gyrus and olfactory bulb, respectively (Altman and Das, 1965; Lois and Alvarez-Buylla, 1994). Neural stem cell proliferation is increased in conditions of brain injury such as stroke (Arvidsson et al, 2002). Vagus nerve stimulation has been shown to increase proliferation of neural progenitor cells in the rat hippocampus (Revesz et al, 2008). Vagus nerve stimulation releases norepinephrine, basic fibroblast growth factor and brain derived neurotrophic factor (Follesa et al, 2007), and 5-HT (Dorr and Debonnel, 2006). Norepinehrine (Bauer et al, 2003; Jhaveri et al, 2010), basic fibroblast growth factor (Maric et al, 2007), brain derived neurotrophic factor (Choi et al, 2009), and 5-HT (Brezun and Daszuta, 1999) have been shown to promote neurogenesis (Figure 2; Pathway A).
5-Hydroxytryptamine and norepinephrine together have been shown to regulate hippocampal neurogenesis by stimulating the sonic hedgehog pathway (Rajendran et al, 2009). The hippocampus also has an extensive cholinergic innervation from the medial septal nucleus, which is part of the basal forebrain cholinergic system (Mohapel et al, 2005). Cholinergic signaling through nAChRα7 promotes survival, maturation, and integration of newborn neurons in the hippocampus (Campbell et al, 2010). Neurogenesis in the hippocampus was impaired by forebrain lesions disrupting the basal forebrain cholinergic network (Figure 2; Pathway B) (Campbell et al, 2010; Cooper-Kuhn et al, 2004; Mohapel et al, 2005). Thus, PNS activation by VNS or the use of nAChRα7 agonists may enhance neurogenesis in the brain, leading to improved functional recovery.
Perspectives and future directions
The data on the neuroprotective roles of VNS, the cholinergic antiinflammatory pathway, and parasympathetic modulation of cerebrovascular tone are promising. However, using the updated recommendations of Stroke Therapy Academic Industry Roundtable (Fisher et al, 2009) as a yardstick of quality and extent of preclinical testing, further progress is required before individual components or combinations of PNS activation could be translated into clinical testing.
The effect of VNS on ischemic stroke was investigated in two different laboratories using rodent transient cerebral ischemia models, with some form of physiological monitoring during experiments (Ay et al, 2009; Miyamoto et al, 2003). In one study (Ay et al, 2009), animal allocation was randomized and assessment of outcome blinded, in contrast to the other study (Miyamoto et al, 2003). Overall, the effect of VNS on ischemic stroke needs to be more rigorously assessed using both permanent and transient cerebral ischemia models in both rodents and gyrencephalic species such as primates, with randomization and blinding of outcome assessment. In the treatment of epilepsy, VNS over broad stimulation parameters (frequency 2 to 10 Hz and higher amplitudes 2.75 to 3.75 mA) have shown no significant cardiorespiratory effects (Binks et al, 2001). In order to develop VNS as a stroke therapy, it is also important to assess its efficacy and safety profile over a broad range of stimulation parameters at different time points after stroke onset. The evidence of neuroprotective effects of SPG stimulation indicates an impressive long therapeutic time window of 24 hours (Solberg et al, 2008). However, this therapeutic modality also needs to be extensively tested and the range of stimulation frequencies, and side effects defined.
In summary, activation of the PNS exerts a broad range of neuroprotective mechanisms in ischemic stroke (Figure 2). This review shows that the PNS can be activated by various methods to provide neuroprotection. Some of the experimental methods of PNS activation such as VNS and SPG stimulation are invasive and may not be very practical for the patient who presents with acute stroke. In developing PNS activation as a stroke therapy, it will be important to assess the efficacy of less invasive and more easily applicable methods of PNS activation such as transcutaneous VNS. In addition, in order for PNS activation to antagonize multiple mechanisms of cerebral ischemia for optimal efficacy, it may need the combination of different modes of PNS activation. For example, as SPG stimulation increases BBB permeability (Yarnitsky et al, 2004a, 2004b), it may act synergistically with nAChRα7 agonists or cholinesterase inhibitors to produce neuroprotection. In conclusion, PNS activation is a promising therapeutic modality for acute ischemic stroke that needs further development.
Acknowledgments
The authors thank Ms Madeline Quinn for her editorial assistance on the manuscript.
The authors declare no conflict of interest.
Footnotes
This work was supported by the National Institutes of Health (NIH) grants, R01 GM053008 and R01 AG028352 (PW).
References
- Agassandian K, Fazan VP, Adanina V, Talman WT. Direct projections from the cardiovascular nucleus tractus solitarii to pontine preganglionic parasympathetic neurons: a link to cerebrovascular regulation. J Comp Neurol. 2002;452:242–254. doi: 10.1002/cne.10372. [DOI] [PubMed] [Google Scholar]
- Agassandian K, Fazan VP, Margaryan N, Dragon DN, Riley J, Talman WT. A novel central pathway links arterial baroreceptors and pontine parasympathetic neurons in cerebrovascular control. Cell Mol Neurobiol. 2003;23:463–478. doi: 10.1023/A:1025059710382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasofu S, Kosasa T, Kimura M, Kubota A. Protective effect of donepezil in a primary culture of rat cortical neurons exposed to oxygen-glucose deprivation. Eur J Pharmacol. 2003;472:57–63. doi: 10.1016/s0014-2999(03)01865-x. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ardesch JJ, Buschman HP, Wagener-Schimmel LJ, van der Aa HE, Hageman G. Vagus nerve stimulation for medically refractory epilepsy: a long-term follow-up study. Seizure. 2007;16:579–585. doi: 10.1016/j.seizure.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Asomugha CO, Linn DM, Linn CL. ACh receptors link two signaling pathways to neuroprotection against glutamate-induced excitotoxicity in isolated RGCs. J Neurochem. 2010;112:214–226. doi: 10.1111/j.1471-4159.2009.06447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay I, Lu J, Ay H, Gregory SA. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459:147–151. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Bansal V, Costantini T, Ryu SY, Peterson C, Loomis W, Putnam J, Elicieri B, Baird A, Coimbra R. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J Trauma. 2010;68:1059–1064. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KE, Barman SM, Boitano S, Brooks H.2010‘Chapter 7. Neurotransmitters & Neuromodulators' (Chapter)Barrett KE, Barman SM, Boitano S, Brooks H: Ganong's Review of Medical Physiology, 23e: http://www.accessmedicine.com.elibrary.einstein.yu.edu/content.aspx?aID=5241648
- Bauer S, Moyse E, Jourdan F, Colpaert F, Martel JC, Marien M. Effects of the alpha 2-adrenoreceptor antagonist dexefaroxan on neurogenesis in the olfactory bulb of the adult rat in vivo: selective protection against neuronal death. Neuroscience. 2003;117:281–291. doi: 10.1016/s0306-4522(02)00757-1. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Binks AP, Paydarfar D, Schachter SC, Guz A, Banzett RB. High strength stimulation of the vagus nerve in awake humans: a lack of cardiorespiratory effects. Respir Physiol. 2001;127:125–133. doi: 10.1016/s0034-5687(01)00252-3. [DOI] [PubMed] [Google Scholar]
- Blomqvist P, Lindvall O, Wieloch T. Lesions of the locus coeruleus system aggravate ischemic damage in the rat brain. Neurosci Lett. 1985;58:353–358. doi: 10.1016/0304-3940(85)90080-1. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bosel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, Megow D, Dirnagl U, Hortnagl H, Fink KB, Endres M. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92:1386–1398. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Campbell NR, Fernandes CC, Halff AW, Berg DK. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci. 2010;30:8734–8744. doi: 10.1523/JNEUROSCI.0931-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso JR, Hurtado O, Pereira MP, Garcia-Bueno B, Menchen L, Alou L, Gomez-Lus ML, Moro MA, Lizasoain I, Leza JC. Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am J Physiol Regul Integr Comp Physiol. 2009;296:R979–R985. doi: 10.1152/ajpregu.90825.2008. [DOI] [PubMed] [Google Scholar]
- Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, Xu L, Duan WH, Xiong ZQ. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke. 2008;39:3042–3048. doi: 10.1161/STROKEAHA.108.521898. [DOI] [PubMed] [Google Scholar]
- Cheyuo C, Wu R, Zhou M, Jacob A, Coppa G, Wang P. Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock. 2010;35:258–65. doi: 10.1097/SHK.0b013e3181f48a37. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985;58:293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Choi SH, Li Y, Parada LF, Sisodia SS. Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol Neurodegener. 2009;4:52. doi: 10.1186/1750-1326-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. 2004;77:155–165. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. 1996;218:41–44. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- Dello RC, Boullerne AI, Gavrilyuk V, Feinstein DL. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J Neuroinflammation. 2004;1:9. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse. 2000;37:273–282. doi: 10.1002/1098-2396(20000915)37:4<273::AID-SYN4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Diansan S, Shifen Z, Zhen G, Heming W, Xiangrui W. Resection of the nerves bundle from the sphenopalatine ganglia tend to increase the infarction volume following middle cerebral artery occlusion. Neurol Sci. 2010;31:431–435. doi: 10.1007/s10072-010-0238-0. [DOI] [PubMed] [Google Scholar]
- Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318:890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, Puligheddu M, Marrosu F, Biggio G. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Fort P, Khateb A, Pegna A, Muhlethaler M, Jones BE. Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea-pig brain. Eur J Neurosci. 1995;7:1502–1511. doi: 10.1111/j.1460-9568.1995.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Furlan AJ, Katzan IL, Caplan LR. Thrombolytic therapy in acute ischemic stroke. Curr Treat Options Cardiovasc Med. 2003;5:171–180. doi: 10.1007/s11936-003-0001-4. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Weir B. Pathway of acetylcholinesterase containing nerves to the major cerebral arteries in rats. J Comp Neurol. 1986;250:245–252. doi: 10.1002/cne.902500211. [DOI] [PubMed] [Google Scholar]
- Hara H, Zhang QJ, Kuroyanagi T, Kobayashi S. Parasympathetic cerebrovascular innervation: an anterograde tracing from the sphenopalatine ganglion in the rat. Neurosurgery. 1993;32:822–827. doi: 10.1227/00006123-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–536. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger N, Fisher M. Stimulating circle of Willis nerve fibers preserves the diffusion-perfusion mismatch in experimental stroke. Stroke. 2007;38:2779–2786. doi: 10.1161/STROKEAHA.107.485581. [DOI] [PubMed] [Google Scholar]
- Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59:S3–14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- Jhaveri DJ, Mackay EW, Hamlin AS, Marathe SV, Nandam LS, Vaidya VA, Bartlett PF. Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors. J Neurosci. 2010;30:2795–2806. doi: 10.1523/JNEUROSCI.3780-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- Kalinin S, Polak PE, Madrigal JL, Gavrilyuk V, Sharp A, Chauhan N, Marien M, Colpaert F, Feinstein DL. Beta-amyloid-dependent expression of NOS2 in neurons: prevention by an alpha2-adrenergic antagonist. Antioxid Redox Signal. 2006;8:873–883. doi: 10.1089/ars.2006.8.873. [DOI] [PubMed] [Google Scholar]
- Kano M, Moskowitz MA, Yokota M. Parasympathetic denervation of rat pial vessels significantly increases infarction volume following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1991;11:628–637. doi: 10.1038/jcbfm.1991.114. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Groschel K, Ringer TM, Redecker C, Cordesmeyer R, Witte OW, Terborg C. Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39:2385–2387. doi: 10.1161/STROKEAHA.107.505420. [DOI] [PubMed] [Google Scholar]
- Kimura T, Yu JG, Edvinsson L, Lee TJ. Cholinergic, nitric oxidergic innervation in cerebral arteries of the cat. Brain Res. 1997;773:117–124. doi: 10.1016/s0006-8993(97)00889-5. [DOI] [PubMed] [Google Scholar]
- Lam CK, Chari M, Su BB, Cheung GW, Kokorovic A, Yang CS, Wang PY, Lai TY, Lam TK. Activation of N-methyl-D-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J Biol Chem. 2010;285:21913–21921. doi: 10.1074/jbc.M109.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Moore RY. Noradrenaline neuron innervation of the neocortex in the rat. Brain Res. 1978;139:219–231. doi: 10.1016/0006-8993(78)90925-3. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- López-Pousa S, Bermejo-Pareja F, Frank A, Hernández F, León T, Rejas-Gutiérrez J. The effect of donepezil in comparison with conventional treatment on cognitive functioning and the performance of the patient in a prospective cohort of patients with Alzheimer's disease treated in routine clinical practice in Spain. Rev Neurol. 2010;51:577–588. [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci. 2009;34:272–280. [PMC free article] [PubMed] [Google Scholar]
- Marcoli M, Cervetto C, Castagnetta M, Sbaffi P, Maura G. 5-HT control of ischemia-evoked glutamate efflux from human cerebrocortical slices. Neurochem Int. 2004;45:687–691. doi: 10.1016/j.neuint.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Maric D, Fiorio PA, Chang YH, Barker JL. Self-renewing and differentiating properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J Neurosci. 2007;27:1836–1852. doi: 10.1523/JNEUROSCI.5141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto O, Pang J, Sumitani K, Negi T, Hayashida Y, Itano T. Mechanisms of the anti-ischemic effect of vagus nerve stimulation in the gerbil hippocampus. Neuroreport. 2003;14:1971–1974. doi: 10.1097/00001756-200310270-00018. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005;26:939–946. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino K, Lin CS, Morse JK, Davis JN. DSP4 treatment worsens hippocampal pyramidal cell damage after transient ischemia. Neuroscience. 1991;43:361–367. doi: 10.1016/0306-4522(91)90300-d. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Moskowitz MA, Maynard KI, Koketsu N, Dawson TM, Bredt DS, Snyder SH. Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibers in cerebral arteries. J Cereb Blood Flow Metab. 1993;13:70–79. doi: 10.1038/jcbfm.1993.9. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Ottani A, Giuliani D, Mioni C, Galantucci M, Minutoli L, Bitto A, Altavilla D, Zaffe D, Botticelli AR, Squadrito F, Guarini S. Vagus nerve mediates the protective effects of melanocortins against cerebral and systemic damage after ischemic stroke. J Cereb Blood Flow Metab. 2009;29:512–523. doi: 10.1038/jcbfm.2008.140. [DOI] [PubMed] [Google Scholar]
- Parada E, Egea J, Romero A, del BL, Garcia AG, Lopez MG. Poststress treatment with PNU282987 can rescue SH-SY5Y cells undergoing apoptosis via alpha7 nicotinic receptors linked to a Jak2/Akt/HO-1 signaling pathway. Free Radic Biol Med. 2010;49:1815–1821. doi: 10.1016/j.freeradbiomed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran R, Jha S, Fernandes KA, Banerjee SB, Mohammad F, Dias BG, Vaidya VA. Monoaminergic regulation of Sonic hedgehog signaling cascade expression in the adult rat hippocampus. Neurosci Lett. 2009;453:190–194. doi: 10.1016/j.neulet.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008;214:259–265. doi: 10.1016/j.expneurol.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205:20–25. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Quarcoo D, Meisel A, Meisel C. Differential affection of intestinal immune cell populations after cerebral ischemia in mice. Neuroimmunomodulation. 2009;16:213–218. doi: 10.1159/000205514. [DOI] [PubMed] [Google Scholar]
- Shi FD, Piao WH, Kuo YP, Campagnolo DI, Vollmer TL, Lukas RJ. Nicotinic attenuation of central nervous system inflammation and autoimmunity. J Immunol. 2009;182:1730–1739. doi: 10.4049/jimmunol.182.3.1730. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Subramanian M, Mesulam MM. Monoaminergic-cholinergic interactions in the primate basal forebrain. Neuroscience. 1999;93:817–829. doi: 10.1016/s0306-4522(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Solberg Y, Yarnitsky D, Borenstein N, Tanne D, Dayan A, Weiss S, Fisher M. Effectiveness of sphenopalatine ganglion stimulation therapy in focal ischemic stroke models: a 24-hour post-stroke window of treatment. Stroke. 2008;39:665. [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and pathways of cerebrovascular vasoactive intestinal polypeptide-positive nerves in rat. J Cereb Blood Flow Metab. 1988;8:697–712. doi: 10.1038/jcbfm.1988.117. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Owman C. Origins and pathways of choline acetyltransferase-positive parasympathetic nerve fibers to cerebral vessels in rat. J Cereb Blood Flow Metab. 1990;10:399–408. doi: 10.1038/jcbfm.1990.70. [DOI] [PubMed] [Google Scholar]
- Tascilar N, Irkorucu O, Tascilar O, Comert F, Eroglu O, Bahadir B, Cakmak GK, Ankarali H, Sayan H. Bacterial translocation in experimental stroke: what happens to the gut barrier. Bratisl Lek Listy. 2010;111:194–199. [PubMed] [Google Scholar]
- Toda N, Ayajiki K, Tanaka T, Okamura T. Preganglionic and postganglionic neurons responsible for cerebral vasodilation mediated by nitric oxide in anesthetized dogs. J Cereb Blood Flow Metab. 2000;20:700–708. doi: 10.1097/00004647-200004000-00007. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol. 1999;412:410–428. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28:4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh P, Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis. 2009;27:247–253. doi: 10.1159/000196823. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Gross Y, Lorian A, Shalev A, Lamensdorf I, Bornstein R, Shorer S, Mayevsky A, Patel KP, Abbott NJ, Mayhan WG. Blood-brain barrier opened by stimulation of the parasympathetic sphenopalatine ganglion: a new method for macromolecule delivery to the brain. J Neurosurg. 2004a;101:303–309. doi: 10.3171/jns.2004.101.2.0303. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Gross Y, Lorian A, Shalev A, Shorer S, Tanaka T, Ayajiki K, Fujimiya M, Okamura T. Increased BBB permeability by parasympathetic sphenopalatine ganglion stimulation in dogs. Brain Res. 2004b;1018:236–240. doi: 10.1016/j.brainres.2004.05.103. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Lorian A, Dayan A, Avnon Y, Krakovsky M, Lamensdorf I. Sphenopalatine ganglion (SPG) stimulation in acute stroke model: a novel method for neuroprotection. Stroke. 2006;37:728. [Google Scholar]
- Yoshida K, Okamura T, Kimura H, Bredt DS, Snyder SH, Toda N. Nitric oxide synthase-immunoreactive nerve fibers in dog cerebral and peripheral arteries. Brain Res. 1993;629:67–72. doi: 10.1016/0006-8993(93)90482-3. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE. Direct catecholaminergic-cholinergic interactions in the basal forebrain. I. Dopamine-beta-hydroxylase- and tyrosine hydroxylase input to cholinergic neurons. J Comp Neurol. 1996;374:535–554. doi: 10.1002/(SICI)1096-9861(19961028)374:4<535::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Lin TR, Hu Y, Fan Y, Zhao L, Stuenkel EL, Mulholland MW. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. J Physiol. 2004;559:729–737. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]