Abstract
Background
Cardiovascular disease is the leading cause of death among diabetics. Vitamin D deficiency is associated with increased risk of cardiovascular disease in this population. To determine the mechanism by which vitamin D deficiency mediates accelerated cardiovascular disease in patients with diabetes, we investigated the effects of active vitamin D on macrophage cholesterol deposition.
Methods and Results
We obtained macrophages from 76 obese, diabetic, hypertensive patients with vitamin D deficiency (25-hydroxyvitamin D < 80 nmol/L)(group A) and four control groups: obese, diabetic, hypertensive patients with normal vitamin D (group B, n=15), obese, non-diabetic, hypertensive patients with vitamin D deficiency (group C, n=25), and non-obese, non-diabetic, non-hypertensive patients with vitamin D deficiency (group D, n=10) or sufficiency (group E, n=10). The same patient’s macrophages from all groups were cultured in vitamin D-deficient or 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) supplemented media and exposed to modified low-density lipoprotein cholesterol. 1,25(OH)2D3 suppressed foam cell formation by reducing acetylated or oxidized low-density lipoprotein cholesterol uptake in diabetics only. Conversely, deletion of the vitamin D receptor in macrophages from diabetic patients accelerated foam-cell formation induced by modified LDL. 1,25(OH)2D3 downregulation of c-Jun N-terminal kinase activation reduced PPARγ expression, suppressed CD36 expression, and prevented oxLDL-derived cholesterol uptake. In addition, 1,25(OH)2D3 suppression of macrophage endoplasmic reticulum stress improved insulin signaling, downregulated SR-A1expression, and prevented oxLDL and AcLDL-derived cholesterol uptake.
Conclusion
These results identify reduced vitamin D receptor signaling as a potential mechanism underlying increased foam-cell formation and accelerated cardiovascular disease in diabetics.
Keywords: Nutrition, Diabetes mellitus, Atherosclerosis and Inflammation
INTRODUCTION
Approximately 20 million Americans have type 2 diabetes, a disease associated with hypertension and increased risk of cardiovascular disease (CVD). This combination of metabolic abnormalities is the leading cause of morbidity and mortality in industrialized countries.1 Several studies indicate that in poorly controlled diabetes, altered insulin signaling and/or hyperglycemia promote unbalanced cholesterol metabolism, which favors oxidized low-density lipoprotein (oxLDL) cholesterol retention in the vascular wall.2 However, the effects of intensive glucose lowering on macrovascular complications in this population are unpredictable and may result in increased mortality.3 Therefore, identification of glucose-independent factors that modulate macrophage cholesterol deposition and vascular infiltration is critical to understanding the development of CVD in diabetics.
Vitamin D deficiency is a largely unacknowledged epidemic associated with CVD. Approximately 1 billion people worldwide have low levels of 25-hydroxyvitamin D (25(OH)D < 80 nmol/L), the principal circulating storage form of vitamin D, and more than half of middle-aged vitamin D–deficient patients develop CVD.4,5 In hypertensive patients, low serum vitamin D levels increase the risk of CVD by 60%.6 In women with type 2 diabetes, the prevalence of vitamin D deficiency is a third higher than that of control subjects, and low vitamin D levels nearly double the risk of developing CVD compared to diabetic patients with normal vitamin D levels.7,8 Similarly, in diabetic patients with mild renal failure, low vitamin D levels increase the relative risk of CVD when compared to their vitamin D-sufficient counterparts.9 Finally, intervention with 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the active form of vitamin D, or paricalcitol (a less calcemic 1,25(OH)2D analog) decreased cardiovascular mortality in patients with end-stage renal disease, suggesting the favorable outcomes from normal vitamin D status in attenuating atherosclerosis.10 Therefore, understanding the mechanism of the accelerated atherosclerosis induced by vitamin D deficiency may be crucial for treating the epidemic of CVD in diabetics.
Increased proinflammatory cytokines in the vessel wall contribute to immune cell recruitment and modified-LDL cholesterol deposition by increasing scavenger receptor expression and cholesteryl ester synthesis and by decreasing cholesterol efflux.11 Active Vitamin D metabolites (1,25(OH)2D3 or its analogs) promote monocyte/macrophage differentiation and diminish proinflammatory cytokine release by immune mononuclear cells in diabetics, suggesting that 1,25(OH)2D3 signaling may regulate monocyte vascular infiltration and macrophage cholesterol retention in the vessel wall in these patients.12,13
Initial studies exploring the influence of 1,25(OH)2D3 on macrophage cholesterol metabolism are contradictory. In human promyelocytic leukemic (HL-60) and monocytic (THP-1) cell lines, 1,25(OH)2D3 decreased scavenger receptor A-1 expression and acetylated-LDL (AcLDL) binding.14, 15 In contrast, in normal subjects, 1,25(OH)2D3 increased cholesteryl ester formation stimulated by AcLDL but only under conditions of lipid deprivation.16 The aim of our study was to determine whether vitamin D deficiency contributes to the increase in macrophage-mediated cholesterol deposition seen in patients with diabetes and to investigate the effects of 1,25(OH)2D3 on macrophage cholesterol deposition in diabetics and non-diabetic matched controls.
METHODS
Population
Our study population included 76 obese, hypertensive adult subjects with type 2 diabetes on medications with an age of 55 ± 2 years, body mass index of 32 ± 1.0; 25(OH)D of 39 ± 5.9 nmol/L, diabetes duration of 5 ± 2.5 years, and hemoglobin A1c level of 8 ± 0.2% (group A). We excluded recently diagnosed diabetes, pregnancy, known CAD, and normal vitamin D levels (serum 25(OH)D ≥ 80 nmol/L). This population was compared to four different control groups: obese, diabetic, hypertensive patients with normal vitamin D levels (serum 25(OH)D 94 ± 10 nmol/L) (group B, n=15), obese, non-diabetic, hypertensive patients with vitamin D deficiency (serum 25(OH)D 34 ± 3 nmol/L) (group C, n=25), and normal weight controls with no history of diabetes or hypertension with either normal vitamin D levels (serum 25(OH)D 97 ± 10 nmol/L) (group D, n=10), or vitamin D deficiency (serum 25(OH)D 32 ± 4 nmol/L) (group E, n=10) (Table 1). Subjects were recruited from the outpatient clinic at Barnes-Jewish Hospital, St. Louis. Participation was voluntary, and each subject was provided with written informed consent, approved by the Human Research Protection Office of Washington University School of Medicine.
Table 1.
Demographic characteristics
| Characteristics | Diabetics Vit. D deficient |
Diabetics Vit. D sufficient |
Obese Non- diabetic Vit. D deficient |
Normal controls Vit. D deficient |
Normal controls Vit. D sufficient |
|---|---|---|---|---|---|
| Group A (76) |
Group B (15) |
Group C (25) |
Group D (10) |
Group E (10) |
|
| Age | 55 ± 2.4 | 59 ± 3 | 50 ± 3.1 | 42 ± 4 | 45 ± 3.4 |
| Women % | 82% | 80% | 80% | 70% | 95% |
| BMI | 32 ± 1 | 31.6 ±1.1 | 31.9± 1.2 | 22± 0.8*†‡ | 23± 1*†‡ |
| African Americans % | 86.9%€ | 80% | 84% | 30% | 30% |
| Smoker % | 22.3% | 20% | 20% | 10% | 10% |
| 25 OH Vitamin D level (nmol/L) | 39 ± 4 | 93 ±10*‡# | 34 ± 3 | 32 ± 4 | 97 ± 10*‡# |
| Systolic Blood Pressure (mmHg) | 132 ± 2.5 | 136 ± 4 | 110 ± 3*† | 104 ± 4.2*† | 113 ± 4*† |
| Diastolic Blood Pressure (mmHg) | 85 ± 8.8 | 74 ± 4 | 75 ± 1.7 | 71 ± 1.5 | 78 ± 5 |
| Duration of Diabetes (years) | 5±2.5 | 9±0.7 | 0 | 0 | 0 |
| -Retinopathy | 1% | 0 | 0 | 0 | 0 |
| -Nephropathy | 7% | 10% | 0 | 0 | 0 |
| -Neuropathy | 10% | 10% | 0 | 0 | 0 |
| Only Diabetic Oral Medications % | 71% | 70% | 0 | 0 | 0 |
| Diabetic Oral Medications plus Long Acting insulin % | 29% | 30% | 0 | 0 | 0 |
| Total Cholesterol | 173 ± 5 | 165 ± 13 | 151 ± 8 | 152 ± 12 | 149 ± 12 |
| Triglycerides | 150 ± 14 | 137 ± 16 | 77 ± 7* | 76 ± 11 | 74 ± 8 |
| Lipid Medications % | 33 % | 40% | 28% | 20% | 10% |
| H1AC | 8 ± 0.2 | 7.8 ± 0.3 | 5.1 ± 0.1*† | 5.4 ± 0.1*† | 5.2 ± 0.6*† |
Data is expressed as mean ± SEM for continuous variables and as a ratio for categorical data.
(p < 0.05 vs. group A,
p < 0.05 vs. group B,
p < 0.05 vs. group C,
p < 0.05 vs. group D by one-way ANOVA with Tukey’s post test analysis for parametric variables.
p < 0.001 by chi-square for multiple categorical variables).
Isolation and Preparation of Primary Human Monocytes
Peripheral monocytes were isolated by standard Ficoll isolation techniques and selected by CD14 marker positivity (Miltenyi Biotec, Auburn, CA). CD14+ /CD11b+ cell purity reached 97% as assessed by flow cytometry (FACStar Plus, BD, Franklin Lakes, NJ). Each patient’s cells were differentiated into macrophages by culturing with 100 ng/mL of M-CSF for 7 days in vitamin D-deficient media (deficient in both 25(OH)D and 1,25(OH)2D; obtained by DMEM media plus 10% charcoal/dextran-treated fetal bovine serum) with or without supplementation of 1,25(OH)2D3 at 10−8 mol/L (courtesy Adriana Dusso, Washington University). Inhibition of JNKp was obtained in macrophages cultured in vitamin D-deficient media stimulated with modified-LDL cholesterol for 6h after preincubation with SP600125 (100uM) for 2 hours (SA Bioscience corporation, Frederick, MD). Induction of ER stress was obtained by adding Thapsigargin (0.25uM) (Sigma, St.Louis, MO) to cultured macrophages for 24 hours in 1,25(OH)2D-supplemented conditions. In a subgroup of diabetic patients from group A, macrophages on either vitamin D-deficient or 1,25(OH)2D3-supplemented media were cultured in high glucose (450 mg/dl) (routine culture conditions used in this study) and normal glucose conditions (100 mg/dl) for 7 days before cholesterol homeostasis and CD36 expression were measured. In these glucose conditions and after stimulation with oxLDL for 6h, macrophages were cell sorted by staining with PE-labeled anti-CD11b and FITC-labeled anti-CD14 (e-Biosciences, San Diego, CA). Then they were evaluated for membrane CD36 expression by using primary anti-CD36 and a secondary IgM CD36 biotin-labeled antibody (BD Bioscience, San Jose, CA). FACScan flow cytometry was performed in 20,000 cells.
Mouse Peritoneal Macrophages
Mouse peritoneal macrophages from CD36−/−, SRA-1−/−, and wild type (WT) mice (n=12 per each group) were isolated 3 days after intraperitoneal injection of 4% thioglycollate solution and cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media (see Supplemental Data).17 Cholesterol uptake was performed after 6h of stimulation with modified cholesterol in mouse macrophages cultured for 7 days in vitamin D-deficient or 1,25(OH)2D3-supplemented media. Foam-cell formation in vivo was determined by measuring total cholesterol and triglyceride in peritoneal macrophages 4h after isolation from LDR−/− mice fed a vitamin D-deficient (n=5) or-sufficient western diet (n=5) for 10 weeks (Harlan, TD 07019). This methodology is also described in Supplemental data.
Macrophage Cholesterol Homeostasis
Foam-cell formation (Oil-red-O stain), cholesteryl ester formation, cholesterol uptake, binding, and efflux were assessed in macrophages after stimulation with oxLDL or AcLDL from same subjects' monocytes cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media for 7 days. Detailed description is presented in Supplemental Data.
Plasmids and Small Interfering RNA
Macrophages obtained from diabetic subjects were cultured for 7 days in vitamin D-deficient or 1,25(OH)2D3-supplemented media and then infected with lentivirus containing either PPARγ-siRNA, VDR-siRNA hairpin, or control-siRNA for 48 hours. Protein, mRNA, and cholesterol uptake were determined 48 hours after recovering from viral infection. Descriptions of lentivirus generation are included in the Supplemental Data.
Gene Expression, Western Blot Analysis and c-Jun N-Terminal Kinase Activity
Quantitative RT-PCR (qPCR) analyses were performed by Sybergreen methodologies. Results were normalized to the housekeeping gene L32. Western blot analysis from macrophage protein extracts were normalized to β-actin expression. AKTp, AKT were determined in 12h serum-starved macrophages before or after insulin incubation (100nmol/L). Detailed description is included in the Supplemental Data. JNKp was also determined by cell-based enzyme-linked immunosorbent assay (ELISA) kit (SA Bioscience, Frederick, MD).
Statistical Analysis
Experiments were carried out with duplicate or triplicate samples. All data are expressed as mean ± SEM for continuous variables and as a ratio for categorical data. Gaussian distribution of continuous variables was verified by KS distance. Statistical significance of differences was calculated using the paired t-test for parametric data involving two groups and ANOVA for parametric data with Tukey’s test for multiple groups. Two-way ANOVA was performed to test the main effects of each factor, and to test for interaction between variables. Chi-square was used for multiple group analysis for categorical variables. Differences were considered statistically significant if p ≤ 0.05.
RESULTS
Population
In group A, we studied 76 obese adult subjects, primarily African-American women, with type 2 diabetes and a concurrent diagnosis of hypertension (80%). 29% of patients were on oral hypoglycemics and long-acting insulin, and the remainder took oral medications only. All groups were similar with respect to age, gender, total cholesterol, lipid medications, and tobacco use. Control groups B and C were similar to group A with regard to body mass index (BMI), ethnicity, cholesterol levels, and lipid medications, and group C had a lower HA1C and better blood pressure control when compared to groups A and B. Control groups D and E had significantly lower BMI and more ethnic diversity than all other groups. Groups D and E had lower systolic blood pressure compared to groups A and B. All parametric variables tested were normally distributed (Table 1).
1,25(OH)2 Vitamin D Prevents Foam-Cell Formation
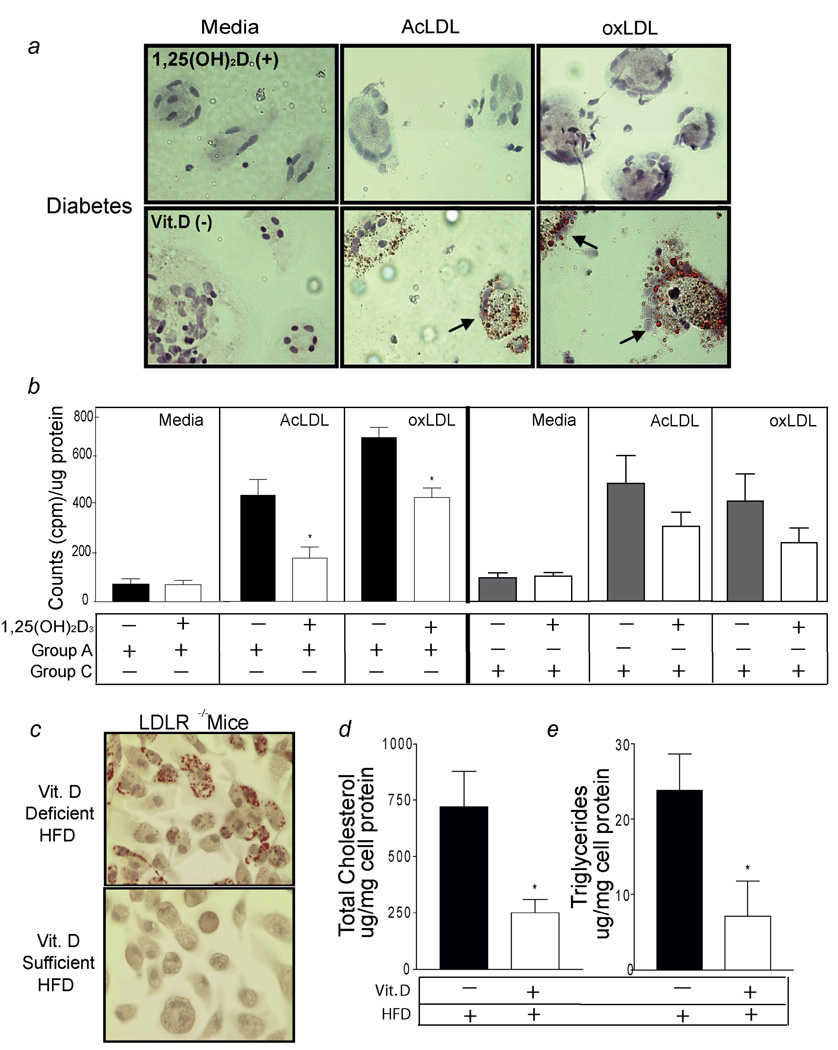
In obese diabetics (group A), macrophages cultured in vitamin D-deficient media exhibited a significant increase in foam-cell formation induced by oxLDL and AcLDL compared to macrophages cultured in 1,25(OH)2D3-supplemented conditions (see arrows Fig. 1a). In group A, 1,25(OH)2D3-treated macrophages exposed to AcLDL or oxLDL had almost 50% less cholesteryl ester formation than macrophages maintained on vitamin D-deficient media (p < 0.01 for both) (Fig. 1b). However, in macrophages obtained from non-diabetic controls (group C), AcLDL or oxLDL-induced cholesteryl ester formation was reduced by 1,25(OH)2D3 but not significantly when compared to macrophages cultured in vitamin D-deficient media (p = 0.1 and p = 0.09, respectively) (Fig. 1b).
Figure 1.
1,25(OH)2D3 prevents foam-cell formation. Macrophages stained with Oil-red-O. a, Diabetic subjects (Group A); Top-panel, 1,25(OH)2D3-treated cells, Bottom-panel, vitamin D-deficient cells. Arrowheads indicate foam-cells. b, Cholesteryl ester formation in macrophages from diabetics (group A) incubated in vitamin D-deficient (black-bars) or 1,25(OH)2D3-supplemented (white-bars) media or in macrophages from non-diabetic, vitamin D-deficient non-diabetic controls (group C) (n = 8 per group) incubated in vitamin D-deficient (gray-bars) or 1,25(OH)2D3-supplemented (white-bars) media (*p < 0.01 vs. vitamin D-deficient). c, Oil-red-O stain, d, Cholesterol, e, Triglycerides from peritoneal macrophages from LDR−/− mice fed vitamin D-deficient or - sufficient high-fat diet (n=5 per group) (*p < 0.05 vs. vitamin D-deficient).
In order to determine the influence of vitamin D status on macrophage foam-cell formation in vivo, we extracted peritoneal macrophages from LDLR−/− mice fed a vitamin D-deficient or a vitamin D-sufficient high fat diet for 10 weeks. Mice on both diets had similar serum cholesterol (1,117 ± 56 vs. 1244 ± 73, p = 0.2) and triglyceride levels (312 ± 27 vs. 312 ± 42, p = 0.8) but serum 25(OH)D levels were significantly lower in mice fed the high-fat, vitamin D-deficient diet (19 ± 3.4 nmol/L vs. 87 ± 10 nmol/L, p < 0.01). Macrophages isolated from vitamin D-sufficient hypercholesterolemic mice exhibited less Oil-red-O droplets and lower total cholesterol and triglycerides when compared to macrophages isolated from vitamin D-deficient mice (Fig.1c, d, e). These observations suggest that a normal vitamin D status may be sufficient to inhibit foam-cell formation in vivo.
1,25(OH)2 Vitamin D Decreases Macrophage Cholesterol Uptake
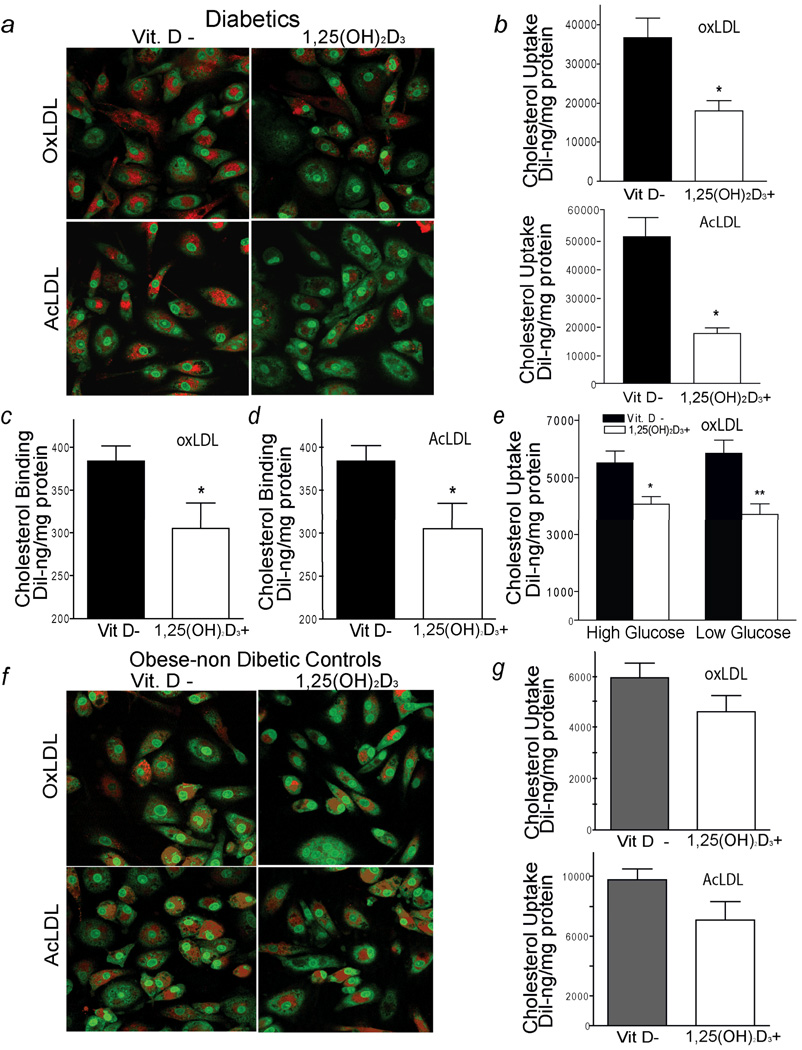
To investigate the mechanism underlying the reduction of foam-cell formation induced by vitamin D in diabetics, we assessed cholesterol uptake and efflux in macrophages cultured in either vitamin D-deficient or 1,25(OH)2D3-supplemented media. Confocal microscopy after fluorescence-labeled Dil-oxLDL or Dil-AcLDL stimulation showed that diabetic-derived macrophages (group A) cultured in 1,25(OH)2D3-supplemented media had decreased oxLDL and AcLDL cholesterol uptake both qualitatively and quantitatively by 40 to 50%, respectively, when compared to macrophages cultured in vitamin D-deficient media (p < 0.01, for both) (Fig. 2 a, b). Of note, macrophages from a subgroup of multiple patients from group A that were cultured in 1,25(OH)2D3 at concentrations of 10−8M showed suppressive effects on cholesterol uptake after stimulation with oxLDL or AcLDL or activation of CYP24 expression (VDR receptor target gene) in contrast to 1,25(OH)2D3 concentrations of 10−10 M or 10−12 M (supplemental Fig. 1 a,b,c). Incubation with 1,25(OH)2D3 at a concentration of 10−8M also suppressed macrophage cholesterol binding induced by Dil-oxLDL or Dil-AcLDL by approximately 20% (Fig. 2 c, d) (p < 0.03, both conditions).
Figure 2.
1,25(OH)2D3 decreases macrophage cholesterol uptake in diabetic subjects. a, Macrophage cholesterol uptake assessed by confocal microscopy in diabetic subjects (group A). Red represents labeled cholesterol uptake after 6h of stimulation with Dil-oxLDL (top-panel) or Dil-AcLDL (bottom-panel); green fluorescence represents nuclear counterstains. b, Quantification of cholesterol uptake in same patient’s macrophages cultured in vitamin D-deficient (black-bars) and 1,25(OH)2D3-supplemented (white-bars) media. Mean fluorescence absorbance after Dil-oxLDL (top-panel) (n=24 subjects) or after Dil-AcLDL (bottom-panel) (n=12 subjects) stimulation (*p < 0.01 vs. vitamin D-deficient). c and d, Cholesterol binding in macrophages stimulated by Dil-oxLDL or Dil-AcLDL at 4°C for 2h (n=8 per experiment) (*p < 0.03 vs. vitamin D-deficient). e, Quantification of cholesterol uptake induced by oxLDL in macrophages in high or normal glucose conditions while on vitamin D-deficient or 1,25(OH)2D3-supplemented media (n=6 per condition) (*p < 0.02 and **p < 0.01 vs. vitamin D-deficient). In non-diabetic subjects (group C): f, Cholesterol uptake assessed by confocal microscopy. Red represents labeled cholesterol uptake after 6h of stimulation with Dil-oxLDL (top-panel) or AcLDL (bottom-panel). g, Quantification of cholesterol uptake after incubation with Dil-oxLDL (top-panel) or Dil-AcLDL (bottom-panel) in same patient’s macrophages cultured in vitamin D-deficient (gray-bars) and 1,25(OH)2D3-supplemented media (white-bars) (n=25 subjects).
In human macrophages, high glucose upregulates and HMG-CoA reductase inhibitors downregulate cholesterol uptake of oxLDL and scavenger receptor expression of CD36.18, 19 In diabetic patients (group A), 1,25(OH)2D3 suppression of oxLDL cholesterol uptake was independent of the glucose conditions (p = 0.2). 1,25(OH)2D3 suppresses oxLDL cholesterol uptake by 30 and 40% in macrophages cultured in high and low glucose conditions, respectively, when compared to macrophages cultured in vitamin D-deficient media with high and low glucose conditions (Fig. 2e) (p < 0.02 and p < 0.01, respectively). In vitamin D-deficient diabetics from group A on HMG-CoA reductase inhibitors, 1,25(OH)2D3 suppresses oxLDL-stimulated cholesterol uptake by 45% compared to macrophages cultured in vitamin D-deficient conditions (supplemental figure 2). These data suggest that 1,25(OH)2D3 regulation of cholesterol metabolism is independent of macrophage glucose conditions and in these cultured conditions is not influenced by a patient’s intake of HMG-CoA reductase inhibitors.
In macrophages from vitamin D-sufficient diabetics (group B), culture in 1,25(OH)2D3-supplemented media also elicited a reduction in oxLDL and AcLDL-induced cholesterol uptake of approximately 45% compared to macrophages cultured in vitamin D-deficient conditions (Supplemental Fig. 3 a, b) (p < 0.01 and p <0.05, respectively). However, in macrophages from vitamin D-deficient, non-diabetic controls (group C), 1,25(OH)2D3 did not significantly reduce cholesterol uptake after induction with oxLDL or AcLDL compared to macrophages maintained on vitamin D–deficient media (Fig. 2 f, g) (p = 0.07 and p = 0.1, respectively). Similarly, 1,25(OH)2D3 did not suppress macrophage oxLDL or AcLDL cholesterol uptake in vitamin D-deficient (group D) or vitamin D-sufficient (group E) normal volunteers (Supplemental Fig.3 c,d). These findings indicate clear differences between control subjects and diabetic subjects in 1,25(OH)2D3 regulation of macrophage cholesterol metabolism.
Cholesterol efflux was determined in macrophages from diabetic subjects from group A after incubation for 24 hours with labeled oxLDL. 1,25(OH)2D3 supplementation did not regulate passive, HDL-stimulated, or apolipoprotein AI-stimulated macrophage cholesterol efflux (supplemental Fig. 4a). 1,25(OH)2D3 supplementation did decrease macrophage ABCA1 mRNA expression by 30% (p < 0.05), but did not suppress ABCG1 and scavenger receptor B1 mRNA expression when compared with cells on vitamin D–deficient media (supplemental Fig. 4b).
Decrease in Macrophage Cholesterol Uptake Induced by Vitamin D (1,25(OH)2D3) is CD36 and SR-A1 Dependent
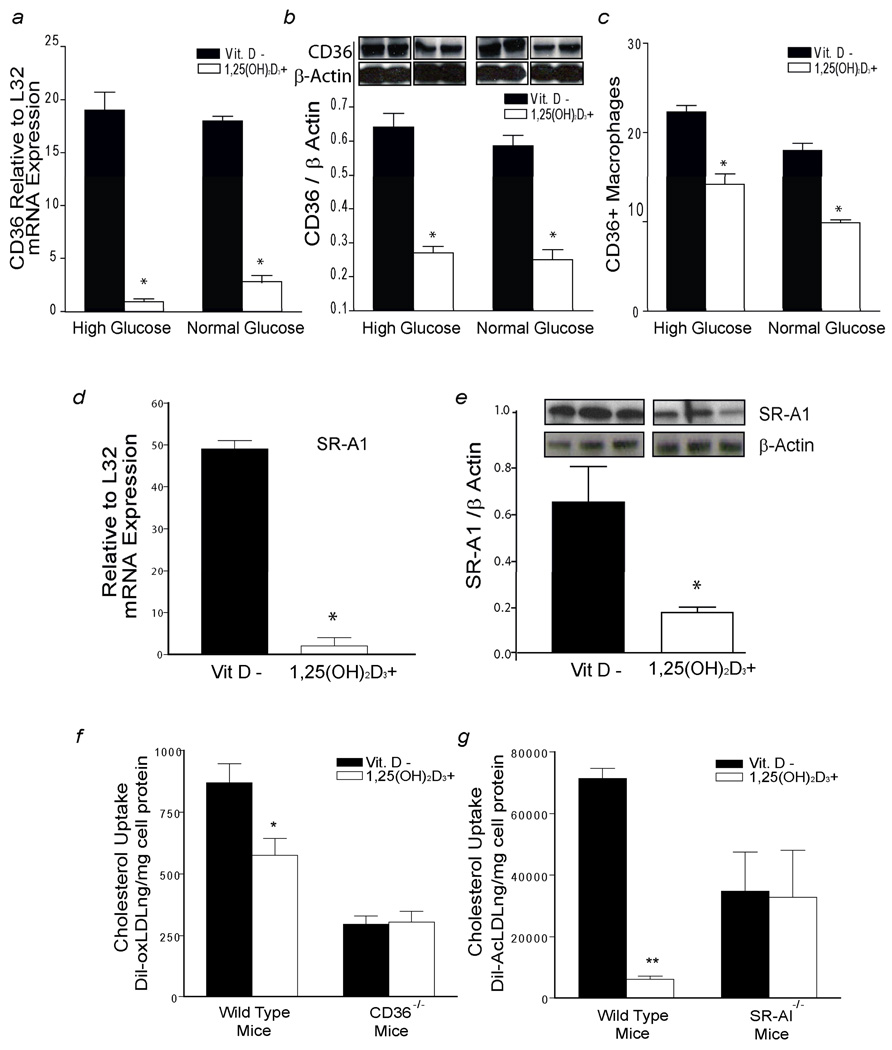
Membrane scavenger receptors SR-A1 and CD36 are essential for recognition and internalization of modified LDL particles.20 In diabetic-derived macrophages (group A) cultured in high and normal glucose, macrophages supplemented with 1,25(OH)2D3 had ~6 fold lower CD36 mRNA and ~40% decreased total and membrane-associated CD36 protein expression after oxLDL stimulation in both glucose conditions compared to macrophages cultured in vitamin D-deficient media (Fig. 3 a,b,c) (p < 0.01 for all). 1,25(OH)2D3 effects on CD36 mRNA, protein, and membrane-associated protein were independent of glucose concentrations (p = 0.3, p = 0.7, p = 0.3 respectively). 1,25(OH)2D3 also decreased macrophage SR-A1 mRNA 20-fold and reduced SR-A1 protein expression after AcLDL stimulation compared to macrophages cultured in vitamin D-deficient media (Fig. 3 d, e) (p < 0.001 for both). However, in macrophages from vitamin D-deficient, non-diabetic controls (group C), 1,25(OH)2D3 did not significantly suppress macrophage CD36 or SR-A1 protein expression (supplemental Fig. 5 a, b).
Figure 3.
1,25(OH)2D3 suppression of cholesterol uptake is CD36 and SR-A1 dependent. Macrophages from diabetic subjects (group A) cultured in vitamin D-deficient (black-bars) or 1,25(OH)2D3-supplemented media (white-bars) with high or normal glucose conditions were used to prepare RNA and protein. After stimulation for 6h with oxLDL: a, qPCR of CD36 mRNA expression relative to L32 expression (n=10) (*p < 0.0001 vs. vitamin D-deficient). b, Densitometric analysis of CD36 expression normalized to β-actin (n=4) (*p < 0.002 vs. vitamin D-deficient). c, Percent of CD14+/CD11b+ macrophages positive for CD36 via flow cytometry (n=4) (*p < 0.001 vs. vitamin D-deficient). In high glucose conditions after stimulation for 6h with AcLDL: d, qPCR of SR-A1 mRNA relative to L32 expression (n=10) (*p < 0.0001 vs. vitamin D-deficient). e, Densitometric analysis of SR-A1 expression normalized to β-actin (*p < 0.001 vs. vitamin D-deficient). f and g, Quantification of cholesterol uptake after stimulation with oxLDL or AcLDL in thioglycollate-elicited macrophages from CD36−/−, SR-A1−/−, and WT mice littermates cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media. Mean fluorescence of Dil absorbance was measured after 6h of incubation with Dil-oxLDL or Dil-AcLDL (n=12 per group per condition) (*p < 0.03, **p < 0.001 vs. vitamin D-deficient).
To clarify the role of CD36 and SR-A1 expression in prevention of foam-cell formation by 1,25(OH)2D3, we measured cholesterol uptake after modified-LDL stimulation in peritoneal macrophages from WT, CD36−/−, and SR-A1−/− mice cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media. 1,25(OH)2D3 suppression of oxLDL and AcLDL-induced cholesterol uptake was dependent on mouse genotype (p < 0.01 for each genotype). In WT mice, 1,25(OH)2D3 suppressed cholesterol uptake induced by oxLDL (Fig. 3f) and AcLDL (Fig. 3g) compared to macrophages on vitamin D-deficient media (p < 0.03 and p < 0.001, respectively). However, the effect of vitamin D deficiency on cholesterol uptake was blunted by the absence of CD36 or SR-A1 receptors in macrophages (Fig. 3f,g). These results suggest that 1,25(OH)2D3 suppression of oxLDL and AcLDL cholesterol uptake is at least partially mediated by CD36 and SR-A1.
1,25(OH)2 Vitamin D Suppression of JNKp Prevents Foam-Cell Formation
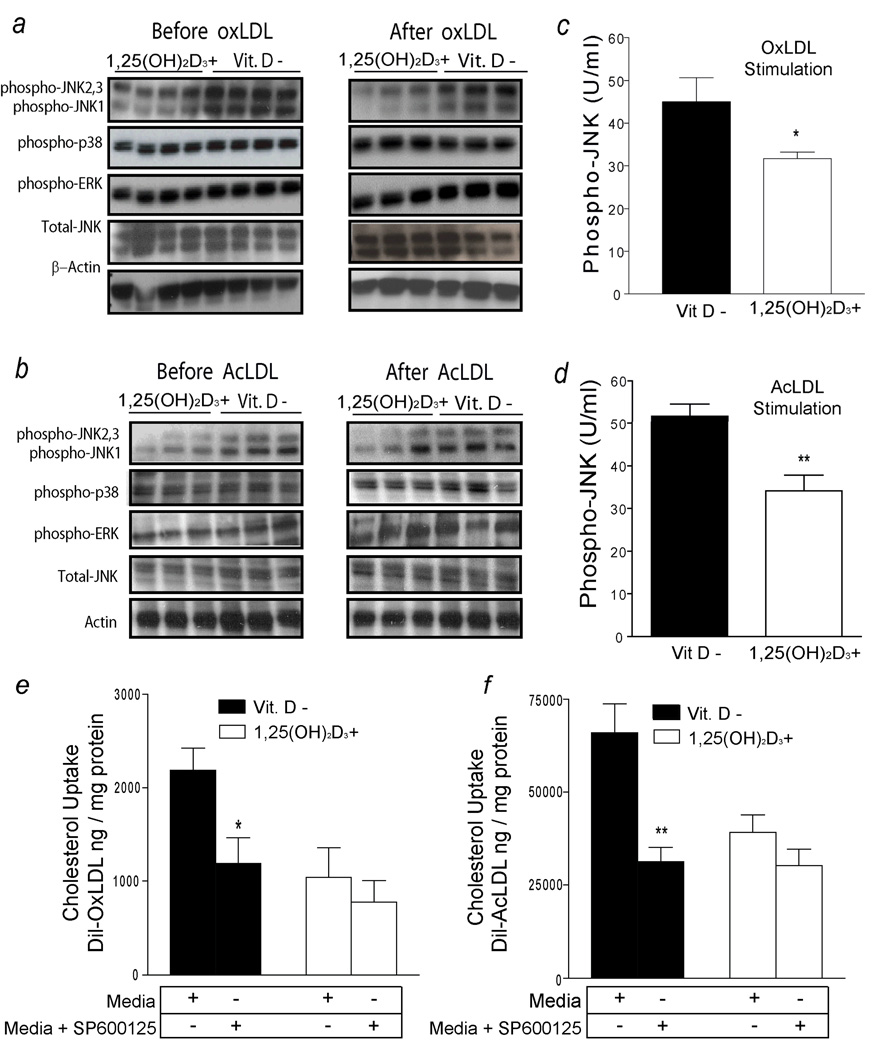
Stress-related JNK is highly activated in human atherosclerotic plaques and is known to mediate CD36 and SR-A1–dependent foam-cell formation in mice.21, 22 OxLDL activates several mitogenic activated protein kinases (MAPKs), including ERKs, JNK, and p38 MAP kinase, but the role of these pathways in vitamin D regulation of oxLDL or AcLDL uptake is unknown.21, 23 In vitamin D-deficient diabetics (group A), macrophages cultured in 1,25(OH)2D3-supplemented media have decreased phosphorylation of JNK1, JNK2, and JNK3 before and after oxLDL or AcLDL stimulation. However, no changes in activation of p38 or ERK1-phosphorylation were found in these subjects (Fig. 4a,b). In this population, JNKp analysis by ELISA confirmed that macrophages cultured in 1,25(OH)2D3-supplemented media have 50% lower JNKp levels after either oxLDL or AcLDL stimulation compared with macrophages cultured in vitamin D-deficient media (p < 0.002 and p < 0.03, respectively) (Fig. 4c,d). No change in activation of MAP kinase family members was present in non-diabetic controls (group C) (supplemental Fig. 5c,d). In macrophages from diabetics (group A), the suppressive effects of JNK inhibition on cholesterol uptake induced by oxLDL or AcLDL were dependent on vitamin D status (p < 0.01 for both). In macrophages cultured in vitamin D-deficient media, incubation with JNKp inhibitor (SP600125) decreased cholesterol uptake stimulated by oxLDL (Fig. 4e) and AcLDL (Fig. 4f) by 50% when compared to vitamin D-deficient macrophages not exposed to the JNK inhibitor (p < 0.03 and p < 0.01, respectively). No additional JNKp downregulation (data not shown) or cholesterol uptake was observed after adding SP600125 to macrophages cultured in 1,25(OH)2D3-supplemented media (Fig. 4e and f). These data suggest that vitamin D downregulation of JNKp is a unifying signaling pathway that suppresses oxLDL and AcLDL cholesterol uptake in diabetic patients.
Figure 4.
JNK phosphorylation is required for 1,25(OH)2D3 suppression of foam-cell formation. a and b, Western blot from macrophages from diabetic subjects (group A) cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media before and after stimulation with oxLDL or AcLDL. Expression of total JNK and β-actin were used as a loading control. c and d, ELISA of JNKp normalized to total JNK in macrophages cultured in vitamin D-deficient (black-bars) and -1,25(OH)2D3-supplemented (white-bars) media after oxLDL (n=10) or AcLDL (n=6) (*p < 0.002; ** p < 0.03 vs. vitamin D-deficient). e and f, Cholesterol uptake in macrophages cultured with or without JNK inhibitor in vitamin D-deficient (black-bars) or 1,25(OH)2D3-supplemented (white-bars) media after stimulation with Dil-oxLDL or Dil-AcLDL (n=8 per group per condition) (*p < 0.03;**p < 0.02 vs. no JNKp inhibitor-treated).
1,25(OH)2 Vitamin D Downregulation of JNKp Suppresses Macrophage oxLDL Cholesterol Uptake via PPARγ
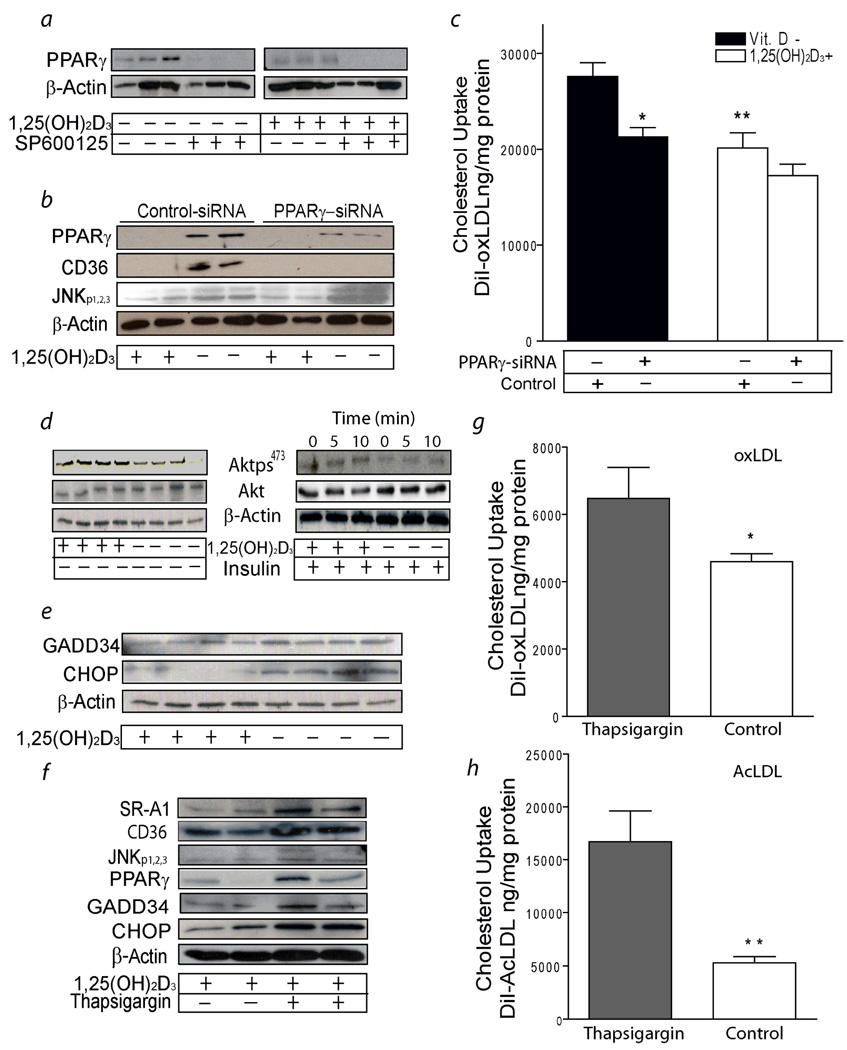
PPARγ is expressed in foam cells of human atherosclerotic lesions.24 PPARγ can be activated by oxLDL and controls macrophage CD36 expression.20 In diabetics (group A), macrophages cultured in 1,25(OH)2D3-supplemented media had significantly less PPARγ protein expression after oxLDL stimulation compared to macrophages cultured in vitamin D-deficient media. Addition of JNKp inhibitor to vitamin D-deficient or 1,25(OH)2D3-supplemented media almost abolished oxLDL-stimulated PPARγ protein expression compared to macrophages without JNK inhibitor (Fig.5a). These data suggest that 1,25(OH)2D3 -mediated downregulation of JNKp suppresses PPARγ expression.
Figure 5.
1,25(OH)2D3 suppresses macrophage ER stress and PPARγ expression in diabetic subjects (group A). a, PPARγ expression from macrophages cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media before and after adding JNK inhibitor (representative of n=6). b, PPARγ, CD36, JNKp expression from macrophages cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media after infection with PPARγ-siRNA or control-siRNA-lentivirus (representative of n=8).c, Cholesterol uptake in macrophages cultured in vitamin D-deficient (black-bars) or 1,25(OH)2D3-supplemented (white-bars) media after stimulation for 6h with with Dil-oxLDL and infection with siRNA lentivirus (n=8) (*p < 0.01; **p < 0.02 vs. vitamin D deficient control-siRNA infected cells). d, AKTp from macrophages cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media before (representative of n=8) and after (representative of n=4) insulin stimulation. Expression of total AKT and β-actin were used for loading control e, GADD34 and CHOP expression from macrophages cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented media. β-actin expression was used for loading control (representative of n=8). f, SR-A1, CD36, JNKp, PPARγ, GADD34 and CHOP expression from macrophages cultured in 1,25(OH)2D3-supplemented media with or without ER induction by thapsigargin (representative of n=8). g and h, Quantification of cholesterol uptake in macrophages cultured in 1,25(OH)2D3-supplemented media with thapsigargin (black-bars) or without thapsigargin (white-bars). Mean fluorescence absorbance after 6h incubation with Dil-oxLDL (g) (n=8) or Dil-AcLDL (h) (n=8). (*p < 0.03, **p < 0.01 vs. thapsigargin treated cells).
In vitamin D-deficient conditions, macrophages from diabetic patients (group A) infected with PPARγ-siRNA lentivirus have almost totally suppressed PPARγ and CD 36 expression without altering JNKp when compared to control-siRNA-infected cells (Fig 5b). Reduction of PPARγ significantly suppressed oxLDL-stimulated cholesterol uptake induced by vitamin D deficiency (p < 0.01) (Fig 5c). However, no interaction between PPARγ inhibition and vitamin D status was identified (p = 0.3). These data suggest that 1,25(OH)2D3-mediated downregulation of JNKp reduces macrophage PPARγ and CD36 expression and suppresses oxLDL-stimulated cholesterol uptake in diabetic patients. PPARγ downregulation did not alter SR-A1 expression or AcLDL-induced cholesterol uptake (data not shown).
1,25(OH)2 Vitamin D Downregulation of Endoplasmic Reticulum Stress Prevents Modified LDL-Stimulated Macrophage Cholesterol Uptake and Suppresses Scavenger Receptor SR-A1 and CD36 Expression
Defective macrophage insulin signaling induces the accumulation of misfolded proteins in the Endoplasmic Reticulum (ER) lumen causing stress.2 Persistent ER stress leads to increased SR-A1 expression and JNK activation.25 In diabetic patients (group A), 1,25(OH)2D3-supplemented media improved macrophage insulin signaling by increasing insulin-induced AKT phosphorylation (Fig. 5d). In addition, 1,25(OH)2D3 significantly suppressed the expression of ER stress protein markers (GADD34, CHOP) (Fig. 5e) and reduced CD36 and SR-A1 expression (Fig. 3a–e). Conversely, induction of ER stress with thapsigargin in 1,25(OH)2D3-treated macrophages increased SR-A1, CD36, PPARγ, GAD34, and CHOP protein expression and promoted JNK activation when compared to macrophages cultured in 1,25(OH)2D3-supplemented media without thapsigargin (Fig. 5f). Thapsigargin-induced ER stress blunted the 1,25(OH)2D3 suppression of oxLDL and AcLDL-induced cholesterol uptake when compared to macrophages cultured in 1,25(OH)2D3-supplemented media without thapsigargin (p < 0.03, p < 0.01, respectively) (Fig. 5g,h). By improving insulin signaling and ER stress in macrophages from diabetic patients, 1,25(OH)2 vitamin D modulates JNK activity and PPARγ expression and suppresses modified LDL cholesterol uptake.
Activation of Vitamin D Receptor (VDR) Signaling Prevents Foam Cell-Formation
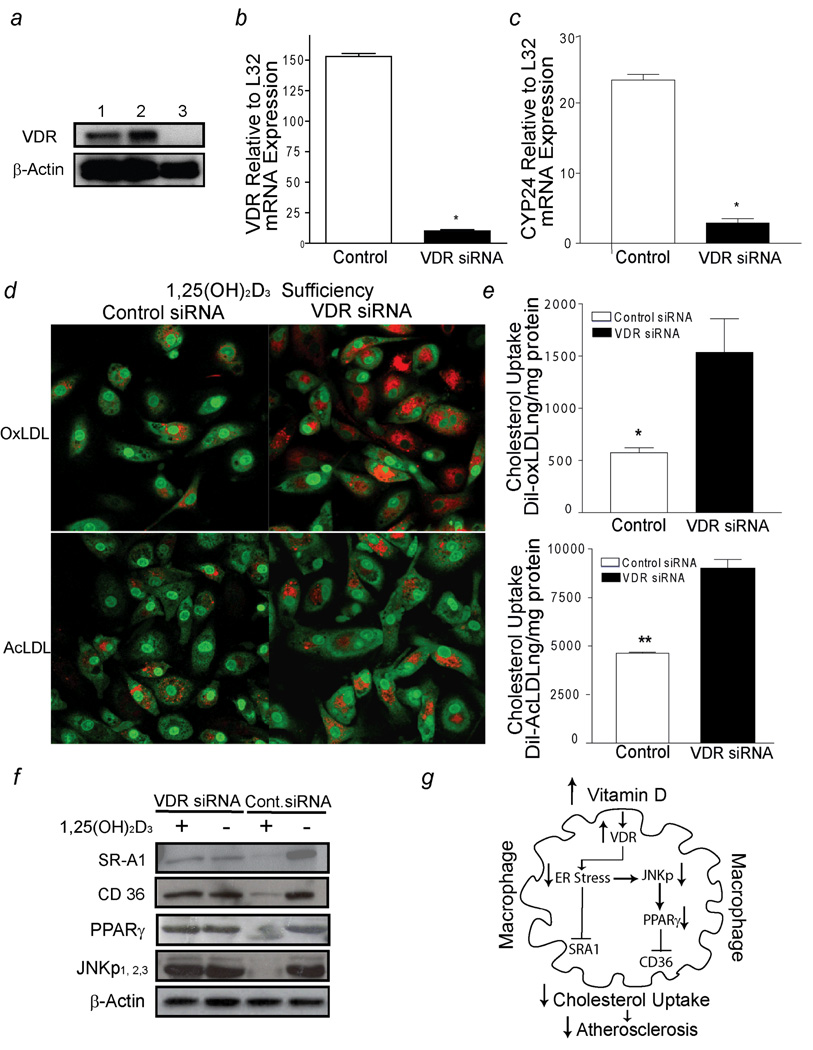
1,25(OH)2D3 acts mostly through, the vitamin D receptor (VDR), a member of the nuclear receptor superfamily of transcriptional regulators, but also through rapid, non-genomic actions upon binding to several other proteins near the plasma membrane of target cells.26 To identify whether the 1,25(OH)2D3 suppressive effects on cholesterol uptake are VDR–dependent, we infected diabetic -derived macrophages (group A) cultured in 1,25(OH)2D3-supplemented media with lentivirus containing either siRNA VDR-hairpins or control-siRNA. VDR-siRNA-infected macrophages showed an 80% reduction in VDR mRNA and protein levels and a six-fold reduction in the mRNA levels of a classical VDR target gene, the 24-hydroxylase (CYP24), compared to control siRNA-infected macrophages (p < 0.001) (Fig. 6 a,b,c).
Figure 6.
1,25(OH)2D3 activation of VDR signaling prevents macrophage cholesterol uptake in diabetics (group A). a, VDR receptor expression (top-panel) and β-actin (bottom-panel). Lines 1–2, siRNA-control infected cells; line 3, VDR-siRNA infected cells. b and c, qPCR of VDR mRNA and CYP24 mRNA expression in response to 1,25(OH)2D3 supplementation in macrophages infected with either VDR-siRNA (black-bars) or control-siRNA (white-bars) lentivirus, respectively (n=8 per group). (* p< 0.0001 vs. control-siRNA macrophages). d, Cholesterol uptake assessed by confocal microscopy. Red represents labeled cholesterol uptake after Dil-oxLDL (top-panel) or Dil-AcLDL (bottom-panel) stimulation; green fluorescence represents nuclear counterstains. e, Cholesterol uptake after incubation with Dil-oxLDL (top-panel) or Dil-AcLDL (bottom-panel) in macrophages cultured in 1,25(OH)2D3-supplemented media after infection with siRNA lentivirus (n=5). (*p <0.01; *p <0.02 vs. VDR-siRNA macrophages). f, SR-A1, CD36, PPARγ, JNKp expression from macrophages infected with either VDR-siRNA or control-siRNA lentivirus cultured in vitamin D-deficient media or 1,25(OH)2D3-supplemented media. g, Mechanistic pathways involved in 1,25(OH)2D3 suppression of foam cell formation.
Confocal microscopy and quantification of cholesterol uptake confirmed that 1,25(OH)2D3 decreased macrophage cholesterol uptake induced by AcLDL and oxLDL by 50% and 60%, respectively, only in macrophages with intact VDR signaling pathways; this response was blunted in macrophages lacking a VDR signaling pathway (p < 0.001 and p < 0.02, respectively) (Fig. 6 d,e). In addition, 1,25(OH)2D3 downregulated CD36, SR-A1, and PPARγ expression as well as JNKp in the presence of intact VDR signaling, but these effects were reduced in VDR-siRNA-infected macrophages (Fig. 6f). These data confirm the importance of the activation of VDR signaling in the regulation of both scavenger receptors and cell signaling pathways involved in macrophage foam-cell formation (Fig. 6g).
DISCUSSION
Despite aggressive lipid-lowering strategies aimed at type 2 diabetics, CVD remains the leading cause of mortality for these individuals. In this study, we demonstrate that activation of vitamin D receptor signaling prevents foam-cell formation by reducing modified-LDL cholesterol uptake in macrophages from diabetic patients. Through suppression of ER stress and JNK activation, 1,25(OH)2D3 downregulates two critical scavenger receptors involved in macrophage cholesterol deposition. Impairment of VDR signaling confirmed acceleration of foam-cell formation in diabetics. Taken together, these results suggest that modulation of vitamin D signaling is a potential therapeutic target to prevent vascular disease progression.
25(OH)D has minimal intrinsic activity and needs to be converted into 1,25(OH)2D to activate VDR. The direct relationship between 25(OH) vitamin D replacement and increased serum 1,25(OH)2D in anephric patients demonstrates that increased local production of 1,25(OH)2D occurs in extra-renal tissues, particularly macrophages.27,28 Therefore, increased local macrophage conversion of 25(OH)D to its active form by vitamin D replacement is a potential therapeutic target to suppress foam-cell formation and vascular disease progression in diabetics.
Macrophage scavenger receptors play a decisive role in transforming macrophages into foam-cells.20 Targeted disruption of the scavenger receptors SR-A1 or CD36 in diet-induced insulin-resistant mouse models confirms the importance of both receptors in the development of atherosclerosis.29 During hyperglycemia and/or an insulin-resistant state, increased scavenger receptor expression promotes foam-cell formation and is considered a link between diabetes and atherosclerosis.18, 30 Previous studies indicated the importance of 1,25(OH)2D3 downregulation of SR-A1 receptor expression in TPA–treated THP-1 macrophages.15 In this study, we provide evidence that 1,25(OH)2D3 activation of VDR decreases macrophage cholesterol uptake by reducing CD36 and SR-A1 expression in diabetics. Furthermore, deletion of macrophage VDR interrupts 1,25(OH)2D3 downregulation of CD36 and SR-A1 expression and accelerates oxLDL and AcLDL cholesterol uptake. These data suggest that activation of VDR regulates a unifying cell signaling pathway that suppresses both scavenger receptor expression and uptake of modified-LDL cholesterol.
Several mechanisms may be involved in 1,25(OH)2D3’s ability to suppress macrophage cholesterol ester accumulation in diabetics, but JNK is particularly important. JNK is activated by stressors such as oxidative stress, fatty acids, and inflammatory cytokines, which are commonly present in insulin-resistant tissues.31 In apolipoprotein E–null mice, pharmacologic inhibition of JNK activity and genetic JNK2 deficiency decreased atherosclerosis, in part due to inhibition of CD36 and SR-A1–dependent foam-cell formation.17, 22 1,25(OH)2D3 modulates JNK signaling activation in response to extracellular stress stimulation.32 In concert, p38/JNK activation regulates VDR gene expression, further supporting the interaction between this signaling pathway and vitamin D.33 In this study, we found that 1,25(OH)2D3 is a natural inhibitor of macrophage JNK phosphorylation in diabetics. 1,25(OH)2D3 downregulation of the JNK pathway suppresses cholesterol uptake by the scavenger receptors CD36 and SR-A1. Furthermore, targeted deletion of VDR interrupts 1,25(OH)2D3's ability to inhibit foam-cell formation and JNK activation. These data suggest that downregulation of JNK stress signaling by VDR activation is a unifying mechanism for both scavenger receptor–induced foam-cell formation and possibly atherogenesis.
PPARγ expression is induced in foam-cells of human atherosclerotic lesions.24 PPARγ plays a critical role in maintaining macrophage cholesterol homeostasis by positively regulating the expression of genes involved in cholesterol storage and efflux.2, 19, 34 Previous observations indicate that 1,25(OH)2D3 is capable of repressing PPARγ expression in adipocytes.35 Consistent with this possibility, we find that 1,25(OH)2D3 downregulation of JNK activation suppresses PPARγ and CD36 expression, reducing oxLDL-derived cholesterol uptake. Conversely, inhibition of macrophage PPARγ expression suppresses oxLDL-derived cholesterol uptake induced by culturing macrophages in vitamin D-deficient media. No interaction was identified between PPARγ inhibition and vitamin D status, but we suspect this was secondary to small sample size. PPARγ inhibition did not prevent 1,25(OH)2D3 suppression of SR-A1 expression and AcLDL derived cholesterol uptake, suggesting that 1,25(OH)2D3 downregulation of JNKp-PPARγ-CD36 only partially explains the 1,25(OH)2D3 effects on foam-cell formation.
In insulin resistant mouse models, persistent metabolic stress activates ER stress regulation of SR-A1 expression and JNK2 activation in macrophages25, 36 Here we show that 1,25(OH)2D3 couples ER stress to regulation of SR-A1 expression and JNK activation in macrophages from diabetic patients. ER stress activation blunts the 1,25(OH)2D3 suppression of JNKp and modified-LDL cholesterol uptake, suggesting that the prevention of ER stress by 1,25(OH)2D3 is critical for limiting macrophage cholesterol accumulation. Previous studies indicated that increased cholesterol trafficking to the ER induces macrophage apoptosis and leads to plaque instability. Activation of p38-CHOP and JNK2 signaling pathways are known apoptotic pathways triggered by ER stress25. Increased CHOP is also shown in macrophages in advanced atherosclerotic lesions in humans37. Thus, 1,25(OH)2D3 suppression of ER stress and foam-cell formation led us to speculate that 1,25(OH)2D3 potentially influences not only the initiation of foam-cell formation, but also the progression of the atherosclerotic plaque.
This study shows clear differences between control subjects and diabetic subjects in 1,25(OH)2D3 regulation of macrophage cholesterol metabolism. In a previous study with normal, non-diabetic subjects, 1,25(OH)2D3 increased cholesteryl ester formation in monocytes stimulated with AcLDL only after 24 hours of lipid deprivation.16 In our study, in the absence of lipid deprivation, 1,25(OH)2D3 did not induce a significant effect on cholesterol metabolism in obese, non-diabetic, hypertensive control subjects. In contrast, robust 1,25(OH)2D3 suppression of foam-cell formation in diabetic subjects was observed. In diabetic subjects and insulin-resistant mice models, defective insulin signaling and elevated JNK activity promotes foam-cell formation.17, 18, 36 Induction of insulin sensitivity reverses abnormal cholesterol metabolism in macrophages.34 In this study we showed that induction of insulin sensitivity and/or downregulation of ER stress-JNK activity by 1,25(OH)2D3 may represent the potential mechanisms whereby 1,25(OH)2D3 suppresses cholesterol metabolism in diabetic subjects.
This study reveals a novel mechanistic link between vitamin D deficiency in macrophages and foam-cell formation in type 2 diabetics. Interventional studies are needed to assess the effects of vitamin D status on CVD in diabetic subjects, as well as the impact of diabetes on the macrophage conversion of 25(OH)D to 1,25(OH)2D.
Clinical Summary.
Cardiovascular disease is the leading cause of death among diabetics. Intensive glucose lowering effects on macrovascular complications in this population are unpredictable and may result in increased mortality. Therefore, identification of glucose-independent factors modulating macrophage cholesterol deposition is critical to understanding the development of CVD in diabetics. Approximately 1 billion people worldwide have 25-hydroxy vitamin D deficiency or insufficiency, and more than half of middle-aged vitamin D–deficient patients develop CVD. In hypertensive patients, low serum vitamin D levels increase the risk of CVD by 60%. In women with type 2 diabetes, the prevalence of vitamin D deficiency is a third higher than that of control subjects, and low vitamin D levels nearly double the risk of developing CVD compared to diabetic patients with normal vitamin D levels. Therefore, understanding the mechanism of accelerated atherosclerosis induced by vitamin D deficiency may be crucial for treating CVD in diabetics. In this study, we demonstrate that active vitamin D suppresses foam-cell formation by reducing acetylated or oxidized low-density lipoprotein cholesterol uptake in diabetics. Through downregulation of macrophage stress-related JNK signaling and suppression of endoplasmic reticulum stress, active vitamin D reduces PPARγ expression, suppresses CD36 and SRA-1 scavenger receptor expression, and prevents macrophage cholesterol deposition. Deletion of VDR confirmed acceleration of foam-cell formation. This study reveals a novel mechanistic link between vitamin D deficiency in macrophages and foam-cell formation in type 2 diabetics and suggests that modulation of vitamin D signaling is a potential therapeutic target to prevent vascular disease progression.
Supplementary Material
Acknowledgments
We thank Drs. Adriana Dusso, Daniel S. Ory, Mark S. Sands, and Clay F. Semenkovich for helpful discussions and review of the manuscript. We also thank Drs. Marco Colonna and Marina Cella for their helpful assistance with flow cytometry analysis.
Founding Sources: This work was supported by the American Diabetes Association (7-08-CR-08), the Washington University Diabetes Research and Training Center (P60DK20579), the Clinical Nutrition Research Unit (P30DK056341), and the David M. and Paula S. Kipnis Scholar in Medicine Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Journal Subject Codes: 96,112,134,135,138,142,143,190
http://clinicaltrials.gov/. Identification Number: NCT00736632
Disclosures: None.
References
- 1.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 2.Liang CP, Han S, Senokuchi T, Tall AR. The macrophage at the crossroads of insulin resistance and atherosclerosis. Circ Res. 2007;100:1546–1555. doi: 10.1161/CIRCRESAHA.107.152165. [DOI] [PubMed] [Google Scholar]
- 3.Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–2633. doi: 10.1056/NEJMe0804182. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24:1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 8.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29:722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 9.Chonchol M, Cigolini M, Targher G. Association between 25-hydroxyvitamin D deficiency and cardiovascular disease in type 2 diabetic patients with mild kidney dysfunction. Nephrol Dial Transplant. 2008;23:269–274. doi: 10.1093/ndt/gfm537. [DOI] [PubMed] [Google Scholar]
- 10.Andress DL. Vitamin D treatment in chronic kidney disease. Semin Dial. 2005;18:315–321. doi: 10.1111/j.1525-139X.2005.18408.x. [DOI] [PubMed] [Google Scholar]
- 11.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 12.Koeffler HP, Amatruda T, Ikekawa N, Kobayashi Y, DeLuca HF. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res. 1984;44:5624–5628. [PubMed] [Google Scholar]
- 13.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Jouni ZE, McNamara DJ. Lipoprotein receptors of HL-60 macrophages. Effect of differentiation with tetramyristic phorbol acetate and 1,25-dihydroxyvitamin D3. Arterioscler Thromb. 1991;11:995–1006. doi: 10.1161/01.atv.11.4.995. [DOI] [PubMed] [Google Scholar]
- 15.Suematsu Y, Nishizawa Y, Shioi A, Hino M, Tahara H, Inaba M, Morii H, Otani S. Effect of 1,25-dihydroxyvitamin D3 on induction of scavenger receptor and differentiation of 12-O-tetradecanoylphorbol-13-acetate-treated THP-1 human monocyte like cells. J Cell Physiol. 1995;165:547–555. doi: 10.1002/jcp.1041650313. [DOI] [PubMed] [Google Scholar]
- 16.Roullet JB, Haluska M, Morchoisne O, McCarron DA. 1,25-Dihydroxyvitamin D3-induced alterations of lipid metabolism in human monocyte-macrophages. Am J Physiol. 1989;257:E290–E295. doi: 10.1152/ajpendo.1989.257.2.E290. [DOI] [PubMed] [Google Scholar]
- 17.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Griffin E, Re A, Hamel N, Fu C, Bush H, McCaffrey T, Asch AS. A link between diabetes and atherosclerosis: Glucose regulates expression of CD36 at the level of translation. Nat Med. 2001;7:840–846. doi: 10.1038/89969. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson AC, Hajjar DP. CD36, oxidized LDL and PPAR gamma: pathological interactions in macrophages and atherosclerosis. Vascul Pharmacol. 2004;41:139–146. doi: 10.1016/j.vph.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Rader DJ, Pure E. Lipoproteins, macrophage function, and atherosclerosis: beyond the foam cell? Cell Metab. 2005;1:223–230. doi: 10.1016/j.cmet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumara G, Belwal M, Ricci R. "Jnking" atherosclerosis. Cell Mol Life Sci. 2005;62:2487–2494. doi: 10.1007/s00018-005-5253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusuhara M, Chait A, Cader A, Berk BC. Oxidized LDL stimulates mitogen-activated protein kinases in smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 1997;17:141–148. doi: 10.1161/01.atv.17.1.141. [DOI] [PubMed] [Google Scholar]
- 24.Ricote M, Huang J, Fajas L, Li A, Welch J, Najib J, Witztum JL, Auwerx J, Palinski W, Glass CK. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemere I, Farach-Carson MC. Membrane receptors for steroid hormones: a case for specific cell surface binding sites for vitamin D metabolites and estrogens. Biochem Biophys Res Commun. 1998;248:443–449. doi: 10.1006/bbrc.1998.8492. [DOI] [PubMed] [Google Scholar]
- 27.Dusso A, Lopez-Hilker S, Rapp N, Slatopolsky E. Extra-renal production of calcitriol in chronic renal failure. Kidney Int. 1988;34:368–375. doi: 10.1038/ki.1988.190. [DOI] [PubMed] [Google Scholar]
- 28.Dusso A, Finch J, Delmez J, Rapp N, Lopez-Hilker S, Brown A, Slatopolsky E. Extrarenal production of calcitriol. Kidney Int Suppl. 1990;29:S36–S40. [PubMed] [Google Scholar]
- 29.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 30.Fukuhara-Takaki K, Sakai M, Sakamoto Y, Takeya M, Horiuchi S. Expression of class A scavenger receptor is enhanced by high glucose in vitro and under diabetic conditions in vivo: one mechanism for an increased rate of atherosclerosis in diabetes. J Biol Chem. 2005;280:3355–3364. doi: 10.1074/jbc.M408715200. [DOI] [PubMed] [Google Scholar]
- 31.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 32.Ravid A, Rubinstein E, Gamady A, Rotem C, Liberman UA, Koren R. Vitamin D inhibits the activation of stress-activated protein kinases by physiological and environmental stresses in keratinocytes. J Endocrinol. 2002;173:525–532. doi: 10.1677/joe.0.1730525. [DOI] [PubMed] [Google Scholar]
- 33.Qi X, Pramanik R, Wang J, Schultz RM, Maitra RK, Han J, DeLuca HF, Chen G. The p38 and JNK pathways cooperate to trans-activate vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to vitamin D(3)-induced growth inhibition. J Biol Chem. 2002;277:25884–25892. doi: 10.1074/jbc.M203039200. [DOI] [PubMed] [Google Scholar]
- 34.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290:E916–E924. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- 36.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.