Abstract
Mammary cancer stem cells (MaCSCs) have been identified as a rare population of cells capable of self-renewal to drive mammary tumorigenesis and metastasis. Nevertheless, relatively little is known about the intracellular signaling pathways regulating self-renewal and metastatic activities of MaCSCs in vivo. Using a recently developed breast cancer mouse model with focal adhesion kinase (FAK) deletion in mammary tumor cells (MFCKO-MT mice), here we present evidence suggesting a compensatory function of Pyk2, a FAK-related kinase, in the regulation of MaCSCs and metastasis in these mice. Increased expression of Pyk2 was found selectively in pulmonary metastatic nodules of MFCKO-MT mice, and its inhibition significantly reduced mammary tumor development and metastasis in these mice. Consistent with the idea of metastasis driven by MaCSCs, we detected selective up-regulation of Pyk2 in MaCSCs, but not bulk mammary tumor cells, of primary tumors developed in MFCKO-MT mice. We further showed that inhibition of Pyk2 in FAK-null MaCSCs significantly decreased their tumorsphere formation and migration in vitro as well as self-renewal, tumorigenicity, and metastatic activity in vivo. Last, we identified PI3K/Akt signaling as a major mediator of FAK regulation of MaCSCs as well as a target for the compensatory function of Pyk2 in FAK-null MaCSCs. Together, these results further advance our understanding of FAK and its related tyrosine kinase Pyk2 in regulation of MaCSCs in breast cancer and suggest that pharmaceutically targeting these kinases may hold promise as a novel treatment for the disease by targeting and eradicating MaCSCs.
Keywords: Breast Cancer, Mouse, Stem Cell, Tumor Metastases, Tyrosine Protein Kinase (Tyrosine Kinase)
Introduction
Cancer stem cells (CSCs)2 are proposed to play important roles in the initiation and progression of breast and several other cancers recently (1–4). According to the CSC concept, although the conventional therapies could destroy the bulk of the tumor mass, even a small amount of residual CSCs could lead to recurrence of the cancer due to their stem cell-like ability for self-renewal and differentiation (2). Several recent studies also suggested that CSCs, such as mammary cancer stem cells (MaCSCs), are more resistant to conventional cancer therapies compared with the bulk of cells in the tumor mass (5–9), which could further decrease the effectiveness of conventional cancer treatment strategies. Therefore, the characterization of key signaling molecules and pathways that regulate MaCSCs will be important for understanding mammary carcinogenesis and development of more effective therapeutic strategies for breast cancer through targeting MaCSCs.
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase that plays a major role in mediating signal transduction by integrins as well as growth factor receptors in the regulation of cell adhesion, migration, survival, proliferation, and differentiation in a variety of cells (10–14). Upon its activation by integrin-mediated cell adhesion or other stimuli, FAK undergoes autophosphorylation at Tyr397 to create a binding site for several Src homology 2 domain-containing molecules, including Src (15, 16) and the p85 subunit of PI3K (17, 18). FAK association and activation of PI3K through autophosphorylated Tyr397 leads to increased production of 3′-phosphorylated phospholipid (17), which activates Akt to promote cell survival by regulating several other proteins (19–23). Consistent with its role in regulation of multiple signaling pathways, FAK has been implicated in the development of breast cancer and other malignancies (24, 25). Recent studies by several groups, including us, showed that ablation of FAK suppressed mammary tumorigenesis and progression in mouse models of breast cancer, demonstrating directly a causal role of FAK in promoting breast cancer in vivo (26–29). Moreover, using a combination of well characterized markers and tumorsphere formation assays (30–34) as well as transplantation experiments, our previous study revealed that inactivation of FAK reduced MaCSCs in primary tumors developed in FAK conditional KO mice, decreased their self-renewal and migration in vitro, and compromised their tumorigenicity and maintenance in vivo (28). These results suggest that deletion of FAK may suppress mammary tumorigenesis and progression by affecting MaCSCs. Nevertheless, it is not clear which of the multiple FAK downstream signaling pathways mediate its regulation of MaCSCs. The potential mechanisms that allow mammary tumorigenesis and metastasis in FAK conditional KO mice, albeit at a reduced level, are not well understood.
Pyk2 is the other member of the FAK subfamily cytoplasmic tyrosine kinases that shares significant sequence homology and a similar structural organization as FAK with a tyrosine kinase domain flanked by non-catalytic domains at both N and C termini. It has been shown to interact with a number of other proteins that also bind to FAK, such as Src (35, 36), paxillin (37, 38), and p130cas (39). Despite the similar structures of these two kinases, there are a number of differences between Pyk2 and FAK. In most adherent cells, FAK, but not Pyk2, is colocalized with integrins in focal contacts (40, 41). Although FAK activation is mainly controlled by integrin-mediated cell adhesion in most cells, tyrosine phosphorylation of Pyk2 is stimulated by integrins in some cells (42, 43) but appears to be independent of integrins in other cells (40, 44). These studies suggested that Pyk2 and FAK may promote both overlapping and distinct signaling pathways, depending on cellular context. Furthermore, although FAK is widely expressed in various tissues, Pyk2 is expressed mainly in the central nervous system and in cells derived from hematopoietic lineages. Interestingly, several reports showed that deletion of FAK can lead to increased expression of endogenous Pyk2 to compensate for FAK functions in embryonic fibroblasts and adult endothelial cells (36, 45), although such compensatory increase of Pyk2 expression was not found upon FAK deletion in several other cells (28, 46, 47).
In the present study, we investigated the potential compensatory function of Pyk2 in the regulation of MaCSCs and their contributions to breast cancer in mouse models. These studies revealed a selective up-regulation of Pyk2 in metastatic nodules and MaCSCs in FAK conditional KO mice, which could compensate for FAK functions in promoting MaCSC self-renewal and mammary tumor metastasis in these mice. They also identified the PI3K/Akt signaling pathway as a major mediator of FAK regulation of MaCSCs and also contribute to Pyk2 compensatory functions in FAK-null MaCSCs. These results provide significant insights into the mechanisms by which FAK and its related tyrosine kinase Pyk2 regulate MaCSCs, implicating that targeting these kinases may serve as an effective therapeutic strategy to eliminate MaCSCs in breast cancer.
EXPERIMENTAL PROCEDURES
Mice
Ctrl-MT and MFCKO-MT mice (i.e. Pos CNT and Target mice, respectively) have been described previously (28). Mice were palpated weekly after weaning, and the size of tumors was measured with a caliper and recorded. In some experiments, FAK inhibitor PF-573,228 and FAK/Pyk2 inhibitor PF-562,271 (provided by Pfizer, Groton, CT) (48,49) were administered orally at a dose of 5 mg/kg daily from the age of 3 weeks. Mice were housed and handled according to local, state, and federal regulations, and all experimental procedures were carried out according to the guidelines of the Institutional Animal Care and Use Committee at the University of Michigan.
Immunohistochemical Staining of Lung Sections and Determination of Lung Metastasis
In some experiments, lungs were harvested from mice and subjected to immunohistochemical staining as described previously (28). The following antibodies were used: FAK (1:200, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)) and Pyk2 (1:200; made by our laboratory as described previously (40)). In other experiments, lung sections were harvested and stained by H&E, and the micrometastatic nodules per section were quantified as described previously (28).
Protein Extraction, SDS-PAGE, and Western Blotting
Lung metastatic nodules were harvested during necropsy and snap frozen in liquid nitrogen and ground with a mortar and pestle, and proteins were extracted using a triple detergent buffer, as described previously (28). Preparation of lysates from MaCSCs or mammary tumor cells and analysis of protein samples in cell lysates and tissue extracts by SDS-PAGE and Western blotting were as described previously (28). The following antibodies were used: FAK (1:500; Santa Cruz Biotechnology, Inc.), phospho-FAK (Tyr397) (1:500; Santa Cruz Biotechnology, Inc.), PyMT (1:500, Santa Cruz Biotechnology, Inc.), Pyk2 (1:500; made by our laboratory as described previously (40)), phospho-Pyk2 (Tyr402) (1:500; Cell Signaling), p130cas (1:500; Santa Cruz Biotechnology, Inc.), phospho-p130cas (Tyr165) (1:500; Cell Signaling), AKT (1:500; Cell Signaling), phospho-AKT (Ser473) (1:500; Cell Signaling), and vinculin (1:1000, Sigma).
Preparation of Mammary Tumor Cells and Fractionation of MaCSCs by FACS
Mammary tumor cells from Ctrl-MT and MFCKO-MT mice were prepared as described previously (28). They were then subjected to FACS to obtain Lin−ALDH+ or Lin−CD24+CD29+CD61+ fractions, which are enriched in MaCSCs, as described previously (28). In some experiments, growth of tumor cells was analyzed directly in medium containing 10% or 0.5% FBS in the presence or absence of inhibitors.
Tumorsphere Formation, Tumor Cell Transplantation Experiments, and Boyden Chamber Assay
Tumorsphere formation, tumor cell transplantation, and a cell migration assay using a modified Boyden chamber (Neuro Probe) were performed as described previously (28). FAK inhibitor PF-573,228 (50 nm) or FAK/Pyk2 inhibitor PF-562,271 (100 nm) was added into the medium at the beginning of tumorsphere culture or Boyden chamber migration assays in some experiments. In other experiments, Akt inhibitor triciribine (50 μm) was included in tumorsphere cultures. For transplant experiments, the inhibitors were administered orally at a dose of 5 mg/kg daily starting at the day of transplantation in some experiments.
Experimental Metastasis Assay Using Tail Vein Injection
Tail vein injection experiments to assess metastatic activity of mammary tumor cells were performed as described previously (50, 51). Briefly, 5 × 104 ALDH+ mammary tumor cells in 100 μl of PBS were injected into the tail vein of 8-week-old female nude mice. Six weeks after injection, the mice were euthanized, and lungs were removed for determination of the number of micrometastatic nodules as described above. In some experiments, FAK inhibitor PF-573,228 or FAK/Pyk2 inhibitor PF-562,271 was administered orally at a dose of 5 mg/kg daily starting at the day of transplantation.
Recombinant Viruses and Infection of Tumor Cells
The psPAX2, pMD2G, and pGIPZ lentiviral vectors encoding shRNA targeting Pyk2 or Akt1 (Pyk2 shRNA-1, catalogue no. RMM4431-98724086; Pyk2 shRNA-2, catalogue no. RMM4431-99204280; Akt shRNA-1, catalogue no. RMM4431-98741567; Akt shRNA-2, catalogue no. RMM4431-101257652; Akt shRNA-3, catalogue no. RMM4431-101257988) were purchased through the University of Michigan shRNA core facility (Open Biosystems, Huntsville, AL). HEK293 cells were transfected with these three vectors, and the media were collected 12 h after transfection. After centrifugation and filtration, the supernatant was used to infect MaCSCs from mammary tumor cells. The recombinant adenoviruses encoding FAK, Y397F, D395A, and P712A/P715A mutants or GFP control were generated using the Adeasy-1 system (Stratagene) as described previously (52). MaCSCs from mammary tumor cells were infected by these adenoviruses at a multiplicity of infection of 100 at suspension condition for 2 h.
Statistical Analysis
Statistical significance was evaluated by a paired t test, using p < 0.05 as indicative of statistical significance. Kaplan-Meier tumor-free survival data were compared using the log rank test.
RESULTS
Increased Expression of Pyk2 in Lung Metastatic Nodules and Its Promotion of Breast Cancer Metastasis in MFCKO-MT Mice
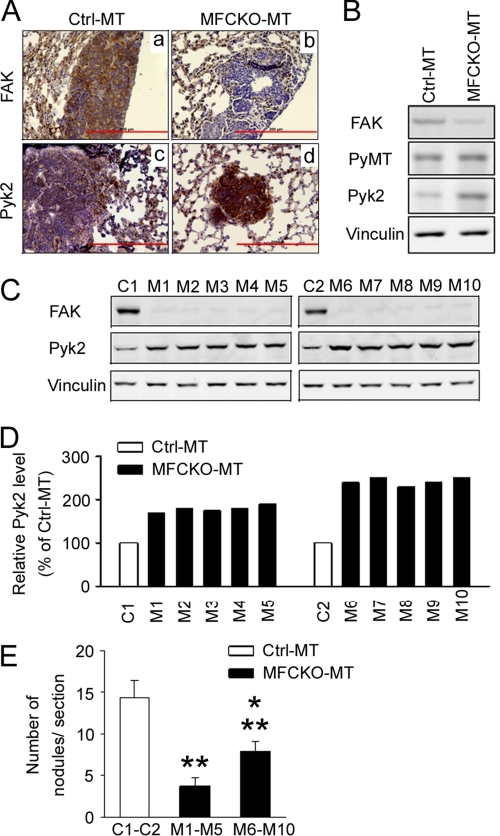
Our previous studies indicated that, similar to a number of other cells, including embryonic endothelial cells (47), deletion of FAK in mammary tumor cells did not change Pyk2 expression in the primary tumors (28). We wondered, however, whether Pyk2 could be up-regulated in metastatic nodules in FAK-null MFCKO-MT mice, where it could compensate for FAK functions to promote the residual breast tumor metastasis in the absence of FAK. To test such a possibility, lung sections from Ctrl-MT and MFCKO-MT mice were examined using immunohistochemistry (Fig. 1A). As expected, FAK expression was detected in metastatic nodules of Ctrl-MT mice but not in those of MFCKO-MT mice (top). Interestingly, an elevated Pyk2 expression was found in metastatic nodules of MFCKO-MT mice compared with those in Ctrl-MT mice (bottom). Consistent with these results, analysis of lysates prepared from the pools of metastatic nodules by Western blotting also revealed increased expression of Pyk2 in FAK-null metastasized tumor cells of MFCKO-MT mice compared with those from Ctrl-MT mice (Fig. 1B). These results suggested that, although it is not up-regulated in the primary tumors as observed previously (28), endogenous Pyk2 expression increases after the deletion of FAK in the lung metastatic nodules in vivo.
FIGURE 1.
Analysis of increased expression of Pyk2 in metastatic nodules of MFCKO-MT mice. A, lung sections containing metastatic nodules in Ctrl-MT (a and c) and MFCKO-MT (b and d) mice were analyzed by immunohistochemistry using antibodies against FAK (a and b) and Pyk2 (c and d). Scale bars, 200 μm. B, lysates prepared from metastatic nodules of four Ctrl-MT or MFCKO-MT mice were pooled and analyzed by immunoblotting using antibodies against various proteins as indicated. C, lysates from metastatic nodules of individual Ctrl-MT (C1 and C2) and MFCKO-MT (M1–M10) mice were analyzed by immunoblotting using antibodies against various proteins as indicated (top). D, the intensity of the Pyk2 bands in C was quantified by densitometry, and the relative intensities (normalized to C1 and C2) are shown. E, lung sections from Ctrl-MT and MFCKO-MT mice were prepared at 7 weeks after the appearance of primary tumors. They were stained with H&E, and the metastatic nodules were quantified under a microscope. The mean number of lung nodules per section for Ctrl-MT mice and the pool of M1–M5 and M6–M10 mice (with 1.5–2-fold and >2-fold Pyk2 expression as compared with Ctrl-MT mice, respectively; see D) is shown. **, p < 0.01 compared with the value from Ctrl-MT mice. *, p < 0.05 compared with the value from the pool of M1–M5 mice. Error bars, S.E.
Although a role for FAK in metastasis is supported by a wealth of data (10–14), it is not clear whether the compensatory expression of Pyk2 upon FAK deletion might also play a role in promoting metastasis in MFCKO-MT mice. To explore a possible function of the increased Pyk2 in metastasis, we first looked for possible correlations between the level of increased Pyk2 expression and the extent of lung metastasis in individual mice. Lysates were collected from metastatic nodules of individual MFCKO-MT mice and analyzed for increased Pyk2 expression in comparison with nodules from Ctrl-MT mice (Fig. 1, C and D). We then determined the number of lung metastatic nodules for each group of mice. We found that MFCKO-MT mice with more increased Pyk2 expression (greater than 2 times control) had more metastatic nodules compared with those with a moderate increase in Pyk2 expression (1.5–2 times control), although both groups showed a reduced number of nodules compared with Ctrl-MT mice (Fig. 1E). These data suggested that the increased Pyk2 expression after FAK deletion might play a role in the promotion of breast cancer metastasis.
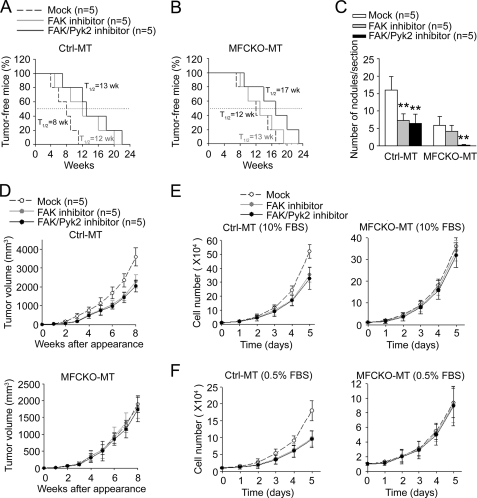
To determine directly the role of Pyk2 in promoting metastasis, Ctrl-MT and MFCKO-MT mice were treated with FAK inhibitor PF-573,228 (IC50 for FAK = 4 nm; IC50 for Pyk2 > 1 μm) or FAK/Pyk2 inhibitor PF-562,271 (IC50 for FAK = 1.5 nm; IC50 for Pyk2 = 13 nm) (48, 49) and monitored for mammary tumor formation, growth, and metastasis, as described under “Experimental Procedures.” Inhibition of FAK by PF-573,228 (50 nm) or both FAK and Pyk2 by PF-562,271 (100 nm) led to a significant suppression of mammary tumorigenesis (Fig. 2A) as well as lung metastasis (Fig. 2C) of Ctrl-MT mice, which is consistent with a number of previous studies showing a role for FAK in breast cancer. In contrast, treatment of MFCKO-MT mice with PF-573,228 did not affect the initiation of mammary tumors in these mice (Fig. 2B), as would be expected due to the absence of FAK in mammary epithelial cells of these mice (28). Interestingly, treatment of the mice with PF-562,271 reduced mammary tumorigenesis in MFCKO-MT mice. Moreover, PF-562,271, but not PF-573,228, treatments abolished lung metastasis in MFCKO-MT mice (Fig. 2C). Together, these data provide strong support for a role of the increased expression of Pyk2 in lung metastasis as well as initiation of mammary tumors after deletion of FAK.
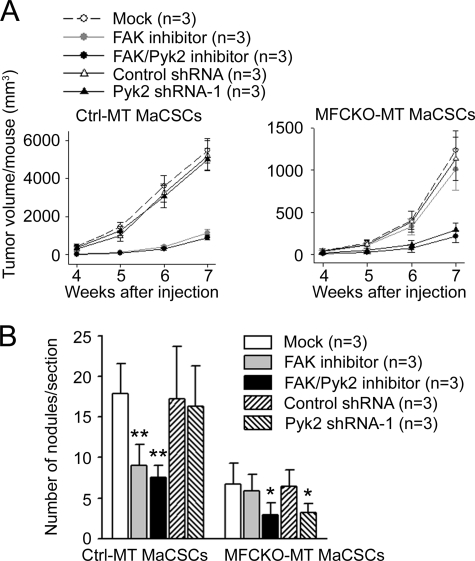
FIGURE 2.
Suppression of mammary tumorigenesis and metastasis by Pyk2 inhibition in MFCKO-MT mice. A and B, Kaplan-Meier analysis of mammary tumor development in Ctrl-MT (A) and MFCKO-MT (B) mice that had been treated with various inhibitors as indicated (n = 5/group). For Ctrl-MT mice, FAK inhibitor or FAK/Pyk2 inhibitor versus mock, p < 0.01 by the log rank test. For MFCKO-MT mice, FAK/Pyk2 inhibitor versus FAK inhibitor or mock, p < 0.01 by the log rank test. C, lung sections from Ctrl-MT and MFCKO-MT mice that had been treated with various inhibitors as indicated (n = 5/group) were prepared at 7 weeks after the appearance of primary tumors. They were stained with H&E, and the metastatic nodules were quantified under a microscope. The mean number of lung nodules per section for each group is shown. In the Ctrl-MT group, **, p < 0.01 compared with mock-treated mice. In the MFCKO-MT group, **, p < 0.01 compared with mock- or FAK inhibitor-treated mice. D, mean cumulative mammary tumor volume ± S.E. (error bars) per mouse at different times after primary tumor appearance for Ctrl-MT (upper) and MFCKO-MT (lower) mice that had been treated with various inhibitors as indicated (n = 5/group). E and F, mammary tumor cells were isolated from Ctrl-MT (left) or MFCKO-MT (right) mice and cultured in normal (E) or low (F) serum conditions with or without the inhibitors, as indicated. For Ctrl-MT mice in D–F, FAK inhibitor or FAK/Pyk2 inhibitor versus mock, p < 0.05 by the two-way analysis of variance.
Previous studies showed that deletion of FAK reduced tumor growth, which may contribute to the decreased lung metastasis in MFCKO-MT mice (28). To evaluate whether the compensatory expression of Pyk2 may also promote metastasis by increased tumor growth, we next measured the mammary tumor growth at weekly intervals after the initial detection of primary tumors with or without inhibitor treatments. Consistent with the previous observation of a role for FAK in tumor growth, treatments of Ctrl-MT mice with PF-573,228 or PF-562,271 significantly reduced mammary tumor growth in these mice (Fig. 2D, upper) compared with the mock treatment. Surprisingly, no difference was found for mammary tumor growth in MFCKO-MT mice with or without the treatments of both inhibitors (Fig. 2D, lower), suggesting that Pyk2 is not required for tumor growth. We then further analyzed the potential role of Pyk2 in the growth of isolated tumor cells from Ctrl-MT and MFCKO-MT mice in vitro. As shown in Fig. 2E, treatment of tumor cells from Ctrl-MT mice by either PF-573,228 or PF-562,271 significantly reduced the growth of these cells under normal culture conditions (left), which is consistent with a role of FAK in mammary tumor growth observed in vivo (28) (see Fig. 2D). In contrast, inhibition of compensatory Pyk2 in tumor cells from MFCKO-MT mice did not affect the growth of these cells (right). Similar results were obtained when the tumor cells were analyzed under low serum conditions (Fig. 2F). Together, these results suggest that the promotion of lung metastasis by compensatory expression of Pyk2 in MFCKO-MT mice was not due to any effect on the growth of primary tumors.
FAK Inactivation Leads to Selective Increased Expression of Pyk2 in MaCSCs
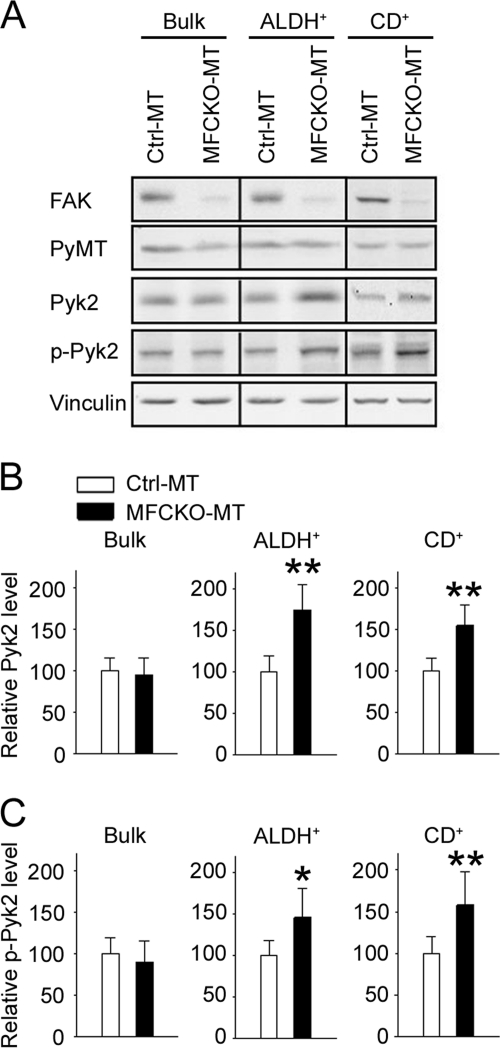
Because our previous data showed no significant change of Pyk2 expression in primary tumors in MFCKO-MT mice, the above observation of increased Pyk2 in lung metastatic nodules and its promotion of metastasis suggested the following two possibilities of compensatory expression of Pyk2 after FAK deletion. It is possible that the Pyk2 up-regulation occurs in a stochastic manner among FAK-null tumor cells but that only those with the increased expression will possess metastatic capability and therefore be detected in the nodules after spreading into lungs. Alternatively, the up-regulation of Pyk2 is not random, and only a select subset of primary tumor cells acquires increased Pyk2 expression and therefore is capable of metastasizing to the lungs. For the latter scenario, it is particularly interesting to consider the possibility of the selective Pyk2 up-regulation in MaCSCs in mammary tumors because they have been suggested to be responsible for metastasis in breast and other cancers (1–4). To explore these possibilities, we first examined the expression of Pyk2 in MaCSCs isolated using ALDH+ as a marker. Mammary tumor cells were labeled by the ALDEFLUOR kit, sorted for ALDH+ cells as described previously (28), and subjected to Western blotting analysis. Although its expression levels were similar in the bulk of primary tumor cells from Ctrl-MT and MFCKO-MT mice, as observed previously (28), Pyk2 showed a significantly increased expression in ALDH+ cells from MFCKO-MT mice compared with those from Ctrl-MT mice (Fig. 3, A and B). Real-time RT-PCR analysis of ALDH+ cells from MFCKO-MT and Ctrl-MT mice showed no significant difference in Pyk2 mRNA levels between these cells, suggesting that the increased Pyk2 expression is likely at the post-transcriptional level (supplemental Fig. S1). Similar results were obtained when MaCSCs were isolated using CD24+CD29+CD61+ as markers (Fig. 3, A and C). Moreover, the activated Pyk2, as detected by anti-PY402, is also increased in FAK-null ALDH+ and CD24+CD29+CD61+ subpopulations of tumor cells compared with control cells with FAK in the same subpopulations (Fig. 3A). These results suggest that Pyk2 is selectively up-regulated in MaCSCs upon deletion of FAK.
FIGURE 3.
Increased Pyk2 expression and phosphorylation in MaCSCs from MFCKO-MT mice. A, lysates from unsorted (Bulk), ALDH+, or CD24+CD29+CD61+ (CD+) subpopulations of tumor cells from Ctrl-MT or MFCKO-MT mice were subjected to immunoblotting using various antibodies as indicated. B and C, the intensity of the Pyk2 (B) and phospho-Pyk2 (p-Pyk2) (C) bands was quantified from three independent experiments by densitometry. The mean ± S.E. (error bars) of relative intensity (normalized to cells from Ctrl-MT mice) is shown. *, p < 0.05; **, p < 0.01 compared with cells from Ctrl-MT mice.
Dependence of FAK-null MaCSC Activities on the Compensatory Pyk2 Expression
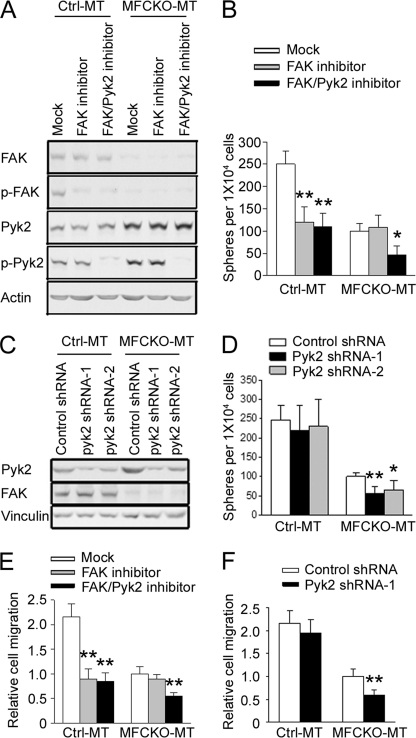
To determine whether the increased Pyk2 expression and phosphorylation can compensate for FAK function in promotion of MaCSCs activities, we treated MaCSCs from Ctrl-MT and MFCKO-MT mice with FAK inhibitor PF-573,228 and FAK/Pyk2 inhibitor PF-562,271 and first examined the effects on their self-renewal using a tumorsphere formation assay in vitro. Fig. 4A shows that PF-573,228 inhibited FAK activation but had no effect on Pyk2, and PF-562,271 abolished both FAK and Pyk2 phosphorylation in both MaCSCs from Ctrl-MT and MFCKO-MT mice, as observed previously in other cells (45, 48, 49). Treatments of MaCSCs from Ctrl-MT mice with PF-573,228 and PF-562,271 both inhibited their tumorsphere formation (Fig. 4B), which is consistent with the previous observation of a role for FAK in self-renewal of MaCSCs (28). As expected, inhibition of FAK by PF-573,228 did not affect tumorsphere formation of MaCSCs from MFCKO-MT mice due to the absence of FAK in these cells. In contrast, treatment of these cells by FAK/Pyk2 inhibitor PF-562,271 significantly reduced their tumorsphere formation, suggesting that the increased Pyk2 expression plays a role in the self-renewal activity of FAK-null MaCSCs in vitro. Consistent with these results using chemical inhibitors, knockdown of Pyk2 expression by two different shRNAs also reduced tumorsphere formation of MaCSCs from MFCKO-MT mice but not those from Ctrl-MT mice (Fig. 4, C and D).
FIGURE 4.
Inhibition of Pyk2 leads to decreased self-renewal and migration of MaCSCs from MFCKO-MT mice in vitro. A, B, and E, freshly isolated tumor cells of Ctrl-MT or MFCKO-MT mice were sorted for ALDH+ cells and analyzed for tyrosine phosphorylation of FAK and Pyk2 (A), tumorsphere formation under suspension culture conditions (B), or by Boyden chamber assays for their migratory capacity (E) in the presence of FAK inhibitor, FAK/Pyk2 inhibitor, or mock treatment. Results are generated from three independent experiments. Values in E are normalized to that of MFCKO-MT cells with mock treatment. In Ctrl-MT group, **, p < 0.01 compared with mock-treated cells. In the MFCKO-MT group, *, p < 0.05; **, p < 0.01 compared with mock- or FAK inhibitor-treated cells. C, D, and F, ALDH+ cells from Ctrl-MT or MFCKO-MT mice were infected with recombinant lentiviruses expressing Pyk2 shRNA-1 (black bars), Pyk2 shRNA-2 (gray bars), or a control shRNA (open bars), as indicated. Aliquots of lysates were analyzed by immunoblotting (C), and cells were subjected to analysis for tumorsphere formation (D) or migration (F). Results are from three independent experiments. Values in F are normalized to that of MFCKO-MT cells with control shRNA treatment. **, p < 0.01; *, p < 0.05 compared with cells infected with lentiviruses expressing control shRNA. Error bars, S.E.
Previous studies also suggested FAK-dependent higher migratory activity of MaCSCs compared with bulk tumor cells (28). Therefore, we measured the effect of inhibition of Pyk2 on the migration of MaCSCs from Ctrl-MT and MFCKO-MT mice by using PF-573,228 and PF-562,271 inhibitors as well as Pyk2 shRNA. As shown in Fig. 4E, treatment with FAK inhibitor PF-573,228 reduced migration of Ctrl-MT MaCSCs but not MFCKO-MT MaCSCs, as expected. On the other hand, FAK/Pyk2 inhibitor PF-562,271 reduced migration of both cells, suggesting a role for the selectively increased Pyk2 in MFCKO-MT MaCSCs. This is further confirmed by the decreased migration of MFCKO-MT MaCSCs, but not Ctrl-MT MaCSCs, after knockdown of Pyk2 expression by shRNA (Fig. 4F).
To complement the above in vitro assays, we performed transplantation experiments to assess the role of increased Pyk2 in FAK-null MaCSCs in self-renewal and tumorigenicity in vivo. Consistent with a role of FAK in self-renewal and tumorigenicity of MaCSCs, treatments of the mice with PF-573,228 and PF-562,271 both reduced tumorigenicity of MaCSCs from Ctrl-MT mice in transplantation assays (Table 1 and Fig. 5A). In contrast, treatment of the recipient mice with FAK/Pyk2 inhibitor PF-562,271, but not FAK inhibitor PF-573,228, decreased tumorigenicity of FAK-null MaCSCs isolated from MFCKO-MT mice. Moreover, knockdown of Pyk2 by shRNA also reduced tumorigenicity of MaCSCs from MFCKO-MT mice without effect on those from Ctrl-MT mice (Table 1 and Fig. 5A). These results suggested a functional role of up-regulated Pyk2 in promoting self-renewal of MaCSCs after FAK deletion.
TABLE 1.
Effects of kinase inhibitors or Pyk2 knockdown on the tumorigenicity of MaCSCs
ALDH+ cells were sorted from mammary tumor cells of Ctrl-MT or MFCKO-MT mice and then injected into the inguinal mammary fat pads of 8-week-old nude mice (5,000 ALDH+ cells from Ctrl-MT or 50,000 ALDH+ cells from MFCKO-MT per site). The recipient mice were treated with vehicle alone (mock), FAK inhibitor PF-573,228, or FAK/Pyk2 inhibitor PF-562,271. In other experiments, ALDH+ cells from Ctrl-MT or MFCKO-MT mice were infected with lentiviruses encoding control shRNA or Pyk2 shRNA-1. The infected cells were then injected into the inguinal mammary fat pads of 8-week-old nude mice. The sites with tumors grown 4 weeks postinjection were counted for each condition.
| Treatment | Tumors/Injections |
||||
|---|---|---|---|---|---|
| Mock | FAK inhibitor | FAK/Pyk2 inhibitor | Control shRNA | Pyk2 shRNA-1 | |
| Ctrl-MT | 6/6 | 3/6 | 3/6 | 5/6 | 5/6 |
| MFCKO-MT | 4/6 | 4/6 | 1/6 | 3/6 | 1/6 |
FIGURE 5.
Decreased tumorigenicity and metastatic activity of FAK-null MaCSCs upon Pyk2 inhibition in vivo. A, ALDH+ tumor cells from Crtl-MT (left) or MFCKO-MT (right) mice were transplanted into the inguinal mammary fat pads of nude mice (n = 6 sites of three mice for each group). The mice were treated with FAK inhibitor, FAK/Pyk2 inhibitor, or mock, and tumor growth was monitored at weekly intervals after transplantation. In some experiments, the cells were infected with recombinant lentiviruses expressing Pyk2 shRNA-1 or a control shRNA, as indicated. Left, **, p < 0.01, recipient mice with FAK inhibitor or FAK/Pyk2 inhibitor treatment versus recipient mice with mock treatment. Right, **, p < 0.01, recipient mice with FAK/Pyk2 inhibitor treatment or with transplant of cells infected by recombinant lentiviruses expressing Pyk2 shRNA-1 versus recipient mice with mock treatment. B, ALDH+ tumor cells from Crtl-MT or MFCKO-MT mice were injected into the tail vein of nude mice as described under “Experimental Procedures.” The recipient mice were treated with FAK inhibitor, FAK/Pyk2 inhibitor, or mock. In some experiments, the cells were infected with recombinant lentiviruses expressing Pyk2 shRNA-1 or a control shRNA, as indicated. Lung sections were prepared from recipient mice at 6 weeks after injection and stained with H&E, and the micrometastatic nodules were quantified under a microscope. The mean number of lung nodules per section of the recipient mice is shown. In the Ctrl-MT MaCSC group, **, p < 0.01 compared with nodule number in mock-treated mice. In the MFCKO-MT MaCSC group, *, p < 0.05, compared with nodule number in mock- or FAK inhibitor-treated mice. Error bars, S.E.
The above data suggesting a role for Pyk2 in compensating FAK functions in self-renewal as well as migration upon FAK deletion provide strong support for the hypothesis that selective up-regulation of Pyk2 in MaCSCs is responsible for the metastasis of mammary tumors in the absence of FAK. To evaluate this possibility directly, we injected Ctrl-MT or MFCKO-MT MaCSCs into the tail vein of recipient nude mice and monitored for metastasis to the lungs with or without treatments of the mice with PF-573,228 or PF-562,271. As shown in Fig. 5B, both inhibitors reduced the number of metastatic nodules generated by Ctrl-MT MaCSCs to a comparable level, consistent with the idea that metastatic activity of these cells is primarily dependent on FAK. On the other hand, only FAK/Pyk2 inhibitor PF-562,271 and not FAK inhibitor PF-573,228 decreased the metastatic activity of MFCKO-MT MaCSCs. Similarly, Pyk2 knockdown reduced metastatic activity of MFCKO-MT MaCSCs but not Ctrl-MT MaCSCs. These results provide further support for a compensatory role of Pyk2 in these FAK-null MaCSCs in vivo.
Role of PI3K/Akt Pathway in the Self-renewal and Tumorigenesis of MaCSCs
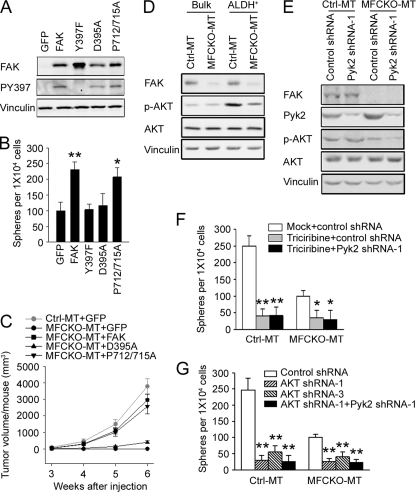
To explore potential downstream targets that mediate regulation of MaCSCs by FAK and the compensatory expression of Pyk2, we analyzed several FAK mutants in FAK-null MaCSCs using a tumorsphere formation assay in vitro. The FAK-null MaCSCs isolated from MFCKO-MT mice were infected by the adenoviruses encoding wild type FAK or various mutants. Western blotting analysis showed comparable expression levels of FAK and its mutants in the infected cells (Fig. 6A). Moreover, the Y397F mutant was not autophosphorylated at Tyr397, whereas FAK and the other mutants were phosphorylated to similar levels at this site, as expected. As shown in Fig. 6B, re-expression of FAK, but not Y397F mutant, rescued the deficiency of FAK-null MaCSCs in tumorsphere formation assays. Interestingly, the D395A mutant, which selectively disrupts FAK interaction with PI3K but not Src, as shown previously (53), also failed to restore tumorsphere formation of these cells. In contrast, the P712A/P715A mutant (lacking binding to p130cas) (54) fully rescued tumorsphere formation of these cells to a level comparable with re-expression of FAK. These results suggested that FAK interaction with p85 of PI3K, but not p130cas, is necessary for FAK promotion of MaCSC self-renewal in vitro.
FIGURE 6.
Role of PI3K/Akt pathway in the regulation of MaCSCs by FAK and compensatory Pyk2 expression. A, ALDH+ tumor cells were obtained from freshly isolated tumor cells of MFCKO-MT mice by FACS as described under “Experimental Procedures.” They were then infected with adenoviruses encoding FAK, its mutants, or GFP alone as a control, as indicated. Lysates from these cells were analyzed for FAK expression, Tyr397 phosphorylation, or vinculin as a loading control. B, ALDH+ tumor cells infected with various adenoviruses as described in A were analyzed for tumorsphere formation under suspension culture conditions. Results are generated from three independent experiments. *, p < 0.05; **, p < 0.01 in comparison with values for cells infected with Ad-GFP. C, ALDH+ tumor cells from Crtl-MT or MFCKO-MT mice were infected with various adenoviruses as indicated. They were transplanted into the fat pads of nude mice (n = 6 for each group), and tumor growth was monitored at weekly intervals after injection. **, p < 0.01, MaCSCs expressing GFP or D395A from MFCKO-MT mice versus MaCSCs expressing GFP from Ctrl-MT mice. D, lysates from unsorted (bulk) or ALDH+ subpopulations of tumor cells from Ctrl-MT or MFCKO-MT mice were subjected to immunoblotting using various antibodies as indicated. E, ALDH+ cells from Ctrl-MT or MFCKO-MT mice were infected with recombinant lentiviruses expressing Pyk2 shRNA-1 or a control shRNA, as indicated. Aliquots of lysates were analyzed by immunoblotting using various antibodies as indicated. F, ALDH+ cells from Ctrl-MT or MFCKO-MT mice were infected with recombinant lentiviruses expressing Pyk2 shRNA-1 or a control shRNA, as indicated. They were then analyzed for tumorsphere formation in the presence of Akt inhibitor triciribine (50 μm) or mock treatment. G, ALDH+ cells from Ctrl-MT or MFCKO-MT mice were infected with recombinant lentiviruses expressing various shRNAs as indicated. The cells were subjected to analysis for tumorsphere formation. Results are from three independent experiments. *, p < 0.05; **, p < 0.01 compared with mock-treated cells infected with lentiviruses expressing control shRNA. Error bars, S.E.
To complement the above in vitro analysis and further investigate the role of FAK signaling through PI3K in regulating MaCSC self-renewal and tumorigenicity in vivo, FAK-null MaCSCs from MFCKO-MT mice with re-expression of FAK, D395A, or P712A/P715A mutant were transplanted into mammary fat pads of recipient nude mice and were monitored for tumorigenesis in comparison with MaCSCs from Ctrl-MT or MFCKO-MT mice infected with adenovirus encoding GFP as controls. At 3 weeks after transplantation, MaCSCs from Ctrl-MT mice generated mammary tumors in the majority of recipient mice, whereas FAK-null MaCSCs from MFCKO-MT mice did not display tumorigenicity in any recipients (Table 2), which is consistent with our previous observations (28). As expected, re-expression of FAK in MaCSCs from MFCKO-MT mice significantly rescued their tumorigenicity. Furthermore, re-expression of P712A/P715A mutant, but not D395A mutant, also rescued their tumorigenicity to a level comparable with re-expression of FAK. Tumor growth in the recipient mice was then followed over the next 3 weeks, providing further support for the lack of ability of D395A mutant to rescue defective FAK-null MaCSCs in contrast to FAK or P712A/P715A mutant (Fig. 6C). Together, these results suggested that FAK interaction with and activation of the PI3K signaling pathway play a major role in mediating its regulation of MaCSC self-renewal and tumorigenicity.
TABLE 2.
Tumorigenicity of FAK-null MaCSCs after re-expression of FAK or mutants
ALDH+ cells were sorted from mammary tumor cells of Ctrl-MT or MFCKO-MT mice and then infected with recombinant adenoviruses encoding FAK, its mutants, or GFP alone as a control, as indicated. The infected cells were injected into the inguinal mammary fat pads of 8-week-old nude mice at 5,000 cells/site. Sites with tumors grown 3 weeks postinjection were counted for each condition. ND, not detected.
| Adenoviruses encoding | Tumors/Injections |
|||
|---|---|---|---|---|
| GFP | FAK | D395A | P712/715A | |
| Ctrl-MT | 5/6 | ND | ND | ND |
| MFCKO-MT | 0/6 | 4/6 | 1/6 | 4/6 |
Previous studies have shown that Akt, a major downstream kinase of PI3K signaling, plays a role in the regulation of MaCSCs by a number of cell surface receptors (55, 56). We therefore examined Akt activation status in mammary tumor cells and MaCSCs from Ctrl-MT and MFCKO-MT mice (Fig. 6D). Although Akt phosphorylation was low and at comparable levels in bulk tumor cells from both Ctrl-MT and MFCKO-MT mice, the activated Akt was found to be significantly increased in MaCSCs compared with the bulk of tumor cells from Ctrl-MT mice. Moreover, this MaCSC-associated Akt activation was decreased upon FAK deletion in MFCKO-MT mice compared with that in Ctrl-MT mice. These results suggested a role for Akt in mediating FAK regulation of MaCSCs.
We noted an increased Akt phosphorylation level in MaCSCs compared with the bulk of tumor cells from MFCKO-MT mice also (Fig. 6D), although FAK is absent in these cells. This raised the possibility that increased Akt phosphorylation may also mediate regulation of MaCSCs by the compensatory expression of Pyk2 in these cells. To further test such a possibility, we examined the effect of Pyk2 knockdown on Akt phosphorylation in these cells. Although it did not affect the activation status of Akt in MaCSCs from Ctrl-MT mice, down-regulation of Pyk2 by shRNA reduced Akt phosphorylation in MaCSCs from MFCKO-MT mice (Fig. 6E). Last, inhibition of Akt activity by its specific inhibitor triciribine decreased tumorsphere formation of MaCSCs from MFCKO-MT mice, which was not further reduced by Pyk2 knockdown in these cells (Fig. 6F and supplementary Fig. S2). In agreement with data using triciribine, infection of the cells with recombinant lentiviruses encoding two different Akt shRNAs, which effectively reduced its expression (supplemental Fig. S3), also reduced tumorsphere formation of MaCSCs from MFCKO-MT mice (Fig. 6G). Together, these results suggest that Akt may also mediate regulation of MaCSCs by compensatory Pyk2 expression after FAK deletion and further highlight the role of PI3K/Akt signaling pathway in MaCSCs and breast cancer.
DISCUSSION
Pyk2 and FAK are structurally related cytoplasmic tyrosine kinases, but there have been conflicting data on whether they can promote overlapping cellular functions or oppose each other's activities due to their distinct subcellular localizations in some cells (36, 41, 45). Interestingly, a number of previous studies suggested that Pyk2 could be up-regulated upon FAK deletion and compensate for FAK functions in these cells, providing strong support for their overlapping functions (36, 45), although other studies did not detect such compensatory expression of Pyk2 in different cells (28, 46, 47). Our results here revealed a selective up-regulation of Pyk2 in MaCSCs and metastatic nodules after FAK deletion in MFCKO-MT mice. Although the mechanisms of the increased Pyk2 expression upon FAK deletion is still unknown, our analysis of MaCSCs from MFCKO-MT and Ctrl-MT mice suggested that it is likely to be at the post-transcriptional level because no difference in Pyk2 mRNA level was detected. More importantly, the functional significance of the compensatory increase in Pyk2 expression in MaCSCs and metastatic nodules is supported by multiple lines of evidence using a combination of various approaches. FAK and FAK/Pyk2 dual inhibitors reduced tumorsphere formation and migration in vitro of MaCSCs from Ctrl-MT mice as well as their self-renewal and tumorigenicity in vivo to a similar extent, suggesting an important role for FAK but a negligible contribution from Pyk2 under these conditions. In contrast, FAK/Pyk2 dual inhibitor as well as Pyk2 shRNA significantly decreased all of these MaCSC functions both in vitro and in vivo for FAK-null MaCSCs from MFCKO-MT mice, whereas FAK inhibitor did not have any effect as expected, demonstrating a role of Pyk2 after FAK deletion. More importantly, inhibition of Pyk2 by the FAK/Pyk2 dual inhibitor, but not FAK inhibitor, also decreased the endogenous mammary tumor formation and metastasis in MFCKO-MT mice, whereas both inhibitors showed comparable activity in Ctrl-MT mice. These results lend further support for the idea of a compensatory function of Pyk2 for FAK in particular physiological and disease processes in vivo (45). They also suggest a more exquisite cell context dependence of this compensatory mechanism (i.e. occurring in a subpopulation, but not the bulk, of mammary tumor cells).
Although increasing evidence suggests the crucial role of MaCSCs in the initiation and progression of breast cancer, relatively little is known about intracellular signaling mechanisms that regulate self-renewal of MaCSCs and their contribution to tumorigenesis and metastasis in vivo. Our studies identified FAK interaction with and activation of PI3K as an important regulatory pathway in MaCSCs. Specific mutation of residue Asp395 to Ala, which disrupts FAK interaction with PI3K selectively (53), abolished the ability of FAK to rescue the defective FAK-null MaCSC self-renewal and tumorigenicity as measured by tumorsphere formation in vitro and transplantation assays in vivo. We further showed that the major PI3K signaling downstream kinase Akt exhibited increased activation in MaCSCs compared with the bulk of tumor cells in Ctrl-MT mice, and this MaCSC-associated Akt activation was significantly decreased upon FAK deletion in MFCKO-MT mice. Consistent with the former observation, a recent study also showed preferential activation of Akt in MaCSCs (i.e. tumor-initiating cells) from a p53-null mammary tumor model (34). Moreover, Akt has been found to play a role in the regulation of MaCSCs by chemokine receptor CXCR1 in a FAK-dependent manner (55) as well as by HER2 (56) in previous studies. These results supporting a role of the FAK/PI3K/Akt signaling pathway in the regulation of MaCSCs are significant because the aberrant activation of the PI3K signaling pathway is one of the most common mutational events in human breast and other cancers (57).
Previous studies also suggested a potential role of p130cas in mediating FAK functions in breast cancer because its tyrosine phosphorylation was found to be significantly reduced in mammary tumor cells after deletion of FAK (27–29). Furthermore, Pylayeva et al. (27) showed that FAK mutant P712A/P715A lacking binding to p130cas was unable to rescue the reduced proliferation and invasion of FAK-null mammary tumor cells in vitro. Surprisingly, however, we found in the present study that P712A/P715A mutant restored defective tumorsphere formation in vitro and, more importantly, self-renewal and tumorigenicity of FAK-null MaCSCs in vivo. This apparent discrepancy highlights the possibly differential requirements of key signaling pathways for MaCSCs and bulk tumor cells. It should be noted that FAK-null tumor cells with re-expression of P712A/P715A mutant were not examined in their tumorigenicity in vivo directly by transplant experiments in the studies by Pylayeva et al. (27). Thus, it is possible that these cells will maintain MaCSC activity to induce tumorigenesis in recipient mice in vivo, despite their reduction in proliferation and invasive activity of total tumor cells in vitro. Because MaCSCs represent a small fraction of total tumor cells, it is conceivable that a significant reduction in proliferation and/or invasion of total tumor cells could be observed even if the MaCSCs (with re-expression of the P712/715A mutant) within the total tumor cells possess proliferative and/or invasive activity similar to the activities of those expressing wild type FAK.
It is also interesting that the P712A/P715A mutant was able to fully rescue the increased anoikis of FAK-null tumor cells (27), suggesting that FAK signaling pathways crucial for cell survival (possibly through FAK/PI3K/Akt, as discussed above, but not p130cas, as shown in this study) are more important for MaCSC self-renewal and tumorigenicity in vivo. In this regard, it is interesting to note that increased Pyk2 expression did not affect the growth of mammary tumors in vivo or isolated tumor cells in vitro in FAK-null cells, although it compensated for FAK promotion of MaCSC self-renewal, tumorigenicity, and metastasis. We also observed that p130cas phosphorylation was similarly reduced in both MaCSCs and bulk tumor cells after FAK deletion and that compensatory Pyk2 expression did not affect its phosphorylation (data not shown). Together, these considerations raise the interesting possibility that different FAK downstream pathways may regulate distinct aspects of breast cancer development and progression (e.g. although it could affect tumor growth through effects on bulk tumor cells, FAK signaling through p130cas is not required for MaCSC and mammary tumorigenesis, which is dependent on FAK/PI3K/Akt signaling).
Pyk2 has been shown to stimulate overlapping and distinct downstream pathways as FAK. It is not clear, however, whether the up-regulated Pyk2 compensates for FAK functions in MaCSCs through the same pathways as FAK or utilizes alternative mechanisms. Our findings on the selective increase in Akt phosphorylation in FAK-null MaCSCs and its corresponding decrease after Pyk2 knockdown suggest that PI3K/Akt pathway may contribute at least in part to the Pyk2 compensation for FAK function in MaCSCs. A recent study showed that elevated expression of Pyk2 upon FAK deletion is linked to increased p190RhoGEF expression and aberrant activation of RhoA pathway and corresponding changes in cell adhesion, migration, and proliferation (58). It remains to be determined whether this or other downstream pathways may also be responsible for Pyk2 regulation of FAK-null MaCSCs.
One of the important and clinically relevant aspects of the CSC concept is the suggestion that metastatic tumor cells are derived from MaCSCs (1–4). This is supported by previous observations of more migratory and invasive activity of MaCSCs compared with bulk and “non-stem” tumor cells (28, 32). Our findings of Pyk2 compensatory up-regulation both in MaCSCs from primary tumors and in tumor cells from lung metastatic nodules, as well as its functions in MaCSCs and mammary tumor metastasis, provide a further link between MaCSCs and metastasis. Because metastasis is responsible for the majority of cancer mortality, these results further highlight the importance of targeting MaCSCs for effective therapy of breast cancer. Moreover, our understanding of the selective compensatory function of Pyk2 in MaCSCs should also aid in the future development of more effective inhibitors targeting FAK for breast cancer treatments.
Supplementary Material
Acknowledgments
We are grateful to the University of Michigan Cancer Center Flow Cytometry core and C. Bian for technical assistance. We thank Drs. Alan Kraker and Donnie Owens at Pfizer for providing FAK inhibitor PF-573,228 and FAK/Pyk2 inhibitor PF-562,271 and our colleagues in the Guan laboratory for critical reading of the manuscript and helpful comments.
This work was supported, in whole or in part, by National Institutes of Health Grants GM052890, CA150926, and HL073394 (to J.-L. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- CSC
- cancer stem cell
- MaCSC
- mammary cancer stem cell.
REFERENCES
- 1. Dalerba P., Cho R. W., Clarke M. F. (2007) Annu. Rev. Med. 58, 267–284 [DOI] [PubMed] [Google Scholar]
- 2. Wicha M. S., Liu S., Dontu G. (2006) Cancer Res. 66, 1883–1890; discussion 1895–1886 [DOI] [PubMed] [Google Scholar]
- 3. Rosen J. M., Jordan C. T. (2009) Science 324, 1670–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stingl J., Caldas C. (2007) Nat. Rev. Cancer 7, 791–799 [DOI] [PubMed] [Google Scholar]
- 5. Li X., Lewis M. T., Huang J., Gutierrez C., Osborne C. K., Wu M. F., Hilsenbeck S. G., Pavlick A., Zhang X., Chamness G. C., Wong H., Rosen J., Chang J. C. (2008) J. Natl. Cancer Inst. 100, 672–679 [DOI] [PubMed] [Google Scholar]
- 6. Bao S., Wu Q., McLendon R. E., Hao Y., Shi Q., Hjelmeland A. B., Dewhirst M. W., Bigner D. D., Rich J. N. (2006) Nature 444, 756–760 [DOI] [PubMed] [Google Scholar]
- 7. Dean M., Fojo T., Bates S. (2005) Nat. Rev. Cancer 5, 275–284 [DOI] [PubMed] [Google Scholar]
- 8. Diehn M., Cho R. W., Lobo N. A., Kalisky T., Dorie M. J., Kulp A. N., Qian D., Lam J. S., Ailles L. E., Wong M., Joshua B., Kaplan M. J., Wapnir I., Dirbas F. M., Somlo G., Garberoglio C., Paz B., Shen J., Lau S. K., Quake S. R., Brown J. M., Weissman I. L., Clarke M. F. (2009) Nature 458, 780–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodward W. A., Chen M. S., Behbod F., Alfaro M. P., Buchholz T. A., Rosen J. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 618–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parsons J. T. (2003) J. Cell Sci. 116, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 11. Schlaepfer D. D., Mitra S. K. (2004) Curr. Opin. Genet. Dev. 14, 92–101 [DOI] [PubMed] [Google Scholar]
- 12. Siesser P. M., Hanks S. K. (2006) Clin. Cancer Res. 12, 3233–3237 [DOI] [PubMed] [Google Scholar]
- 13. Schaller M. D. (2001) Biochim. Biophys. Acta. 1540, 1–21 [DOI] [PubMed] [Google Scholar]
- 14. Cohen L. A., Guan J. L. (2005) Curr. Cancer Drug Targets 5, 629–643 [DOI] [PubMed] [Google Scholar]
- 15. Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. (1994) Mol. Cell. Biol. 14, 1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xing Z., Chen H. C., Nowlen J. K., Taylor S. J., Shalloway D., Guan J. L. (1994) Mol. Biol. Cell 5, 413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen H. C., Guan J. L. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10148–10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H. C., Appeddu P. A., Isoda H., Guan J. L. (1996) J. Biol. Chem. 271, 26329–26334 [DOI] [PubMed] [Google Scholar]
- 19. Hennessy B. T., Smith D. L., Ram P. T., Lu Y., Mills G. B. (2005) Nat. Rev. Drug Discov. 4, 988–1004 [DOI] [PubMed] [Google Scholar]
- 20. Luo J., Manning B. D., Cantley L. C. (2003) Cancer Cell 4, 257–262 [DOI] [PubMed] [Google Scholar]
- 21. Frisch S. M., Vuori K., Ruoslahti E., Chan-Hui P. Y. (1996) J. Cell Biol. 134, 793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan P. C., Lai J. F., Cheng C. H., Tang M. J., Chiu C. C., Chen H. C. (1999) J. Biol. Chem. 274, 26901–26906 [DOI] [PubMed] [Google Scholar]
- 23. Sonoda Y., Matsumoto Y., Funakoshi M., Yamamoto D., Hanks S. K., Kasahara T. (2000) J. Biol. Chem. 275, 16309–16315 [DOI] [PubMed] [Google Scholar]
- 24. Golubovskaya V. M., Cance W. G. (2007) Int. Rev. Cytol. 263, 103–153 [DOI] [PubMed] [Google Scholar]
- 25. McLean G. W., Carragher N. O., Avizienyte E., Evans J., Brunton V. G., Frame M. C. (2005) Nat. Rev. Cancer 5, 505–515 [DOI] [PubMed] [Google Scholar]
- 26. Lahlou H., Sanguin-Gendreau V., Zuo D., Cardiff R. D., McLean G. W., Frame M. C., Muller W. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 20302–20307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pylayeva Y., Gillen K. M., Gerald W., Beggs H. E., Reichardt L. F., Giancotti F. G. (2009) J. Clin. Invest. 119, 252–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo M., Fan H., Nagy T., Wei H., Wang C., Liu S., Wicha M. S., Guan J. L. (2009) Cancer Res. 69, 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Provenzano P. P., Inman D. R., Eliceiri K. W., Beggs H. E., Keely P. J. (2008) Am. J. Pathol. 173, 1551–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang M., Behbod F., Atkinson R. L., Landis M. D., Kittrell F., Edwards D., Medina D., Tsimelzon A., Hilsenbeck S., Green J. E., Michalowska A. M., Rosen J. M. (2008) Cancer Res. 68, 4674–4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ginestier C., Hur M. H., Charafe-Jauffret E., Monville F., Dutcher J., Brown M., Jacquemier J., Viens P., Kleer C. G., Liu S., Schott A., Hayes D., Birnbaum D., Wicha M. S., Dontu G. (2007) Cell Stem Cell 1, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kouros-Mehr H., Bechis S. K., Slorach E. M., Littlepage L. E., Egeblad M., Ewald A. J., Pai S. Y., Ho I. C., Werb Z. (2008) Cancer Cell 13, 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iliopoulos D., Lindahl-Allen M., Polytarchou C., Hirsch H. A., Tsichlis P. N., Struhl K. (2010) Mol. Cell 39, 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang M., Atkinson R. L., Rosen J. M. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 3522–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diehl J. A., Zindy F., Sherr C. J. (1997) Genes. Dev. 11, 957–972 [DOI] [PubMed] [Google Scholar]
- 36. Sieg D. J., Ilić D., Jones K. C., Damsky C. H., Hunter T., Schlaepfer D. D. (1998) EMBO. J. 17, 5933–5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li X., Earp H. S. (1997) J. Biol. Chem. 272, 14341–14348 [DOI] [PubMed] [Google Scholar]
- 38. Salgia R., Avraham S., Pisick E., Li J. L., Raja S., Greenfield E. A., Sattler M., Avraham H., Griffin J. D. (1996) J. Biol. Chem. 271, 31222–31226 [DOI] [PubMed] [Google Scholar]
- 39. Astier A., Avraham H., Manie S. N., Groopman J., Canty T., Avraham S., Freedman A. S. (1997) J. Biol. Chem. 272, 228–232 [DOI] [PubMed] [Google Scholar]
- 40. Zheng C., Xing Z., Bian Z. C., Guo C., Akbay A., Warner L., Guan J. L. (1998) J. Biol. Chem. 273, 2384–2389 [DOI] [PubMed] [Google Scholar]
- 41. Zhao J., Zheng C., Guan J. (2000) J. Cell Sci. 113, 3063–3072 [DOI] [PubMed] [Google Scholar]
- 42. Li J., Avraham H., Rogers R. A., Raja S., Avraham S. (1996) Blood 88, 417–428 [PubMed] [Google Scholar]
- 43. Ma E. A., Lou O., Berg N. N., Ostergaard H. L. (1997) Eur. J. Immunol. 27, 329–335 [DOI] [PubMed] [Google Scholar]
- 44. Raja S., Avraham S., Avraham H. (1997) J. Biol. Chem. 272, 10941–10947 [DOI] [PubMed] [Google Scholar]
- 45. Weis S. M., Lim S. T., Lutu-Fuga K. M., Barnes L. A., Chen X. L., Göthert J. R., Shen T. L., Guan J. L., Schlaepfer D. D., Cheresh D. A. (2008) J. Cell Biol. 181, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Braren R., Hu H., Kim Y. H., Beggs H. E., Reichardt L. F., Wang R. (2006) J. Cell Biol. 172, 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen T. L., Park A. Y., Alcaraz A., Peng X., Jang I., Koni P., Flavell R. A., Gu H., Guan J. L. (2005) J. Cell Biol. 169, 941–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Slack-Davis J. K., Martin K. H., Tilghman R. W., Iwanicki M., Ung E. J., Autry C., Luzzio M. J., Cooper B., Kath J. C., Roberts W. G., Parsons J. T. (2007) J. Biol. Chem. 282, 14845–14852 [DOI] [PubMed] [Google Scholar]
- 49. Roberts W. G., Ung E., Whalen P., Cooper B., Hulford C., Autry C., Richter D., Emerson E., Lin J., Kath J., Coleman K., Yao L., Martinez-Alsina L., Lorenzen M., Berliner M., Luzzio M., Patel N., Schmitt E., LaGreca S., Jani J., Wessel M., Marr E., Griffor M., Vajdos F. (2008) Cancer Res. 68, 1935–1944 [DOI] [PubMed] [Google Scholar]
- 50. Hauck C. R., Hsia D. A., Puente X. S., Cheresh D. A., Schlaepfer D. D. (2002) EMBO. J. 21, 6289–6302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang C., Yoo Y., Fan H., Kim E., Guan K. L., Guan J. L. (2010) J. Biol. Chem. 285, 29398–29405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park A. Y., Shen T. L., Chien S., Guan J. L. (2009) J. Biol. Chem. 284, 9418–9425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reiske H. R., Kao S. C., Cary L. A., Guan J. L., Lai J. F., Chen H. C. (1999) J. Biol. Chem. 274, 12361–12366 [DOI] [PubMed] [Google Scholar]
- 54. Cary L. A., Han D. C., Polte T. R., Hanks S. K., Guan J. L. (1998) J. Cell Biol. 140, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ginestier C., Liu S., Diebel M. E., Korkaya H., Luo M., Brown M., Wicinski J., Cabaud O., Charafe-Jauffret E., Birnbaum D., Guan J. L., Dontu G., Wicha M. S. (2010) J. Clin. Invest. 120, 485–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Korkaya H., Paulson A., Iovino F., Wicha M. S. (2008) Oncogene 27, 6120–6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wood L. D., Parsons D. W., Jones S., Lin J., Sjöblom T., Leary R. J., Shen D., Boca S. M., Barber T., Ptak J., Silliman N., Szabo S., Dezso Z., Ustyanksky V., Nikolskaya T., Nikolsky Y., Karchin R., Wilson P. A., Kaminker J. S., Zhang Z., Croshaw R., Willis J., Dawson D., Shipitsin M., Willson J. K., Sukumar S., Polyak K., Park B. H., Pethiyagoda C. L., Pant P. V., Ballinger D. G., Sparks A. B., Hartigan J., Smith D. R., Suh E., Papadopoulos N., Buckhaults P., Markowitz S. D., Parmigiani G., Kinzler K. W., Velculescu V. E., Vogelstein B. (2007) Science 318, 1108–1113 [DOI] [PubMed] [Google Scholar]
- 58. Lim Y., Lim S. T., Tomar A., Gardel M., Bernard-Trifilo J. A., Chen X. L., Uryu S. A., Canete-Soler R., Zhai J., Lin H., Schlaepfer W. W., Nalbant P., Bokoch G., Ilic D., Waterman-Storer C., Schlaepfer D. D. (2008) J. Cell Biol. 180, 187–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.