Abstract
Previously, we reported that α1,6-fucosyltransferase (Fut8)-deficient (Fut8−/−) mice exhibit emphysema-like changes in the lung and severe growth retardation due to dysregulation of TGF-β1 and EGF receptors and to abnormal integrin activation, respectively. To study the role of α1,6-fucosylation in brain tissue where Fut8 is highly expressed, we examined Fut8−/− mice using a combination of neurological and behavioral tests. Fut8−/− mice exhibited multiple behavioral abnormalities consistent with a schizophrenia-like phenotype. Fut8−/− mice displayed increased locomotion compared with wild-type (Fut8+/+) and heterozygous (Fut8+/−) mice. In particular, Fut8−/− mice showed strenuous hopping behavior in a novel environment. Working memory performance was impaired in Fut8−/− mice as evidenced by the Y-maze tests. Furthermore, Fut8−/− mice showed prepulse inhibition (PPI) deficiency. Intriguingly, although there was no significant difference between Fut8+/+ and Fut8+/− mice in the PPI test under normal conditions, Fut8+/− mice showed impaired PPI after exposure to a restraint stress. This result suggests that reduced expression of Fut8 is a plausible cause of schizophrenia and related disorders. The levels of serotonin metabolites were significantly decreased in both the striatum and nucleus accumbens of the Fut8−/− mice. Likewise, treatment with haloperidol, which is an antipsychotic drug that antagonizes dopaminergic and serotonergic receptors, significantly reduced hopping behaviors. The present study is the first to clearly demonstrate that α1,6-fucosylation plays an important role in the brain, and that it might be related to schizophrenia-like behaviors. Thus, the results of the present study provide new insights into the underlying mechanisms responsible for schizophrenia and related disorders.
Keywords: Brain, Carbohydrate Function, Gene Knockout, Glycoprotein, Glycosylation, Oligosaccharide, Fut8, Glycosyltransferase, Fucosyltransferase
Introduction
α1,6-Fucosyltransferase (Fut8)2 catalyzes the transfer of a fucose residue from GDP-fucose to position 6 of the innermost GlcNAc residue to form α1,6-fucose in hybrid and complex N-linked oligosaccharides of glycoproteins as shown in Fig. 1 (1). α1,6-Fucosylated glycoproteins are widely distributed in mammalian tissues, especially in the brain (2). In fact, the majority of N-glycans present in mouse brain tissue are α1,6-fucosylated (3). We recently investigated the physiological functions of α1,6-fucose by using Fut8-deficient (Fut8−/−) mice (4). Fut8−/− mice showed severe growth retardation. They also suffered from emphysema-like changes in their lungs that appeared to be due to a lack of α1,6-fucosylation of the transforming growth factor-β1 (TGF-β1) receptor, which consequently resulted in dysregulation of TGF-β1 receptor activation and signaling (4), and down-regulation of expression of vascular endothelial cell growth factor receptor-2 (5). In addition, deletion of the core fucose from the IgG1 molecule reportedly enhances antibody-dependent cellular cytotoxicity activity by 50–100-fold. This result indicates that α1,6-fucose is an important sugar chain in terms of antibody-dependent cellular cytotoxicity activity (6). Moreover, the loss of α1,6-fucosylation down-regulates both EGF receptor-mediated cell signaling pathways (7) and integrin α3β1-mediated cell adhesion (8). Taken together, these results suggest that α1,6-fucose plays a key role in regulating important physiological functions via modification of functional proteins.
FIGURE 1.
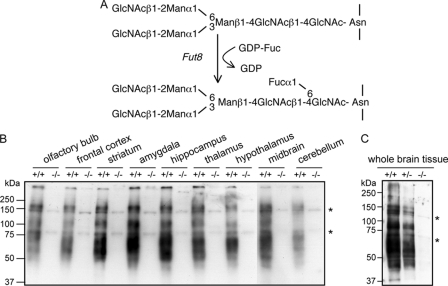
Expression of α1,6-fucosylation in brain tissues. A, reaction pathway for the synthesis of α1,6-fucose. GlcNAc, N-acetylglucosamaine; Man, mannose; Fuc, fucose; GDP-Fuc, guanosinodiphospho-fucopyranoside. Homogenates of various brain regions from Fut8+/+ and Fut8−/− mice (B) and whole brain tissues from Fut8+/+, Fut8+/−, and Fut8−/− mice (3 months old) (C) were subjected to a lectin blot analysis using AAL as described under “Experimental Procedures.” Asterisk indicates nonspecific bands.
Complex N-glycans are required for the development of the embryo, and the complete lack of N-glycans in GnT-I-deficient mice is lethal with defects in neural tube formation (9). These observations suggest that high-mannose N-glycans are insufficient for normal ontogeny. On the other hand, restriction of N-glycan branching to the formation of only hybrid structures by GnT-II inactivation in mice results in a very low rate of survival to adulthood and a postnatal phenotype that is similar to human “congenital disorder of glycosylation (CDG) IIa,” which manifests as severe multisystemic defects, psychomotor abnormalities, and mental retardation (10, 11). Furthermore, the specific elimination of GnT-I and GnT-II genes in neuronal cell types reveals that hybrid N-glycans are essential for neuronal and postnatal viability in mice, whereas complex N-glycans appear dispensable in this cell lineage (12). On the other hand, the importance of fucosylation has recently been underlined by identification of the monogenetic inherited human disease CDG-llc, also termed “leukocyte adhesion deficiency II,” which is caused by defective Golgi GDP-fucose transporter (SLC35C1) activity (13, 14). CDG-llc patients show hypofucosylation of glycoproteins and present clinically with mental and growth retardation, persistent leukocytosis, and severe infections (15). These symptoms can be partially corrected by oral fucose (16).
Schizophrenia is a common, chronic and severe brain disorder characterized by episodic positive symptoms such as delusions, hallucinations, and thought disorders, and/or persistent negative symptoms such as flattened affect, impaired attention, social withdrawal, and selective cognitive deficits in attention, learning, and memory (17). It ranks as one of the leading causes of disability worldwide, as it afflicts 0.5 to 1% of the world's population (18). Schizophrenia seems to have strong genetic component. Indeed, more than 130 genes reportedly predispose to schizophrenia, but few of these results have been replicated, and fewer still have biological support (17). Although evidence has accumulated for plausible candidate genes for schizophrenia, e.g. neuregulin1 (NRG1), disrupted in schizophrenia 1 (DISC1), and dystrobrevin-binding protein 1 (DTNBP1), etc., substantial controversy remains regarding both the meaning of the positive genetic findings and implications for therapeutic strategies (19). Some studies suggest that interactions between genetics and environmental factors increase the risk that an individual will develop schizophrenia. For example, a functional polymorphism in the gene encoding catechol-O-methyltransferase, which is thought to affect the availability of dopamine in the cortex, increases the risk for the development of psychosis associated with cannabis use during adolescence (20). Similarly, serious obstetric complications might interact with variants of genes that are regulated by hypoxia and/or genes that are involved in vascular function to influence the risk of developing schizophrenia (21).
The unique structure of the α1,6-fucosylated hybrid, the formation of which is catalyzed by Fut8, is highly expressed in both mouse and rat brain tissues and the expression pattern of N-glycans is altered during brain development (22, 23). The present study examined the effects of α1,6-fucosylation on the central nervous system. We characterized Fut8−/− mice using neurological and behavioral assays that included open field and hopping tests, a social interaction assessment, the Y maze test, and the prepulse inhibition (PPI) of startle test. The Fut8−/− mice exhibited multiple behavioral abnormalities with a schizophrenia-like phenotype.
EXPERIMENTAL PROCEDURES
Animals
All animal experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences, Tohoku Pharmaceutical University. The generation of Fut8−/− mice by a gene-targeting technique has been described previously (4). F1 heterozygous mice were mated with C57BL/6 mice to produce F2 generation mice. We used mice with ∼87% of C57BL/6 genetic background (F3 generation mice), because we found that mice that have ∼93% of C57BL/6 genetic background (F4 generation mice) rarely lived longer than 1 month. In the present study, we switched to mice with an ICR genetic background (F7 generation mice, backcrossed 7 times), and the survival rate of these mice was about 30%. Wild-type littermates and Fut8−/− mice were obtained by intercrossing the heterozygous animals. All experiments were conducted with male mice 2.5–3.5 months old, except those (3.5–4.5 months old) used for analyses of monoamine turnover (Fig. 6). Mice were housed in a room with a 12-h light/dark cycle (lights on at 7:00 a.m.) with access to food and water. Behavioral testing was performed between 10:00 a.m. and 5:00 p.m.
FIGURE 6.
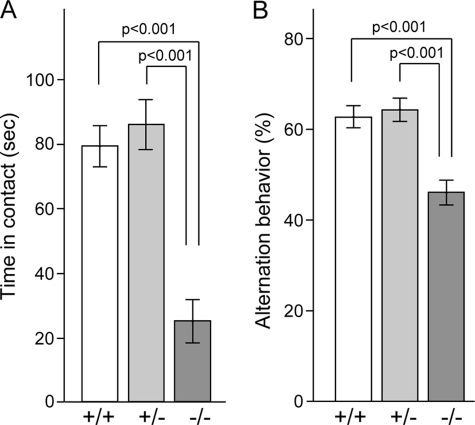
Impairment of social behavior and spontaneous alternation behavior. A, social interaction of pairs of male mice (2.5–3.5 months old) of the same genotype (Fut8+/+, n = 6; Fut8+/−, n = 6; Fut8−/−, n = 10) was observed for 10 min in an open arena. Bars represent total contact time. B, spontaneous alternation behavior during Y-maze test in mice (n = 10 for each group) was observed 8 min as described under “Experimental Procedures.”
Lectin Blotting Analysis
After mice (3 months old) were sacrificed, whole brain tissues or various brain regions, such as the olfactory bulb, prefrontal cortex, striatum, amygdale, hippocampus, thalamus, hypothalamus, midbrain, and cerebellum were rapidly removed and then homogenized in 4 volumes of PBS with a digital homogenizer (As One, CO., Osaka, Japan). After centrifugation at 900 × g for 10 min, the supernatants were collected and used for lectin blotting. Whole cell lysates (2.5 μg/each lane) were subjected to 10% SDS-PAGE, followed by transfer to PVDF membranes. The membranes were blocked with 5% BSA in TBST overnight at 4 °C, and then incubated with 0.5 μg/ml of biotinylated aleuria aurantia lectin (AAL) (Seikagaku Corp., Japan), which preferentially recognizes the Fucα1,6-GlcNAc structure, in TBST for 1 h at room temperature. After washing with TBST four times, lectin-reactive proteins were detected using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and an ECL kit.
Mass Spectrometry
The brain tissues from 3-month-old mice were dissolved in a small amount of 50 mm ammonium bicarbonate. After cooling the solution to 4 °C, a 3-fold volume of acetone was added to the solution, and the mixture was kept in an ice bath for 2 h to precipitate proteins. After centrifugation at 12,000 × g for 10 min, the precipitate was collected. An aliquot (200 μg equivalent) of the precipitate was treated by using glycan purification kit BlotGlyco (Sumitomo Bakelite, Co., Tokyo, Japan) according to the manufacturer's protocol. Briefly, the proteins were treated by reduction and alkylation followed by trypsinization. To the obtained mixture, peptide N-glycosidase F (EC 3.5.1.52, Takara Bio, Otsu, Japan) was added, and the mixture was incubated at 37 °C overnight. Then, the released glycans were captured by BlotGlyco beads, and sialic acids were methylesterified with 3-methyl-1-p-tolytriazene. Finally, the captured glycans were released in derivatized form with a labeling reagent, aoWR (24). MS spectra were acquired in the positive ion mode using a MALDI-TOF mass spectrometer (Reflex IV; Bruker Daltonics, Bremen, Germany). Ions were generated with a pulsed 337-nm nitrogen laser and accelerated to 20 kV. All spectra were obtained in the reflectron mode with delayed extraction of 200 ns. MS/MS spectra were acquired using a MALDI-Q-RT-TOF mass spectrometer (AXIMA-QIT, Shimadzu Corp., Japan). Argon was used as the collision gas. For sample preparation, 0.5 μl of an analyte solution was deposited on the target plate and allowed to dry. Then, 0.5 μl of a 2,5-dihydroxybenzoic acid (Wako Pure Chemical Industries Ltd., Osaka, Japan) solution (10 mg/ml in 30% ethanol) was used to cover the analyte on the target plate, followed by drying.
Measurement of Locomotor Activity
The locomotor activity was measured by using an Animex Auto MK-110 (Muromachi Kikai Co., Ltd., Tokyo, Japan), as described previously (25). Briefly, each mouse was placed at top of an Animex, which is an activity cage. As the mouse moved, it produced a signal due to variations in inductance and capacity of the apparatus resonance circuit. These signals were automatically converted into numbers. The number of activity counts was recorded every 5 min over a 120-min period. To detect hopping, each mouse was placed in a cylinder (8 cm in diameter, 25 cm deep) large enough for mouse movement. Behavior was recorded for 90-min using a video camera. The hopping activities were then scored.
Social Interaction Test In a Novel Environment
To investigate the habituation response to a novel mouse, a social interaction test in a novel environment was performed with 10–12-week-old male mice. Two mice of identical genotypes, which were previously housed in different cages, were introduced in a new environment (i.e. a box with dimensions of 40 × 40 × 30 cm3) and allowed to explore freely during the 10-min trial. Social behavior was monitored using a video camera attached to the top of the box. The total duration of contact (close following, inspection, anogenital sniffing, and other social body contact) was measured manually.
Y-maze Spontaneous Alternations
This task is based on exploration of novelty and was employed to measure spatial memory. Testing was carried out in a black Plexiglas maze composed of three arms with an inter-arm angle of 120 degrees connected to a central area. The arms were 50 cm long and 8 cm wide. The triangular center area measured 8 × 8 × 8 cm3. The floor of the maze was cleaned with paper soaked in 70% ethanol after each test. Each mouse was placed at the end of one fixed arm facing the central area and allowed to freely explore the three arms for an 8-min testing period. An arm entry was defined as the animal placing all four paws in that arm, having entered the center area from a different arm. Entry from the center area into the previously visited arm was not considered an arm entry. The sequence of arm entries was manually recorded. An alternation was defined as visits into all three arms in succession. The percentages of alternations were calculated as the number of alternations divided by number of entries minus 2 and multiplied by 100.
Sensitivity to Haloperidol and Atomoxetine
The effects of a typical antipsychotic drug, i.e. haloperidol (Sigma), and a non-stimulant drug approved for the treatment of attention-deficit hyperactivity disorder, i.e. atomoxetine (Sigma), on locomotor activity were tested. The pharmacological agents were injected intraperitoneally 20 min before monitoring the hopping activity in the clear plastic cylinder (8 cm in diameter, 25 cm deep). Activity was recorded for 60 min using a video camera, and hopping activity was scored. Atomoxetine was dissolved in saline and administered at a dose of 0.5 mg/kg of body weight, whereas haloperidol was dissolved in a drop of 1% ascorbic acid and administered at 0.1 mg/kg of body weight.
PPI of the Acoustic Startle Response and Restraint Stress
Tests were conducted using a SR-LAB system (SR-LAB, San Diego Instruments, San Diego, CA) that consisted of a Plexiglas tube (105 mm length; 38 mm inner diameter) in a sound-attenuated chamber. Each animal was tested individually. The testing session started with a 5-min acclimatization to the startle chamber in the presence of 65 db background white noise, which was provided by a small electric fan for ventilation. A duration of 40 ms of white noise was used as the startle stimulus for all trial types. The startle response was recorded for 160 ms (measuring the response every 1 ms) starting with the onset of the prepulse stimulus. A piezoelectric accelerometer affixed to the animal enclosure frame was used to detect and transduce the motion that resulted from the response of the animal. To measure PPI, mice were presented with a 73-, 76-, 79-, 82-, or 85-db prepulse followed by a 120-db pulse (40 ms in length) 100 ms later. In the restraint stress test, mice were subjected to a cylindrical mouse restrainer (10.0 cm length; 2.5 cm diameter) for 3 h, and then PPI was tested as described above. PPI was calculated as a percentage score: PPI (%) = (1 × [(startle response for pulse with prepulse)/(startle response for pulse alone)]) × 100.
High-performance Liquid Chromatography (HPLC) Assessment of the Brain Content of Monoamines and Metabolites
Mice were euthanized by decapitation, after which the striatum, nucleus accumbens, or prefrontal cortex were immediately dissected and frozen in liquid nitrogen. Wet tissue samples were weighed and stored at −80 °C until subsequent analysis. The tissue was homogenized in 0.1 m HClO4 containing 100 ng/ml of 3,4-dihydroxybenzylamine as an internal standard. Homogenates were centrifuged for 10 min at 10,000 × g. Supernatants were filtered through a 0.22-μm filter. Concentration of dopamine, serotonin, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindoleacetic acid were determined by HPLC with electrochemical detection, as described previously (26).
Statistical Analysis
Data collected were analyzed using GraphPad Prism and Microsoft Excel software. Data are mean ± S.E. A Student's t test was used to compare the two groups. For comparison between groups, a two-way analysis of variance was used.
RESULTS
α1,6-Fucosylation of glycoprotein, which is widely distributed in mammalian tissues, is altered under pathological conditions, such as hepatocellular carcinoma and liver cirrhosis (27, 28). We previously reported that Fut8 plays an important role in lung tissues. Fut8 deficiency leads to emphysema-like changes due to dysregulation of the TGF-β1 receptor and signaling (4). However, although expression of Fut8 is the highest in brain tissue, the function of Fut8 in the brain remains unknown.
Loss of α1,6-Fucosylation in Fut8−/− Brain Tissues
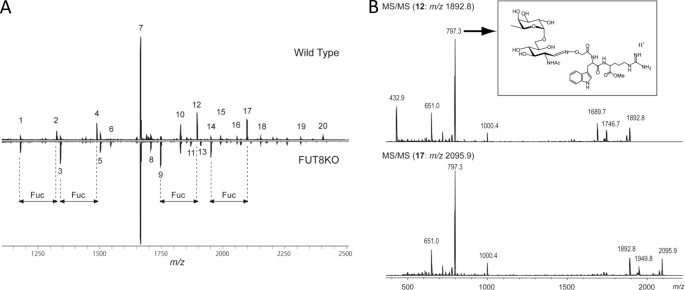
The reaction products of Fut8, i.e. α1,6-fucosylated N-glycans (Fig. 1A), were analyzed using AAL lectin, which preferentially recognizes α1,6-fucose (29). To examine whether the products of α1,6-fucosylation are widely expressed in brain tissues, we analyzed the following brain regions: olfactory bulb, prefrontal cortex, striatum, amygdale, hippocampus, thalamus, hypothalamus, midbrain, and cerebellum. As shown in Fig. 1B, several regions that diffuse at ∼50–250 kDa were strongly stained with AAL in all tissues from Fut8+/+ mice, but the reactive staining was lost in tissues from Fut8−/− mice. The intensities of AAL lectin staining for whole brain tissue from Fut8+/− mice were at the middle levels between Fut8+/+ and Fut8−/− mice (Fig. 1C). Furthermore, N-linked glycans on proteins in brain were compared between Fut8−/− mice and the corresponding wild type. N-Linked glycans were released from each part by peptide N-glycosidase F (Roche Diagnostics), and derivatized by BlotGlyco (Sumitomo Bakelite, Co., Tokyo, Japan) according to the manufacturer's protocol. The derivatized glycans were analyzed by MALDI-TOF mass spectrometer. The glycan analysis of hippocampus was shown in Fig. 2 and Table 1 as an example. Although four signals 2, 4, 12, and 17, which correspond to monofucosylated glycans completely disappeared, signals 1, 3, 9, and 14, which are non-fucosylated glycans, significantly increased in the spectrum of the Fut8−/− tissues. Fucosylated position of glycans 2 and 4 could be unambiguously estimated by both the biosynthetic pathway and the glycan compositions. To confirm the fucosylated position in glycans 12 and 17, tandem MS spectra were acquired. As shown in Fig. 2B, the signal at m/z 797.4, which should be generated from glycans containing a core fucose was observed as the most intense signal in both the spectra. This indicated that a fucose attached to the reducing end GlcNAc in these glycans. Similar results were obtained from glycan analysis of other parts of the brain (data not shown). Thus we concluded that glycans with a core fucose disappeared and the glycans without a core fucose increased in the brain of Fut8−/− mice.
FIGURE 2.
Glycan analyses using mass spectrometry. A, MALDI-TOF MS spectra of glycans in hippocampus from 3-month-old mice. Upper, wild type; lower, Fut8 knock-out. The details of signals were summarized in Table 1. B, MS/MS spectra of mono-fucosylated glycans 12 and 17. In both spectra, the most intense product ion (m/z 797.3) was assigned as the structure in the rectangle.
TABLE 1.
Assignment of the signals in the MS spectra as shown in Fig. 2A
| Signala | Observed | Calculated | Composition |
|---|---|---|---|
| m/z | |||
| 1 | 1178.47 | 1178.49 | Hex2HexNAc2 |
| 2 | 1324.49 | 1324.55 | Hex2HexNAc2dHex1 |
| 3 | 1340.48 | 1340.54 | Hex3HexNAc2 |
| 4 | 1486.54 | 1486.60 | Hex3HexNAc2dHex1 |
| 5 | 1502.53 | 1502.59 | Hex4HexNAc2 |
| 6 | 1543.55 | 1543.62 | Hex3HexNAc3 |
| 7 | 1664.58 | 1664.65 | Hex5HexNAc2 |
| 8 | 1705.61 | 1705.67 | Hex4HexNAc3 |
| 9 | 1746.63 | 1746.70 | Hex3HexNAc4 |
| 10 | 1826.63 | 1826.70 | Hex6HexNAc2 |
| 11 | 1867.66 | 1867.73 | Hex5HexNAc3 |
| 12 | 1892.68 | 1892.76 | Hex3HexNAc4dHex1 |
| 13 | 1908.67 | 1908.75 | Hex4HexNAc4 |
| 14 | 1949.69 | 1949.78 | Hex3HexNAc5 |
| 15 | 1988.66 | 1988.75 | Hex7HexNAc2 |
| 16 | 2054.71 | 2054.81 | Hex4HexNAc4dHex1 |
| 17 | 2095.74 | 2095.84 | Hex3HexNAc5dHex1 |
| 18 | 2150.69 | 2150.81 | Hex8HexNAc2 |
| 19 | 2312.74 | 2312.86 | Hex9HexNAc2 |
| 20 | 2403.82 | 2403.95 | Hex4HexNAc5dHex2 |
a All glycans were observed as [M + H]+.
Novelty-induced Hyperactivity
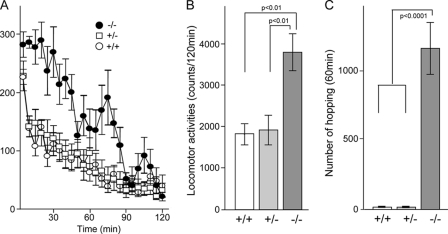
Novelty-induced hyperactivity has been viewed as a preclinical model of the positive symptoms of schizophrenia and psychomotor agitation in particular (30–32). We used an Animex auto MK-110 (Fig. 3, A and B) to measure hopping in a cylinder (Fig. 3C) to assess locomotor response to a novel environment in Fut8−/− mice. When mice were placed into the Animex cage, all mice showed higher novelty-induced activity in the first 5 min (Fig. 3A). The Fut8+/+ and Fut8+/− mice calmed down within 10 min. The decrease in activity presumably reflected habituation to the test procedure and novel environment. However, Fut8−/− mice still continued to exhibit an increase in activity for 90 min. The total duration of locomotor activity of Fut8−/− mice during 120 min was significantly greater than that of the Fut8+/+ mice (Fig. 3B). Hopping movements were recorded for 90 min using a video camera, and then the number of hopping movements was determined. Compared with Fut8+/+ and Fut8+/− mice, Fut8−/− mice showed a significant increase in novelty-induced hopping (Fig. 3C).
FIGURE 3.
Novelty-induced hyperactivity. A and B, locomotor activity was measured using an Animex Auto MK-110 as described under “Experimental Procedures.” The time spent in motion was assessed at 5-min intervals. Data are mean ± S.E.; n = 10 for Fut8+/+, Fut8+/−, and Fut8−/− mice (2.5–3.5 months old). C, hopping behavior of Fut8−/− mice in the cylinder. Hopping movements were observed for 60 min for each animal. Data are mean ± S.E.; n = 20 for Fut8+/+, Fut8+/−, and Fut8−/− mice (2.5–3.5 months old).
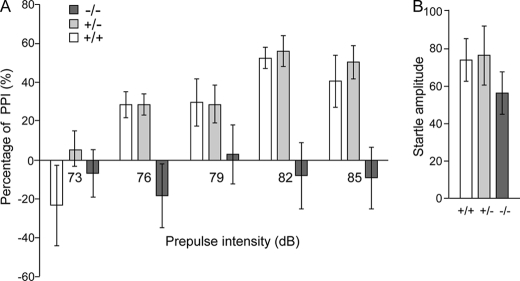
Impaired PPI
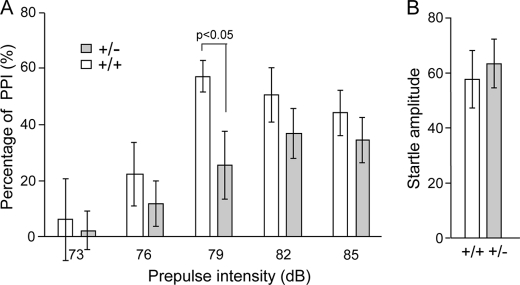
The PPI of startle is a cross-species measure that measures the ability of a non-startling “pre-stimulus” to inhibit the response to a startling stimulus. There have been numerous reports of PPI deficits in schizophrenia patients (33–35), as well as in several mouse models (36–38). Thus, the PPI deficits associated with schizophrenia are the most thoroughly characterized and the most widely replicated. In the test, a brief, low intensity acoustic stimulus (the prepulse) inhibits the startle reflex caused by a loud stimulus. Fut8−/− mice show complete deficits in PPI compared with the Fut8+/+ and Fut8+/− mice (Fig. 4A). Startle amplitudes were not significantly different between Fut8−/− mice and Fut8+/+ littermates (Fig. 4B). These results suggest that PPI is impaired in Fut8−/− mice. However, we also recognized the possibility that the novelty-induced hyperactivity described above interfered with the measurement of PPI. Exposure to various mild stressors has been shown to activate dopamine-containing neuronal systems (39). Therefore, to examine the importance of Fut8 on PPI, we focused on the PPI in Fut8+/− mice after exposure to restraint stress in a cylindrical mouse restrainer for 3 h. Of particular interest, Fut8+/− mice showed a significant PPI deficiency when presented with a 79-db prepulse, and a decrease in PPI following pre-pulses at all of other intensities compared with Fut8+/+ littermates tested under the same conditions (Fig. 5). These results, taken together, strongly suggest that Fut8 might play a causal role in the disorder.
FIGURE 4.
Impaired PPI in Fut8−/− mice. Comparison of prepulse inhibition of startle reflex (A) and startle amplitude (B) among Fut8+/+, Fut8+/−, and Fut8−/− mice. Data are mean ± S.E.; n = 10 for each group of mice (2.5–3.5 months old).
FIGURE 5.
Restraint stress-induced PPI deficiency in Fut8+/− mice. A, the effects of restraint stress (3 h) on prepulse inhibition of the startle reflex were assayed under different prepulse intensities (A) or without a prepulse (B). Data are mean ± S.E.; n = 10 for each group of mice (2.5–3.5 months old).
Alterations in Social Interaction and Short-term Memory
Deficits in social interaction are hallmarks of schizophrenia (40, 41). We adapted a novel environment task, such that pairs of mice with the same genotype and same sex were allowed to explore a novel environment for 10 min. As compared with Fut8+/+ and Fut8+/− mice, Fut8−/− mice spent significantly less time engaging in active contact, such as sniffing or following each other, during the 10-min social interaction test (Fig. 6A). To examine whether short-term memory was altered in Fut8−/− mice, we performed a Y-maze task. As Fig. 6B shows, spontaneous alterations were reduced in Fut8−/− mice compared with Fut8+/+ and Fut8+/− mice. It is worth noting that there were no significant differences in the number of total arm entries among these mice.
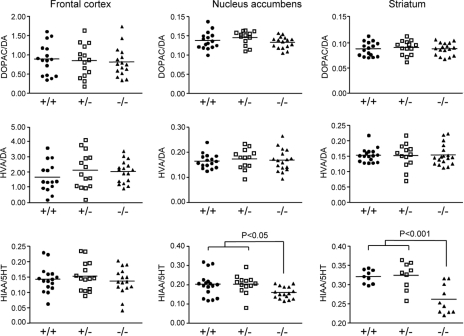
Analyses of Monoamine Turnover in Fut8−/− Mice
Because locomotor hyperactivity is commonly associated with increased dopaminergic tone (42), the effect of Fut8 deficiency on monoamine turnover was determined by HPLC. Levels of both dopamine and dopamine metabolites were not significantly changed in the striatum of Fut8−/− mice compared with Fut8+/+ or Fut8+/− mice. Similar results were obtained for other brain tissues, including the frontal cortex and nucleus accumbens (Fig. 7, upper and middle panels), indicating that there were no significant differences in either dopamine synthesis or dopamine metabolites between Fut8−/− and Fut8+/+ or Fut8+/− mice. Intriguingly, levels of 5-hydroxytryptamine (5-HT; serotonin) metabolites were significantly decreased in the striatum and nucleus accumbens of the Fut8−/− mice compared with those in the Fut8+/+ and Fut8+/− mice (lower panel). Based on these results, we hypothesize that the balance between dopaminergic and serotonergic signaling might be disrupted in the Fut8−/− mice.
FIGURE 7.
Comparison of tissue contents of monoamines and their metabolites in the cortex, nucleus accumbens, and striatum of Fut8+/+, Fut8+/−, and Fut8−/− mice. The turnover rates of monoamine neurotransmitters in the cortex, nucleus accumbens, and striatum of Fut8+/+, Fut8+/−, and Fut8−/− mice (3.5–4.5 months old) were assayed by HPLC as described under “Experimental Procedures.” Data are mean ± S.E.; n = 15. DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; 5-HIAA, 5-hydroxyindolacetic acid.
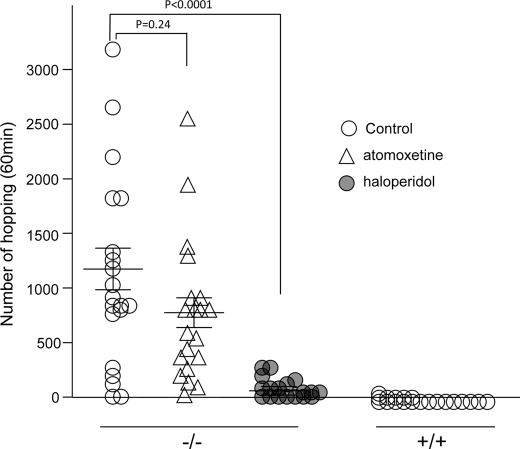
Amelioration of Hopping Hyperactivity and Monoamine Turnovers by Treatment with Haloperidol
The typical antipsychotic drug haloperidol, which is a dopamine D2 receptor antagonist (43), was tested for its ability to attenuate the hyperactive behavior of Fut8−/− mice. Haloperidol (0.1 mg/kg) effectively reduced the hyperactivity of Fut8−/− mice to the level observed in wild-type mice (Fig. 8). This dose did not impair locomotor activity in Fut8+/+ mice, and Fut8−/− mice did not show spontaneous catalepsy. On the other hand, treatment with atomoxetine, which is a non-stimulant approved for the treatment of attention deficit hyperactivity disorder (44), did not significantly inhibit the hyperactivity of Fut8−/− mice, also suggesting that Fut8 might play a role in schizophrenia-like disorders.
FIGURE 8.
Effects of haloperidol and atomoxetine on hopping behavior of Fut8−/− mice. The mice (2.5–3.5 months old) were injected intraperitoneally with haloperidol at a dose of 0.1 mg/kg, atomoxetine at 0.5 mg/kg, or with the same volume of 1% ascorbic acid as a control. Twenty min following injection, the mice were placed in the cylinder for measurement of hopping movements during a 60-min test period. Data are mean ± S.E.; n = 20.
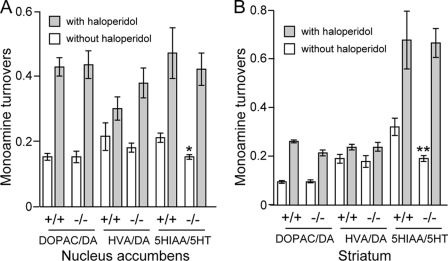
Furthermore, haloperidol might normalize the balance between the dopamine and serotonin systems in the Fut8−/− mice as shown in Fig. 9. It is known that dopamine metabolism is evaluated in several regions such as striatum and nucleus accumbens by treatment with haloperidol, whereas the administration usually does not affect serotonin metabolism (45–47). However, the present study showed that haloperidol significantly increased not only dopamine metabolism but also the serotonin metabolism (Fig. 9). In fact, haloperidol has also been found to have a lower affinity with 5-HA2A, a serotonin receptor (48). Thus, the discrepancy could explain why loss of α1,6-fucosylation on 5-HA2A may result in its conformation change, which in turn enhances its binding with haloperidol. In fact, deletion of the α1,6-fucose from the IgG1 molecule reportedly enhances binding of the IgG Fc domain to an Fc receptor (FcgRIIIa) on natural killer cells, and then increases antibody-dependent cellular cytotoxicity activity (6, 49). Taken together, the altered balance between dopamine and serotonin systems could be causally related to the abnormal behavior of the Fut8−/− mice.
FIGURE 9.
Effects of haloperidol on monoamine turnovers in the nucleus accumbens and striatum of Fut8+/+ and Fut8−/− mice. The mice (2.5–3.0 months old) were injected intraperitoneally with haloperidol at a dose of 0.1 mg/kg or with the same volume of 1% ascorbic acid as a control. One hour following injection, the turnover rates of monoamine neurotransmitters in the nucleus accumbens and striatum were assayed by HPLC as described under “Experimental Procedures.” Data are mean ± S.E.; n = 8. *, p < 0.05; **, p < 0.001 versus Fut8+/+ mice. DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; 5-HIAA, 5-hydroxyindolacetic acid.
DISCUSSION
We performed a comprehensive behavioral analysis of Fut8-deficient mice. These mice exhibited increased locomotor activity, decreased social interaction, and impaired alertness, as assessed by the PPI test. Furthermore, the enhanced locomotor activity of Fut8−/− mice was significantly reduced to normal levels of Fut8+/+ mice by treatment with the typical antipsychotic drug haloperidol, which is a dopamine D2 receptor antagonist. These results suggest that α1,6-fucosylation plays an important role in brain function, and regulates psychotic conditions.
The biological basis for the psychotic signs and symptoms associated with schizophrenia is not known. Although hyperlocomotion and stereotypy are commonly considered behaviors of increased dopaminergic tone (42, 50), studies of dopamine release and metabolism have generated conflicting results, and several groups have not observed alterations in dopamine transmission (51–53). In Fut8−/− mice, dopamine turnover was unchanged, whereas 5-HT turnover was decreased. In fact, the importance of 5-HT in controlling locomotor activity has been demonstrated in a study with 5-HT1B-receptor knock-out mice, which show hyperlocomotor activity and aggressive behavior (54). A similar phenomenon has also been observed in PACAP knock-out mice, which show slightly decreased 5-HIAA levels in the cortex and striatum with abnormal jumping behavior and other psychomotor behavioral abnormalities (30). Recently, it has been reported that 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on the locomotor activity induced by the hallucinogen 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (55). Thus, the balance between the dopamine and 5-HT systems seems to be important for normal motor activity, and alterations in any of the parameters that control this delicate homeostasis might underlie hyperactive states. This may explain, at least in part, the locomotor hyperactivity of Fut8−/− mice. On the other hand, it also has been postulated that glutamatergic and dopaminergic neurons share common postsynaptic targets in the striatum with opposing effects, i.e. glutamate exerts an excitatory influence on these target neurons, whereas dopamine exerts an inhibitory influence (56). This hypothesis has been confirmed in N-methyl-d-aspartate receptor knock-out mice, which show hyperlocomotor activity, which is attenuated by both typical and atypical antipsychotics (52). In fact, the glutamate receptor agonist LY404039 has demonstrated clinical efficacy for the treatment of schizophrenia, suggesting a hypoglutamate state during the illness (57).
Previously, we reported that α1,6-fucosylation affects many target proteins, such as α3β1 integrin, EGF, and TGF-β1 receptors (7, 8). α1,6-Fucosylation is required for α3β1 integrin-mediated cell adhesion and cell signaling. In brain tissue, reelin may arrest neuronal migration and promote normal cortical lamination by binding α3β1 integrin and modulating integrin-mediated cellular adhesion (58). On the other hand, in mouse brain tissues, most complex type N-glycans contain α1,6-fucose, as shown in the present study (Fig. 2) as well as in the glycan profiling data base from the Consortium for Functional Glycomics (CFG). It is well accepted that multiple genes of each with a small effect, rather than a single causative gene, act in concert with nongenetic factors to increase the risk of mental disorders (59). Therefore we believe that there may be many other functional molecules, such as dopamine and serotonin transporters or receptors, which also depend on the α1,6-fucosylation for their biological functions. Thus, the lack of α1,6-fucosylation of each target molecule has a small effect, but collectively, the absence of α1,6-fucosylation has a significant effect on behavior. A detailed characterization of the functions of α1,6-fucosylation on those receptors is required in further studies. In fact, the modification of sialic acid residues facilitates homo- and hetero-oligomerization of the serotonin transporter to regulate the serotonergic system through uptake and clearance of serotonin released from the nerve terminal (60). γ-Aminobutyric acid transporters also require N-glycosylation for correct folding, protein stability, and intracellular trafficking (61). Moreover, knock-out of GnT-V, which forms β1,6-GlcNAc branching in N-glycans, altered social interactions and increased depression-like behavior in the mouse model (62). On the other hand, in humans, reduced fucosylation of glycoproteins in CDG-llc patients, due to a defective Golgi GDP-fucose transporter, results in growth and mental retardation, which is similar to the results for Fut8−/− mice in the present study. Importantly, some CDG-llc patients experience partial alleviation of symptoms following oral fucose therapy (16, 63). It should be noted that, in the present study, Fut8+/− mice exposed to restraint stress showed a PPI deficiency, whereas Fut8+/+ littermates did not. It was unclear why only the 79-db prepulse intensity (not higher or lower values) induced a significantly different response. Perhaps a prepulse at lower values is not enough to get full PPI, whereas a prepulse at higher values may get signals that are too strong for PPI to distinguish between wild-type mice and Fut8-deficient mice. Thus, the prepulse intensity at 79-db in the present study could be an appropriate stimulus. In fact, the phenomenon was also observed in a dominant-negative DISC1 transgenic mouse, for which the decreased PPI only occurred at 74-db, one of four prepulse intensities (64). Taken together, these results strongly suggest that alterations in α1,6-fucosylation might underlie certain cases of human schizophrenia.
In summary, the Fut8-deficient mice described here support a model in which decreased Fut8 expression leads to extensive behavioral changes that are characteristics of schizophrenia. Continued studies of these mice will undoubtedly lead to a better understanding of the role of α1,6-fucosylation of many receptors related to this disease. Thus, the results of the present study provide new insight into the underlying mechanism responsible for schizophrenia and related disorders.
Acknowledgment
We thank Azusa Tomioka for technical assistance.
This work was supported by Scientific Research Grants-in-aid 21370059 (to J. G.) and 22590071 (to T. F.) from the Japan Society for the Promotion of Science and the Academic Frontier Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
- Fut8
- α1,6-fucosyltransferase
- PPI
- prepulse inhibition
- TGF-β1
- transforming growth factor-β1
- GnT-III
- N-acetylglucosaminyltransferase III
- GnT-V
- N-acetylglucosaminyltransferase V
- 5-HT
- 5-hydroxytryptamine (serotonin)
- CDG
- congenital disorder of glycosylation
- AAL
- aleuria aurantia lectin.
REFERENCES
- 1. Wilson J. R., Williams D., Schachter H. (1976) Biochem. Biophys. Res. Commun. 72, 909–916 [DOI] [PubMed] [Google Scholar]
- 2. Uozumi N., Yanagidani S., Miyoshi E., Ihara Y., Sakuma T., Gao C. X., Teshima T., Fujii S., Shiba T., Taniguchi N. (1996) J. Biol. Chem. 271, 27810–27817 [DOI] [PubMed] [Google Scholar]
- 3. Shimizu H., Ochiai K., Ikenaka K., Mikoshiba K., Hase S. (1993) J. Biochem. 114, 334–338 [DOI] [PubMed] [Google Scholar]
- 4. Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., Uozumi N., Ihara S., Lee S. H., Ikeda Y., Yamaguchi Y., Aze Y., Tomiyama Y., Fujii J., Suzuki K., Kondo A., Shapiro S. D., Lopez-Otin C., Kuwaki T., Okabe M., Honke K., Taniguchi N. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 15791–15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X., Fukuda T., Li W., Gao C. X., Kondo A., Matsumoto A., Miyoshi E., Taniguchi N., Gu J. (2009) J. Biochem. 145, 643–651 [DOI] [PubMed] [Google Scholar]
- 6. Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., Hanai N., Shitara K. (2003) J. Biol. Chem. 278, 3466–3473 [DOI] [PubMed] [Google Scholar]
- 7. Wang X., Gu J., Ihara H., Miyoshi E., Honke K., Taniguchi N. (2006) J. Biol. Chem. 281, 2572–2577 [DOI] [PubMed] [Google Scholar]
- 8. Zhao Y., Itoh S., Wang X., Isaji T., Miyoshi E., Kariya Y., Miyazaki K., Kawasaki N., Taniguchi N., Gu J. (2006) J. Biol. Chem. 281, 38343–38350 [DOI] [PubMed] [Google Scholar]
- 9. Ioffe E., Stanley P. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 728–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaeken J., Schachter H., Carchon H., De Cock P., Coddeville B., Spik G. (1994) Arch. Dis. Child. 71, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y., Tan J., Sutton-Smith M., Ditto D., Panico M., Campbell R. M., Varki N. M., Long J. M., Jaeken J., Levinson S. R., Wynshaw-Boris A., Morris H. R., Le D., Dell A., Schachter H., Marth J. D. (2001) Glycobiology 11, 1051–1070 [DOI] [PubMed] [Google Scholar]
- 12. Ye Z., Marth J. D. (2004) Glycobiology 14, 547–558 [DOI] [PubMed] [Google Scholar]
- 13. Lübke T., Marquardt T., Etzioni A., Hartmann E., von Figura K., Körner C. (2001) Nat. Genet. 28, 73–76 [DOI] [PubMed] [Google Scholar]
- 14. Lübke T., Marquardt T., von Figura K., Körner C. (1999) J. Biol. Chem. 274, 25986–25989 [DOI] [PubMed] [Google Scholar]
- 15. Marquardt T., Brune T., Lühn K., Zimmer K. P., Körner C., Fabritz L., van der Werft N., Vormoor J., Freeze H. H., Louwen F., Biermann B., Harms E., von Figura K., Vestweber D., Koch H. G. (1999) J. Pediatr. 134, 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marquardt T., Lühn K., Srikrishna G., Freeze H. H., Harms E., Vestweber D. (1999) Blood 94, 3976–3985 [PubMed] [Google Scholar]
- 17. Ross C. A., Margolis R. L., Reading S. A., Pletnikov M., Coyle J. T. (2006) Neuron 52, 139–153 [DOI] [PubMed] [Google Scholar]
- 18. Freedman R. (2003) N. Engl. J. Med. 349, 1738–1749 [DOI] [PubMed] [Google Scholar]
- 19. Lewis D. A., Sweet R. A. (2009) J. Clin. Invest. 119, 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caspi A., Moffitt T. E., Cannon M., McClay J., Murray R., Harrington H., Taylor A., Arseneault L., Williams B., Braithwaite A., Poulton R., Craig I. W. (2005) Biol. Psychiatry 57, 1117–1127 [DOI] [PubMed] [Google Scholar]
- 21. Nicodemus K. K., Marenco S., Batten A. J., Vakkalanka R., Egan M. F., Straub R. E., Weinberger D. R. (2008) Mol. Psychiatry 13, 873–877 [DOI] [PubMed] [Google Scholar]
- 22. Ishii A., Ikeda T., Hitoshi S., Fujimoto I., Torii T., Sakuma K., Nakakita S., Hase S., Ikenaka K. (2007) Glycobiology 17, 261–276 [DOI] [PubMed] [Google Scholar]
- 23. Nakakita S., Natsuka S., Okamoto J., Ikenaka K., Hase S. (2005) J. Biochem. 138, 277–283 [DOI] [PubMed] [Google Scholar]
- 24. Furukawa J., Shinohara Y., Kuramoto H., Miura Y., Shimaoka H., Kurogochi M., Nakano M., Nishimura S. (2008) Anal. Chem. 80, 1094–1101 [DOI] [PubMed] [Google Scholar]
- 25. Nakagawasai O., Oba A., Sato A., Arai Y., Mitazaki S., Onogi H., Wakui K., Niijima F., Tan-No K., Tadano T. (2009) Life Sci. 84, 512–516 [DOI] [PubMed] [Google Scholar]
- 26. Nakazawa T., Yasuda T., Ueda J., Ohsawa K. (2003) Biol. Pharm. Bull. 26, 474–480 [DOI] [PubMed] [Google Scholar]
- 27. Miyoshi E., Noda K., Yamaguchi Y., Inoue S., Ikeda Y., Wang W., Ko J. H., Uozumi N., Li W., Taniguchi N. (1999) Biochim. Biophys. Acta 1473, 9–20 [DOI] [PubMed] [Google Scholar]
- 28. Noda K., Miyoshi E., Gu J., Gao C. X., Nakahara S., Kitada T., Honke K., Suzuki K., Yoshihara H., Yoshikawa K., Kawano K., Tonetti M., Kasahara A., Hori M., Hayashi N., Taniguchi N. (2003) Cancer Res. 63, 6282–6289 [PubMed] [Google Scholar]
- 29. Matsumura K., Higashida K., Ishida H., Hata Y., Yamamoto K., Shigeta M., Mizuno-Horikawa Y., Wang X., Miyoshi E., Gu J., Taniguchi N. (2007) J. Biol. Chem. 282, 15700–15708 [DOI] [PubMed] [Google Scholar]
- 30. Hashimoto H., Shintani N., Tanaka K., Mori W., Hirose M., Matsuda T., Sakaue M., Miyazaki J., Niwa H., Tashiro F., Yamamoto K., Koga K., Tomimoto S., Kunugi A., Suetake S., Baba A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13355–13360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pletnikov M. V., Ayhan Y., Xu Y., Nikolskaia O., Ovanesov M., Huang H., Mori S., Moran T. H., Ross C. A. (2008) Mol. Psychiatry 13, 173–186, 115 [DOI] [PubMed] [Google Scholar]
- 32. van den Buuse M., Wischhof L., Lee R. X., Martin S., Karl T. (2009) Int. J. Neuropsychopharmacol. 12, 1383–1393 [DOI] [PubMed] [Google Scholar]
- 33. Braff D. L., Geyer M. A., Swerdlow N. R. (2001) Psychopharmacology 156, 234–258 [DOI] [PubMed] [Google Scholar]
- 34. Kumari V., Fannon D., Geyer M. A., Premkumar P., Antonova E., Simmons A., Kuipers E. (2008) Cortex 44, 1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ludewig K., Geyer M. A., Vollenweider F. X. (2003) Biol. Psychiatry 54, 121–128 [DOI] [PubMed] [Google Scholar]
- 36. Miyakawa T., Leiter L. M., Gerber D. J., Gainetdinov R. R., Sotnikova T. D., Zeng H., Caron M. G., Tonegawa S. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8987–8992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moy S. S., Perez A., Koller B. H., Duncan G. E. (2006) Brain Res. 1089, 186–194 [DOI] [PubMed] [Google Scholar]
- 38. Stefansson H., Sigurdsson E., Steinthorsdottir V., Bjornsdottir S., Sigmundsson T., Ghosh S., Brynjolfsson J., Gunnarsdottir S., Ivarsson O., Chou T. T., Hjaltason O., Birgisdottir B., Jonsson H., Gudnadottir V. G., Gudmundsdottir E., Bjornsson A., Ingvarsson B., Ingason A., Sigfusson S., Hardardottir H., Harvey R. P., Lai D., Zhou M., Brunner D., Mutel V., Gonzalo A., Lemke G., Sainz J., Johannesson G., Andresson T., Gudbjartsson D., Manolescu A., Frigge M. L., Gurney M. E., Kong A., Gulcher J. R., Petursson H., Stefansson K. (2002) Am. J. Hum. Genet. 71, 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carlson J. N., Fitzgerald L. W., Keller R. W., Jr., Glick S. D. (1991) Brain Res. 550, 313–318 [DOI] [PubMed] [Google Scholar]
- 40. Kopelowicz A., Liberman R. P., Zarate R. (2006) Schizophr. Bull. 32, Suppl. 1, S12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Piskulic D., Olver J. S., Norman T. R., Maruff P. (2007) Psychiatry Res. 150, 111–121 [DOI] [PubMed] [Google Scholar]
- 42. Gainetdinov R. R., Wetsel W. C., Jones S. R., Levin E. D., Jaber M., Caron M. G. (1999) Science 283, 397–401 [DOI] [PubMed] [Google Scholar]
- 43. Kapur S., Barsoum S. C., Seeman P. (2000) Neuropsychopharmacology 23, 595–598 [DOI] [PubMed] [Google Scholar]
- 44. Garnock-Jones K. P., Keating G. M. (2009) Paediatr. Drugs 11, 203–226 [DOI] [PubMed] [Google Scholar]
- 45. Karoum F., Egan M. F. (1992) Br. J. Pharmacol. 105, 703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kitaichi K., Yamada K., Hasegawa T., Furukawa H., Nabeshima T. (1994) Jpn. J. Pharmacol. 66, 181–189 [DOI] [PubMed] [Google Scholar]
- 47. Sitges M., Guarneros A. (1998) Eur. Neuropsychopharmacol. 8, 105–111 [DOI] [PubMed] [Google Scholar]
- 48. Roth B. L., Sheffler D. J., Kroeze W. K. (2004) Nat. Rev. Drug Discov. 3, 353–359 [DOI] [PubMed] [Google Scholar]
- 49. Shields R. L., Lai J., Keck R., O'Connell L. Y., Hong K., Meng Y. G., Weikert S. H., Presta L. G. (2002) J. Biol. Chem. 277, 26733–26740 [DOI] [PubMed] [Google Scholar]
- 50. Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. (1996) Nature 379, 606–612 [DOI] [PubMed] [Google Scholar]
- 51. Druhan J. P., Rajabi H., Stewart J. (1996) Synapse 24, 135–146 [DOI] [PubMed] [Google Scholar]
- 52. Mohn A. R., Gainetdinov R. R., Caron M. G., Koller B. H. (1999) Cell 98, 427–436 [DOI] [PubMed] [Google Scholar]
- 53. Pierce R. C., Meil W. M., Kalivas P. W. (1997) Psychopharmacology 133, 188–195 [DOI] [PubMed] [Google Scholar]
- 54. Saudou F., Amara D. A., Dierich A., LeMeur M., Ramboz S., Segu L., Buhot M. C., Hen R. (1994) Science 265, 1875–1878 [DOI] [PubMed] [Google Scholar]
- 55. Halberstadt A. L., van der Heijden I., Ruderman M. A., Risbrough V. B., Gingrich J. A., Geyer M. A., Powell S. B. (2009) Neuropsychopharmacology 34, 1958–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seeman P. (2009) J. Psychiatry Neurosci. 34, 143–149 [PMC free article] [PubMed] [Google Scholar]
- 57. Patil S. T., Zhang L., Martenyi F., Lowe S. L., Jackson K. A., Andreev B. V., Avedisova A. S., Bardenstein L. M., Gurovich I. Y., Morozova M. A., Mosolov S. N., Neznanov N. G., Reznik A. M., Smulevich A. B., Tochilov V. A., Johnson B. G., Monn J. A., Schoepp D. D. (2007) Nat. Med. 13, 1102–1107 [DOI] [PubMed] [Google Scholar]
- 58. Dulabon L., Olson E. C., Taglienti M. G., Eisenhuth S., McGrath B., Walsh C. A., Kreidberg J. A., Anton E. S. (2000) Neuron 27, 33–44 [DOI] [PubMed] [Google Scholar]
- 59. Hyman S. E. (2000) Bull. W. H. O. 78, 455–463 [PMC free article] [PubMed] [Google Scholar]
- 60. Ozaslan D., Wang S., Ahmed B. A., Kocabas A. M., McCastlain J. C., Bene A., Kilic F. (2003) J. Biol. Chem. 278, 43991–44000 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61. Cai G., Salonikidis P. S., Fei J., Schwarz W., Schülein R., Reutter W., Fan H. (2005) FEBS J. 272, 1625–1638 [DOI] [PubMed] [Google Scholar]
- 62. Soleimani L., Roder J. C., Dennis J. W., Lipina T. (2008) Genes Brain Behav. 7, 334–343 [DOI] [PubMed] [Google Scholar]
- 63. Hidalgo A., Ma S., Peired A. J., Weiss L. A., Cunningham-Rundles C., Frenette P. S. (2003) Blood 101, 1705–1712 [DOI] [PubMed] [Google Scholar]
- 64. Hikida T., Jaaro-Peled H., Seshadri S., Oishi K., Hookway C., Kong S., Wu D., Xue R., Andradé M., Tankou S., Mori S., Gallagher M., Ishizuka K., Pletnikov M., Kida S., Sawa A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14501–14506 [DOI] [PMC free article] [PubMed] [Google Scholar]