Abstract
Aggregation-prone polyglutamine (polyQ) expansion proteins cause several neurodegenerative disorders, including Huntington disease. The pharmacological activation of cellular stress responses could be a new strategy to combat protein conformational diseases. Hydroxylamine derivatives act as co-inducers of heat-shock proteins (HSPs) and can enhance HSP expression in diseased cells, without significant adverse effects. Here, we used Caenorhabditis elegans expressing polyQ expansions with 35 glutamines fused to the yellow fluorescent protein (Q35-YFP) in body wall muscle cells as a model system to investigate the effects of treatment with a novel hydroxylamine derivative, NG-094, on the progression of polyQ diseases. NG-094 significantly ameliorated polyQ-mediated animal paralysis, reduced the number of Q35-YFP aggregates and delayed polyQ-dependent acceleration of aging. Micromolar concentrations of NG-094 in animal tissues with only marginal effects on the nematode fitness sufficed to confer protection against polyQ proteotoxicity, even when the drug was administered after disease onset. NG-094 did not reduce insulin/insulin-like growth factor 1-like signaling, but conferred cytoprotection by a mechanism involving the heat-shock transcription factor HSF-1 that potentiated the expression of stress-inducible HSPs. NG-094 is thus a promising candidate for tests on mammalian models of polyQ and other protein conformational diseases.
Keywords: C. elegans, Chaperone Chaperonin, Drug Action, Heat-Shock Protein, Huntington Disease, Polyglutamine Disease, Chaperone Co-inducers, Hydroxylamine Derivatives, Neurodegenerative Disease, Protein Misfolding
Introduction
Aberrant protein folding leading to the formation of toxic protein conformers and aggregates causes several human neurodegenerative disorders, including amyotrophic lateral sclerosis and Alzheimer, Parkinson, and Huntington diseases (1, 2). Different toxic proteins are involved in the onset of these devastating diseases, but in all cases the first clinical symptoms typically appear at mid-age or later in life, pointing to a close relationship between neurodegeneration and aging. The molecular link between the two processes is poorly understood, but there is mounting evidence that it is related to an age-dependent decline in the capacity of the cells to maintain protein homeostasis (proteostasis). Proteostasis is essential to cellular function and is achieved by a complex network of molecular interactions balancing protein biosynthesis, folding, trafficking, assembly/disassembly, and clearance (3–6). Strategies aimed at maintaining or restoring proteostasis may thus retard the onset of protein conformational diseases and slow their progression (4–7).
Molecular chaperones, many of which are heat-shock proteins (HSPs)3 (8), play a central role in the protein quality control of the cells (9, 10) and delay neurodegeneration associated with protein misfolding in neurones (5, 7, 11–13). The activity of members of the chaperone network, as well as the capacity of the cells to express molecular chaperones in response to stress, progressively declines with age. Weakening of the chaperone network may thus significantly contribute to the age-dependent loss of proteostasis, resulting in accelerated neurodegeneration (13, 14). Because molecular chaperones stand in a first line of cellular defenses against aberrant protein interactions that trigger pathogenic cascades, the development of therapies capable of activating the chaperone network is a promising approach to combat protein conformational disorders (11–13).
Polyglutamine (polyQ) diseases comprise at least nine neurodegenerative disorders such as Huntington disease (HD), spinobulbar muscular atrophy and spinocerebellar ataxia-1, which are associated with mutant genes containing (CAG)n trinucleotide repeats encoding polyQ expansions in otherwise unrelated proteins (15–17). PolyQ expansions render the host proteins prone to misfolding, oligomerization, and aggregation (18, 19). In all polyQ diseases, there is a pathogenic threshold of the polyQ tract length that causes disease. The exact length is different in each disease but is typically in the range of 35–45 glutamine repeats. Above the pathogenic threshold there is a strong inverse correlation between the polyQ tract length and the age of onset of the diseases. Near the threshold there is a large variability with regard to the age of disease onset among individuals sharing the same CAG repeat length in their pathogenic genes (16, 20, 21). The variability depends on both genetic and environmental factors (21, 22).
PolyQ proteins are by themselves poor pharmacological targets (23), underscoring the importance of developing indirect treatment strategies. There is ample evidence for the presence in the cells of disease modifiers, among which molecular chaperones, that strongly influence the course of the pathology of protein conformational diseases and could thus be attractive therapeutic targets (3, 24). Numerous studies using various polyQ proteins and model systems of polyQ diseases have shown that molecular chaperones can effectively modulate the aggregation and/or alleviate the toxicity of mutant polyQ proteins (12, 25, 26). Noticeably, genetic overexpression of HSPs was found to have positive effects on disease progression in spinobulbar muscular atrophy and spinocerebellar ataxia-1 transgenic mice (27, 28). Because of the limitations of gene therapies, clinical applications appeal for a pharmacological approach to up-regulate the expression of HSPs in protein misfolding diseases. Similar to heat-shock, many chemical compounds can induce isothermally the expression of HSPs (7, 29). Some of these compounds, including nontoxic hydroxylamine derivatives (30, 31), are not potent inducers of molecular chaperones per se, but amplify the expression of HSPs induced by mild physical or physiological stresses. These so-called HSP co-inducers are interesting drugs because they can enhance HSP chaperone expression in diseased cells, without significant adverse effects (31, 32).
Caenorhabditis elegans is a valuable model organism to study neurodegenerative disorders associated with protein misfolding (33). Here, we used a C. elegans model system of polyQ diseases, in which the expression of polyQ expansions in body wall muscle cells causes animal paralysis that develops in a polyQ length- and age-dependent manner (20), to test the efficiency of treatment with a novel hydroxylamine derivative, NG-094, in conferring protection against polyQ proteotoxicity. This model system was originally established to examine directly the effects of polyQ on the protein aggregation and toxicity. Although other domains of toxic polyQ proteins can contribute to specific disease progression, polyQ expansions alone can recapitulate many of the characteristics of polyQ-related diseases such as polyQ length- and age-dependent toxicity (5, 20). We used the above indicated model system to determine the effects of NG-094 on these properties. We show that NG-094 is remarkably effective in ameliorating polyQ-dependent paralysis and that the drug confers protection against polyQ proteotoxicity, even when administered after disease onset, by a mechanism involving heat-shock factor HSF-1-controlled expression of molecular chaperones.
EXPERIMENTAL PROCEDURES
Strains and General Methods
All C. elegans strains were maintained and handled using standard methods (34). Strains expressing polyglutamine expansions fused to the yellow fluorescent protein (YFP) in body wall muscle cells have been described previously (20). The specific strains used in this study were: Q0 (AM134[rmIs126(Punc-54::q0::yfp)]) and Q35 (AM140[rmIs132(Punc-54::q35::yfp)]). Additional strains were obtained from the Caenorhabditis Genetics Center (University Minneapolis, MN): CL2070 (dvIs70 Is[(hsp16.2::gfp; rol-6(su1006)]) (35), TJ356 (zIs356 Is[daf-16::daf16-gfp; rol-6]) (36), and BC10060 (sEx884 [rCesC12C8.1::gfp + pCeh361]) (37). Age-synchronized populations were obtained as described elsewhere (20). Unless indicated otherwise, all experiments were performed starting with embryos isolated from animals grown at 20 °C on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 strain. Animals used for experiments that were older than 3–4 days since hatching were grown on NGM plates containing 5 mg/liter 5-fluoro-2′-deoxyuridine (FUDR; Sigma) to inhibit progeny development. Nematodes used for reproduction or life span assays were not treated with FUDR. NG-094 was a gift from N-Gene Research Laboratories, Inc. (Budapest, Hungary) and was administered by adding the indicated amounts to the NGM. Animals were heat-shocked by transferring worm plates to an incubator set at the desired temperature.
Motility Assays
The motility of the nematodes was assessed as described previously (38). Briefly, age-synchronized animals were washed off the plates and washed three times with M9 solution. 100–200 animals in a small volume of solution were then spotted at the center of a 5-cm NGM plate containing a 3–5-mm thick ring of E. coli OP50 strain of ∼3.5 cm in diameter along the edge. The motility index was defined as the percentage of animals that successfully reached the bacteria in 60 min.
Reproductive Profile
The reproductive profile, which is the total number of progeny born to a single worm over time, was determined essentially as described elsewhere (39). Briefly, gravid adults cultured at 20 °C were first placed on NGM plates that were supplemented or not with NG-094 and allowed to lay eggs for 3 h at 20 °C. The adults were then removed from the plates and the progeny grown at 22 °C. Forty-five hours after the adults were removed (time 0) randomly selected animals were individually placed onto separate plates and grown at 22 °C. Animals were transferred to new plates every 12 h until the end of the reproductive phase, and the resulting progeny was allowed to grow for about 48 h at 22 °C until counted for progeny measurements.
Life Span Assays
Life span assays were conducted at 22 °C and performed essentially as described previously (39). Each assay was performed starting with 80 individuals placed on the 3rd day since hatching onto eight NGM plates (10 individuals/plate). The SPSS 16.0 for windows software was used for statistical analysis and determination of means and percentiles. p values were calculated using the standard chi-squared-based log-rank (Mantel-Cox) test. The maximum life span was calculated as the mean life span of the eight worms of the population with the longest life spans.
RNA Interference (RNAi) Experiments
Bacteria-mediated RNAi experiments were conducted using feeding protocols according to standard methods. RNAi feeding strains were from the Ahringer RNAi library (40). Control bacteria expressing the empty vector alone were generated by transforming HT115(DE3) bacteria with the L4440 vector (41), using standard methods. Bacterial strains were obtained from GeneService (Cambridge, UK) and the L4440 plasmid from Addgene (Cambridge, MA). RNAi bacteria were grown at 37 °C in LB with 100 μg/ml carbenicillin and then seeded onto NGM-carbenicillin (25 μg/ml) plates supplemented with 1 mm isopropyl β-d-1-thiogalactopyranoside. To ensure that DAF-16 expression was already inhibited when the animals came into contact with the drug, experiments on the effects of NG-094 in daf-16 RNAi-treated worms were performed using embryos isolated from animals grown at 20 °C on RNAi bacteria. A different protocol was used for the experiments with hsf-1 RNAi because embryos laid by hsf-1 RNAi-treated animals developed into small sterile adults, as observed previously (42). To find out whether NG-094 acts in an HSF-1-dependent manner, we therefore first cultured animals on control or hsf-1 RNAi bacteria in the absence of the drug, starting from the first larval stage (L1) of the animals. L1 larvae were obtained by allowing embryos to develop overnight in M9 solution. After about 60 h of growth on the RNAi plates, animals were transferred to fresh RNAi plates, supplemented or not with NG-094. Measurements were performed on the sixth day since hatching. These experiments were conducted at 20 °C because 6-day-old Q35 animals grown at 22 °C on hsf-1 RNAi plates were too severely paralyzed to be used for motility assays.
Worm Images, Fluorescence Microscopy, and Aggregate Quantification
Images of animals cultured on NGM plates were captured using a Leica MZ16 FA stereomicroscope equipped with a Leica DC300F camera controlled by the Leica IM50 imaging software. Fluorescence microscopy was performed using a Leica DM5000B microscope equipped with a Leica DFC 340 FX camera. Images were recorded using the Leica Application Suite (LAS AF Lite) software. For microscopy, animals were mounted on a 2% agarose pad on a glass slide and immobilized in 1 mm levamisole. PolyQ aggregates were defined as discrete structures with boundaries distinguishable from surrounding fluorescence on all sides.
Immunoblotting Analysis
Extracts were prepared by sonication of animals that were resuspended in PBS. Laemmli sample buffer was then added to the lysates to a final concentration of 1% SDS. Samples were heated for 5 min at 95 °C and analyzed by 12% SDS-PAGE and Western blot analysis. Blots were probed with the following primary antibodies: anti-GFP (1:10,000; Santa Cruz Biotechnology), anti-HSP16-2 (1:20,000, kind gift of Dr. C. D. Link, University of Colorado), anti-HSP40 (1:5,000, Stressgen Biotechnologies), anti-HSP70 (1:5,000, Agrisera), anti-HSP90 (1:35,000, Stressgen Biotechnologies), and anti-α-tubulin (1:500, Sigma). Antibody binding was visualized by binding of horseradish peroxidase-coupled secondary antibodies and chemiluminescence (Western blotting detection reagents; Bio-Rad).
RNA Isolation and Quantitative RT-PCR (qPCR)
Total RNA was isolated from synchronized populations of ∼5,000 animals. Total RNA was extracted using TRIzol reagent (Invitrogen) and purified using an RNeasy kit (Qiagen). cDNA was generated using Superscript II reverse transcriptase (Invitrogen). For qPCRs, dilutions of 1:20 were used. SYBR Green real-time quantitative PCR experiments were performed as described in the manual using ABI Prism 7900HT (Applied Biosystems, Foster City, CA). Quantification was achieved using SDS2.4 software (Applied Biosystems), normalizing to control levels of Act-4 or 18 S cDNA. The primer pair used for DAF-16 was 5′-GAGACGACTACAAAGGCT-3′ and 5′-GTTCGGGGACGGAAAGA-3′. The sequences of all the other primer pairs used in this study are listed elsewhere (43).
NG-094 Concentration in Animal Tissue
For the determination of the concentration of NG-094 in animal tissue, worm samples were homogenized in bidistilled H2O, and extracts were centrifuged at 10,000 rpm for 10 min at 10 °C. The concentration of the drug in the supernatant was then analyzed by HPLC-MS/MS on a Sciex 3200 Q-Trap instrument. The HPLC instrument was an Agilent HP1100 chromatograph equipped with a Waters Symmetry C18 column (2.1 × 150 mm, 5 μm), thermostatted at 40 °C. The mobile phase was a mixture (98:2) of 0.1% acetic acid in H2O and 0.1% acetic acid in methanol. The flow rate was 300 μl min−1 and the injected volume 5 μl. The hydroxylamine amine derivative BGP-15 was used as an internal standard.
Statistical Analyses
All data are expressed at the mean ± S.E. Statistical significance was calculated with an ANOVA with post hoc Tukey's test and Student's t test, using the SPSS 16.0 software. Significance was accepted at the level p < 0.05.
RESULTS
The Temperature of Culture Affects PolyQ Protein Aggregation and Toxicity
We reasoned that if proteostasis was not only challenged by toxic polyQ proteins, but also by other factors, the possible therapeutic benefits of treatment with hydroxylamine amine derivatives would be better revealed. Temperature is a well known stress factor that increases the intrinsic propensity of labile proteins to misfold and aggregate (7). Therefore, we first set out to characterize how the temperature of culture affects polyQ protein aggregation and toxicity in C. elegans expressing Q35-YFP in body wall muscle cells (Q35 animals), compared with animals expressing YFP alone (Q0 animals). We chose to perform experiments with Q35 animals because Q35-YFP responds readily to changes in the cellular protein homeostasis and thus represents a highly sensitive polyQ length at the threshold for age-dependent aggregation and toxicity (20, 44). Q35-YFP behavior allowed us to test for both positive and negative effects of hydroxylamine amine derivatives on Q35-YFP-related toxicity.
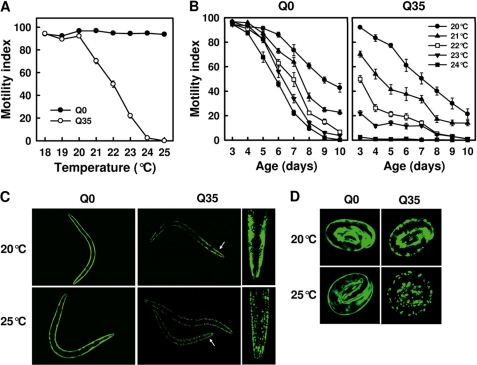
Growing the nematodes at different temperatures (T) in the range between 18 °C and 25 °C from embryo until the 3rd day since hatching barely affected the motility of Q0 animals (Fig. 1A). At T ≤ 20 °C, Q35 animals showed an unimpaired motility similar to that observed in Q0, but rising temperature above 20 °C resulted in a spectacular loss of motility, with the animals displaying severe paralysis at T ≥ 24 °C (Fig. 1A). The temperature increase from 20 °C to 24 °C also led to an acceleration of age-dependent loss of motility. The motility of Q0 animals was reduced by 50%, either after 9 days at 20 °C or after 7 days at 24 °C (Fig. 1B, left), whereas Q35 animals displayed a 50% loss of motility already after 7 days at 20 °C or after 3 days at 22 °C (Fig. 1B, right).
FIGURE 1.
Temperature of culture affects polyQ protein aggregation and toxicity. A, effect of culture temperature on the motility of 3-day-old C. elegans animals expressing polyQ-YFP fusion proteins with 0 (Q0) or 35 glutamine residues (Q35) in body wall muscle cells. B, effect of culture temperature on age-dependent changes in the motility of Q0 and Q35 animals. Values in A and B are means ± S.E. (error bars) of 12 motility assays from two independent experiments, two replicates/experiment, and three assays/experiment. Note that cohort sizes decreased as some animals died during the experiment. This was mainly observed for Q35 animals grown at elevated temperature. Dead animals were excluded from the analysis. C, fluorescence microscopy images of Q0 and Q35 animals (2 days post-hatch) cultured at 20 °C or 25 °C. The white arrow indicates the region of the head also shown at higher magnification. Note that the head of the Q35 animal cultured at 25 °C is slightly rotated. Images were taken using the 10× objective of a Leica DM5000B microscope. D, fluorescence microscopy images of embryos laid by Q0 and Q35 animals cultured at 20 °C or 25 °C. Images were taken using the 20× objective of a Leica DM5000B microscope.
In Q0 animals, a diffuse fluorescence distribution was observed in all YFP-expressing cells, regardless of whether the animals were cultured at 20 °C or 25 °C. In contrast, Q35 animals exhibited focal fluorescence distribution at 25 °C, whereas at 20 °C they showed polymorphic distribution, with diffuse fluorescence in some cells and foci in others (Fig. 1C). The change from diffuse to focal fluorescence in C. elegans expressing polyQ-YFP corresponds to a conversion of the biochemical state of the polyQ protein from soluble to aggregate (20, 44). Noticeably, although at 20 °C the animals of the two strains displayed a similar body size, at 25 °C, Q35 animals were smaller than Q0 animals (Fig. 1C). Embryos laid by Q0 animals showed diffuse fluorescence distribution, irrespective of their parents being grown at 20 °C or 25 °C (Fig. 1D). In contrast, embryos laid by Q35 animals displayed focal fluorescence when their parents were grown at 25 °C, whereas they mainly exhibited diffuse fluorescence when born from 20 °C-grown parents (Fig. 1D). Collectively, these results indicate that the expression of polyQ proteins renders the animals very sensitive to normally innocuous mildly elevated temperatures and that growing the animals at elevated physiological temperatures in turn accelerates and exacerbates polyQ protein aggregation and toxicity.
NG-094 Protects against PolyQ Proteotoxicity
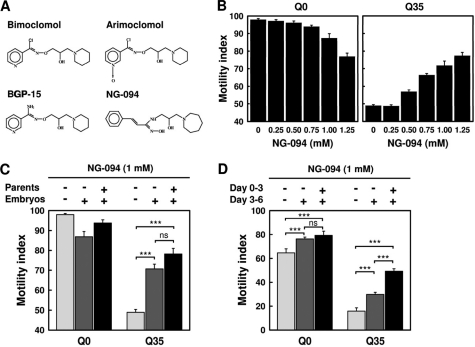
The hydroxylamine derivatives bimoclomol, arimoclomol, and BGP-15 were shown in animal model systems to be effective in the treatment of various protein conformational diseases (31, 45–47). Here, we investigated the effects of treatment with the novel hydroxylamine derivative NG-094 (Fig. 2A) on polyQ-mediated cytotoxicity. We chose to perform tests on the possible therapeutic benefits of NG-094 using animal grown at 22 °C for 3 days because Q35 animals showed under these conditions a 50% loss of motility, compared with Q0 animals (Fig. 1A). NG-094 was found to improve the motility of Q35 animals considerably, in a dosage-dependent manner (Fig. 2B, right). It should be noted that Q0 animals progressively displayed a somewhat reduced motility at NG-094 concentrations that exceeded 0.5 mm (Fig. 2B, left). Up to 1 mm, this effect remained marginal, and it disappeared when the animals became older (see below). Therefore, 1 mm NG-094 in the growth medium was chosen as the standard concentration in further experiments. HPLC-MS/MS analyses showed that nematodes grown in the presence of 1 mm NG-094 contained 1.2 ± 0.2 μm (n = 6) of the drug in their tissues.
FIGURE 2.
NG-094 alleviates polyQ-dependent animal paralysis. A, chemical structure of different hydroxylamine derivatives. B, motility index of 3-day-old Q0 and Q35 animals cultured at 22 °C and treated with different concentrations of NG-094. C, motility index of 3-day-old Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094, as indicated. Parent animals were cultured at 20 °C. D, motility index of 6-day-old Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094, as indicated. Values are means ± S.E. (error bars) of nine motility assays from three independent experiments and three assays per experiment. ***, p < 0.001; ns, not significant.
As demonstrated above, applying NG-094 starting from the time of hatching significantly ameliorated the motility of Q35 animals. Pretreatment of parent animals with NG-094 further improved the motility, albeit not in a statistically significant manner (Fig. 2C). Motility assays performed on the 6th day since hatching showed that NG-094 significantly reduces polyQ-mediated toxicity in the longer term. Remarkably, Q35 animals also displayed an improved motility when NG-094 was only applied starting from the 3rd day since hatching (Fig. 2D), indicating that the drug can slow disease progression even when administered after disease onset. NG-094 also delayed the age-dependent loss of motility in Q0 animals (Fig. 2D).
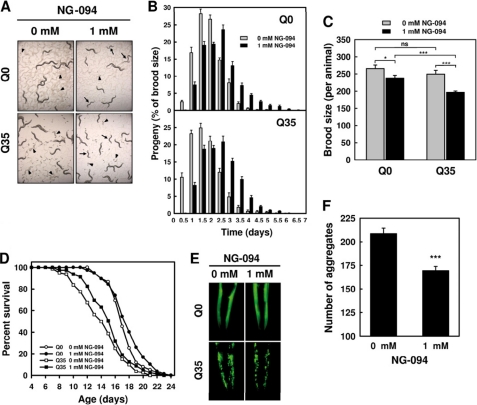
The development of both Q0 and Q35 animals was found to be slower in the presence of the drug (Fig. 3A), with NG-094-treated animals exhibiting a delayed egg-laying onset and a mildly altered reproductive schedule (Fig. 3B). The brood size per animal displayed by NG-094-treated Q0 and Q35 animals was, respectively, 10 and 20% lower than in untreated animals (Fig. 3C), indicating a marginal reduction of the fitness of the nematodes. Without NG-094, the brood size per animal was similar in Q0 and Q35 animals (Fig. 3C), but egg-laying onset started earlier in Q35 animals (Fig. 3B), indicative of a proteotoxic stress. This is consistent with our observation that Q35 animals aged more rapidly, their mean life span being about 20% shorter (14.2 ± 0.4 days) than that of Q0 animals (17.4 ± 0.3 days) (Fig. 3D and supplemental Table S1). NG-094 delayed aging of Q35 animals, and there was a tendency (p < 0.097) toward a significant longer mean life span for NG-094-treated Q35 animals (15.2 ± 0.3 days) compared with their untreated counterparts (14.2 ± 0.4 days). NG-094-treated Q35 animals exhibited an ∼20% longer maximum life span (20.7 ± 0.4 days) compared with their untreated counterparts (17.6 ± 0.3 days) (p < 0.0001). The drug had a comparatively marginal effect on the life span characteristics of Q0 animals (Fig. 3D and supplemental Table S1).
FIGURE 3.
NG-094 confers cytoprotection without significant adverse effects on the fitness of the nematodes. A, images of 3-day-old Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. Arrows indicate animals with a delayed development, and arrowheads indicate embryos. B, reproductive profile of Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. Values are means ± S.E. (error bars) of at least 15 individuals. C, brood size per animal for Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. Values are means ± S.E. of at least 15 individuals. *, p < 0.05; ***, p < 0.001; ns, not significant. D, survival of Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. Statistical data are given in supplemental Table S1. E, fluorescence microscopy images of the head of 3-day-old Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. Images were taken using the 10× objective of a Leica DM500B microscope. F, number of Q35-YFP aggregates exhibited by Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. Values are means ± S.E. of 50 animals. ***, p < 0.001.
The accumulation inside the cells of polyQ protein aggregates visible by a light microscope is a hallmark of polyQ diseases (2, 12). NG-094 did not affect the diffuse fluorescence distribution exhibited by Q0 animals (Fig. 3E). Neither did the focal fluorescence distribution displayed by Q35 animals seem to be affected by the drug (Fig. 3E), but treated animals exhibited on average 20% less Q35-YFP aggregates than their untreated counterparts (Fig. 3F). Collectively, these results show that NG-094 confers protection against polyQ proteotoxicity without significant adverse effects on the fitness of the nematodes.
NG-094 Enhances Heat-induced Accumulation of HSPs
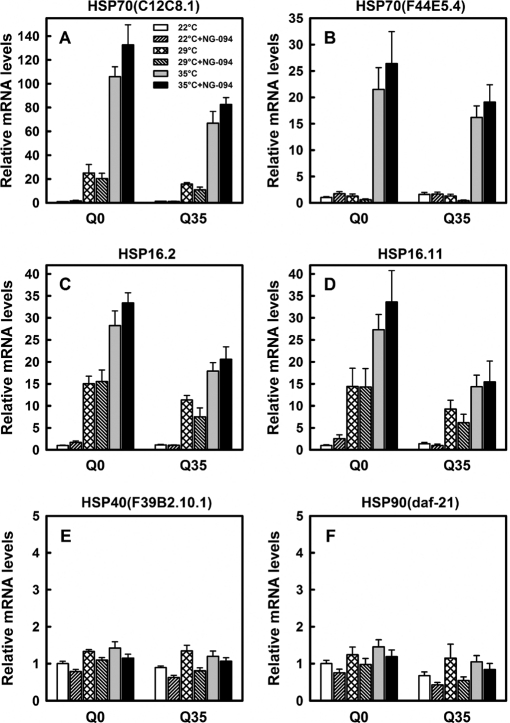
To determine whether NG-094 acts as a co-inducer of HSPs, we examined the effects of NG-094 on the expression of a set of HSP genes in Q0 and Q35 animals that were subjected or not to a heat-shock (HS) treatment. qPCR analyses revealed that a 90-min HS at 35 °C strongly up-regulated the gene expression of HSP70(C12C8.1), HSP70(F44E5.4), HSP16.2, and HSP16.11 (Fig. 4, A–D), whereas it hardly affected the gene expression of HSP40(F39B2.10.1) and HSP90(daf-21) (Fig. 4, E and F). A milder HS at 29 °C did not enhance the gene expression of HSP70(F44E5.4) (Fig. 4B), but significantly up-regulated the expression of other heat-inducible HSP genes (Fig. 4, A, C, and D). Noticeably, heat-induced changes in the expression of HSP genes were somewhat less pronounced in Q35 than in Q0 animals, particularly when the nematodes were heat-shocked at 35 °C. The qPCR analyses showed that, unexpectedly, NG-094 has no significant effects on heat-induced transcription of HSP genes (Fig. 4, A–D).
FIGURE 4.
Heat- and NG-094-induced changes in the transcription of HSP genes. Relative mRNA levels of HSP70(C12C8.1) (A), HSP70(F44E5.4) (B), HSP16.2 (C), HSP16.11 (D), HSP40(F39B2.10.1) (E), and HSP90(daf-21) (F) measured by RT-qPCR for 3-day-old Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. Analyses were performed for animals that on the 3rd day since hatching were either kept at 22 °C or heat-shocked at 29 °C or 35 °C for 90 min. Data were normalized to Act-4 levels. Values were calculated relative to the value of untreated Q0 animals. Similar results were obtained when data were normalized to 18 S mRNA levels (data not shown). Data are means ± S.E. (error bars) of four independent biological samples.
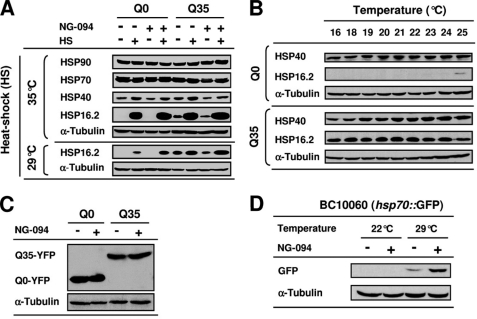
Semiquantitative Western blot analyses showed that HSP90 levels were similar in Q0 and Q35 animals and did not change when the nematodes were heat-shocked at 35 °C, irrespective of NG-094 treatment (Fig. 5A). On the other hand, HS at 35 °C led to an accumulation of small HSPs (sHSPs) and increased HSP40 levels (Fig. 5A). The antibody targeted against HSP70 used in this study and others that we tested did not permit detection of heat-induced changes in HSP70 levels (see below), probably because the antibodies did not sufficiently distinguish inducible HSP70 from constitutively expressed HSP70 (Fig. 5A).
FIGURE 5.
NG-094 enhances heat-induced accumulation of HSPs. A, Western blot analysis of the levels of HSPs in 3-day-old Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. Analyses were performed for animals that on the 3rd day since hatching were either kept at 22 °C or heat-shocked at 35 °C for 2 h or at 29 °C for 3 h. Animals were collected after a 6-h period of recovery at 22 °C. B, expression levels of HSP40 and HSP16.2 in Q0 and Q35 animals cultured at different temperatures from embryo until early adulthood. C, expression levels of YFP and Q35-YFP in 3-day-old Q0 and Q35 animals cultured at 22 °C and treated or not with 1 mm NG-094. D, expression levels of GFP in 3-day-old BC10060 nematodes expressing a C12C8.1(hsp70)::GFP transgene. Animals were cultured at 22 °C and treated or not with 1 mm NG-094. Analyses were performed for animals that on the 3rd day since hatching were either kept at 22 °C or heat-shocked at 29 °C for 3 h. Animals were collected after a 6-h period of recovery at 22 °C. α-Tubulin was used as a loading control.
When the nematodes were neither heat-shocked nor treated with NG-094, the levels of HSP40 and HSP16.2 were clearly higher in Q35 than in Q0 (Fig. 5A). The levels of Q35-YFP-dependent accumulation of HSPs were found to be largely indifferent to the temperature of culture (Fig. 5B). NG-094 treatment diminished the constitutively higher levels of HSP40 and HSP16.2 in non-heat-shocked Q35 animals (Fig. 5A), suggesting that the drug reduces the cellular stress caused by chronic protein misfolding. Western blot analyses showed that this effect of NG-094 was not related to changes in the expression level of Q35-YFP (Fig. 5C), indicating that NG-094 alleviates polyQ-mediated cytotoxicity.
A mild HS at 29 °C revealed that NG-094 influences the cellular response to stress. NG-094 considerably enhanced the accumulation of HSP16.2 induced by mild HS (Fig. 5A), indicating that the drug acts as a co-activator of sHSPs. This was confirmed in CL2070 nematodes expressing a hsp16.2::GFP transgene (supplemental Fig. S1). We further observed that NG-094 greatly increased the accumulation of GFP induced by HS at 29 °C in BC10060 nematodes expressing a C12C8.1(hsp70)::GFP transgene (Fig. 5D), showing that NG-094 also co-induces HSP70. Amplification of heat-induced accumulation of HSPs by NG-094 was not detected in animals subjected to extreme HS at 35 °C (Fig. 5A and supplemental Fig. S1A; not shown for C12C8.1(hsp70)::GFP)), probably because the strong HS, by itself, resulted in a close to maximum accumulation of inducible HSPs. Together, these findings suggest that the cytoprotective effect of NG-094 is related, at least in part, to the capacity of the drug to potentiate the activation of stress-inducible HSP genes when the expression of specific molecular chaperones is needed to maintain or restore proteostasis.
Cytoprotection by NG-094 Requires HSF-1 Activity, whereas DAF-16 Activity Is Dispensable
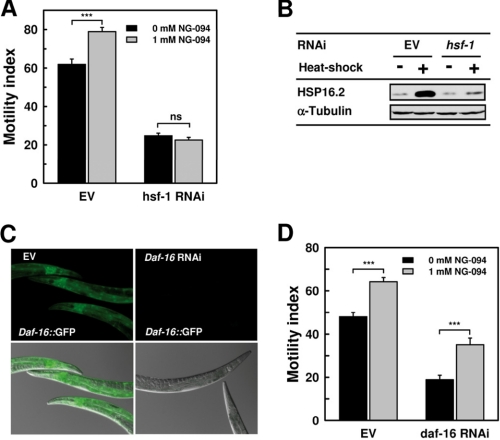
In C. elegans, HSF-1 is essential for the activation of inducible HSP genes and other stress-response genes (42, 48). As expected, suppressing HSF-1 activity by RNAi strongly exacerbated paralysis in Q35 animals (Fig. 6A) and effectively inhibited stress-induced accumulation of HSPs, as judged from the strongly dampened heat-induced expression of HSP16.2 (Fig. 6B). Noticeably, NG-094 failed to ameliorate polyQ-dependent paralysis in hsf-1 RNAi-treated Q35 animals (Fig. 6A).
FIGURE 6.
Cytoprotection by NG-094 requires HSF-1 but not DAF-16 activity. A, motility index of 6-day-old Q35 animals that were grown at 20 °C on either control bacteria (EV) or hsf-1 RNAi bacteria and treated or not with 1 mm NG-094. NG-094 was administered starting from the 3rd day since hatching. Values are means ± S.E. (error bars) of nine motility assays from three independent experiments and three assays per experiment. ***, p < 0.001; ns, not significant. B, Western blot analysis of the expression level of HSP16.2 in 6-day-old Q35 animals that were grown on control bacteria (EV) or hsf-1 RNAi bacteria. Analyses were performed for animals that on the 6th day since hatching were either kept 20 °C or heat-shocked at 35 °C for 2 h. Animals were collected after a 6-h period of recovery at 22 °C. α-Tubulin was used as a loading control. C, images of 3-day-old TJ356 nematodes expressing a daf-16::GFP transgene. Animals were grown at 22 °C on control bacteria (EV) or daf-16 RNAi bacteria. Shown are fluorescence images (upper panels) and overlays of fluorescence and diffraction contrast images (lower panels). All pictures were taken with the same exposure. D, motility index of 3-day-old Q35 animals that were grown at 22 °C on control bacteria (EV) or daf-16 RNAi bacteria and treated or not with 1 mm NG-094. Values are means ± S.E. of nine motility assays from three independent experiments and three assays per experiment. ***, p < 0.001.
There is mounting evidence that the insulin/insulin-like growth factor 1 signaling (ILS) pathway is a major factor linking proteotoxicity-mediated neurodegeneration and aging (49). In C. elegans, reduced ILS protects against proteotoxicity by a mechanism that requires the activity of both the forkhead transcription factor DAF-16 and HSF-1 (20, 48, 50). Growing the nematodes on daf-16 RNAi bacteria efficiently inhibited DAF-16 expression, as demonstrated by the complete abrogation of GFP fluorescence in daf-16 RNAi-treated TJ356 animals expressing a daf-16::GFP transgene (Fig. 6C). The daf-16 RNAi treatment further reduced the motility of Q35 animals from 50% to 20%. Noticeably, NG-094 remained very effective in alleviating polyQ-dependent animal paralysis despite suppression of DAF-16 activity (Fig. 6D). Consistent with earlier findings (48), we observed that daf-16 RNAi partially inhibited the expression of heat-inducible sHSPs without significant effects on the expression of heat-inducible HSP70s, whereas hsf-1 RNAi strongly suppressed the expression of inducible HSPs (supplemental Fig. S2). Collectively, these findings indicate that NG-094 does not reduce ILS, but confers protection against polyQ proteotoxicity by a mechanism involving HSF-1.
DISCUSSION
Here, we showed that when Q35 animals are grown above 20 °C, Q35-YFP protein aggregation and toxicity are dramatically accelerated and exacerbated: the higher the temperature, the more severe the disease. Our findings reveal that even a weak cellular stress caused by environmental challenges can greatly enhance polyQ proteotoxicity and that, in turn, the expression of polyQ proteins renders the animals highly sensitive to environmental stresses. This provides additional evidence that the protein folding environment of the cells is delicately balanced and is very vulnerable to changes initiated by toxic polyQ proteins (38). Our results also provide experimental evidence that, in addition to genetic disease modifiers (44, 51), environmental factors may significantly account for the large variability in symptoms and age of onset of polyQ diseases observed near the pathogenic threshold of the polyQ tract length that causes disease among individuals sharing the same CAG repeat length in their pathogenic genes (21–22). Moreover, indicative of an important role for cellular defense mechanisms against polyQ proteotoxicity, phenotypes such as the paralysis displayed by young Q35 animals at T > 20 °C were also observed at T < 20 °C when intermediate-length polyQ expansions were co-expressed with temperature-sensitive mutant proteins that do not by themselves induce a phenotype at T < 20 °C (38). This indicates that toxic polyQ proteins and other factors generating homeostatic demands in the cells are synergistic in their destabilizing effects on proteostasis.
Molecular chaperones are involved in various cellular processes and are essential to prevent misfolding of nascent polypeptide chains, to facilitate their co- and post-translational folding, to assist in assembly and disassembly of protein oligomers, and to regulate the translocation of proteins across membranes (9, 10). Moreover, chaperones such as HSP70 can act as ATP-fuelled polypeptide unfoldases capable of forcefully converting misfolded proteins into natively refoldable or degradable conformers (52). It is therefore likely that at T > 20 °C Q35 animals exhibited a strong phenotype already in early adulthood because the combination of Q35-YFP expression and elevated temperatures generated an excessive flux of conformationally challenged or misfolded proteins that, in conjunction with an intense recruitment of molecular chaperones by nascent chains and other labile polypeptides during growth and development, overwhelmed the capacity of the cells to maintain proteostasis.
Consistent with earlier findings with C. elegans-expressing Q82-GFP (53) or the human β-amyloid peptide (Aβ1–42) involved in Alzheimer disease (35), Q35-YFP caused activation of the stress-sensing machinery of C. elegans, leading to an apparent constitutive expression of sHSPs and inducible HSP40. For temperatures between 16 °C and 25 °C, Q35-YFP-dependent accumulation of HSPs was largely unaffected by the temperature, suggesting that it is not associated with a cytoprotective response, but rather reflects a polyQ-dependent deregulation of an otherwise transient cellular stress response, with possible deleterious consequences for the organism (53). Although the reasons for the polyQ-dependent accumulation of HSPs need further clarifications, one can assume that the cellular machineries controlling proteostasis and countering proteotoxicity fail to respond adequately to a chronic imbalance of the protein folding homeostasis of the cells caused by polyQ proteins and that even mild environmental stresses can aggravate the problem, thereby accelerating disease progression. Treatments that activate the cellular machineries regulating proteostasis may thus reduce polyQ-mediated cytotoxicity.
Nontoxic hydroxylamine derivatives, such as bimoclomol and its derived analogues arimoclomol and BGP-15 have been shown in animal model systems to confer protection against a number of protein-conformational disorders by acting as co-inducers of various HSPs (30, 31, 45–47). The mechanisms by which hydroxylamines can amplify the expression of stress-inducible HSPs are not well understood, but it seems that the drugs interfere with membrane hyperstructures via their highly specific lipid interactions (54), which appear sufficient to enhance stress signals that activate HSP genes (31, 46). Treatment with hydroxylamines may thus be considered for “membrane-lipid therapies,” potentiating the capacity of the cells to detect and respond to various stress conditions (31, 55).
Here, we showed that the novel hydroxylamine derivative NG-094 can markedly reduce polyQ proteotoxicity and confer therapeutic benefits even when administrated after disease onset. This is important, given that patients suffering from protein conformational diseases usually start therapies after symptom appearance. Cytoprotection was observed at fairly low concentrations of NG-094 in animal tissues (1 μm range) with only marginal effects on the fitness of the nematodes. These characteristics make NG-094 a good candidate for the treatment of polyQ diseases. Consistent with the scenario that hydroxylamines confer cytoprotection by acting as co-activators of HSPs, NG-094 considerably enhanced heat-induced expression of HSP70. This suggests that stimulation of stress-dependent activation of HSP70s played an important role in the amelioration of polyQ-mediated animal paralysis by NG-094. Indeed, there is ample evidence that HSP70 and its co-chaperone HSP40 effectively modulate the aggregation and/or suppress the toxicity of polyQ proteins and other disease-causing misfolding-prone proteins (12, 25, 26, 56), for example by unfolding toxic misfolded polypeptides (52, 57). We observed that NG-094 acts also as a co-inducer of sHSPs, which play a key role in the establishment of stress resistance and longevity in C. elegans (47, 58) and were found to protect against polyQ and Aβ1–42 toxicity in C. elegans models of HD and Alzheimer disease (48, 59). Enhanced accumulation of sHSPs following HS was also observed in long lived and stress-resistant C. elegans mutants with reduced ILS (60). It is unlikely that NG-094 confers protection by reducing ILS because the drug was effective in alleviating polyQ-dependent paralysis despite daf-16 RNAi treatment and did not extend the life span of Q0 animals.
There is a large body of evidence that sHSPs, which are not ATPase chaperones, contribute to proteostasis by binding proteins in nonnative conformations, thereby preventing potentially toxic aggregations and pathogenic interactions with essential cellular proteins. It seems that sHSPs create a reservoir of nonnative proteins, which may become actively unfolded by the ATP-dependent HSP70/HSP40 system and thus given a new chance to fold into a native functional protein or to be degraded by the proteasome (7, 52, 61, 62).
Given that various classes of molecular chaperones often work in concert, the pharmacological activation of multiple endogenous chaperones and co-chaperones is expected to be more effective than the genetic and perhaps ectopic overexpression of particular chaperones for the treatment of protein conformational diseases (7, 8, 29). In support of this view, the genetic overexpression of HSP27 or of inducible HSP70 (63–65) had only marginal effects on disease progression in the R6/2 mouse model of HD. On the other hand, treatment with geranylgeranylacetone (66) or the HSP90 inhibitor 17-DMAG (67), both up-regulating the expression of many HSPs in vivo, were reported to ameliorate the disease phenotype effectively in a mouse model of spinobulbar muscular atrophy. Likewise, 17-DMAG was found to have beneficial effects in an in vitro cellular model of HD (68). A potential problem of the pharmacological activation of HSPs is that the beneficial effects associated with a chronic overexpression of HSPs may be accompanied by deleterious effects caused by the deregulation of the diverse physiological functions carried out by the molecular chaperones in the cell (12). In this regard, hydroxylamines are particularly interesting drugs because they can specifically potentiate the expression of HSPs that is naturally induced in the cells in response to stress stimuli, thus circumventing potential problems associated with a general and chronic up-regulation of HSPs (31, 32).
Because hydroxylamines act in such a specific manner, they may potentiate the expression of different HSPs, depending on the nature of the disease-causing agent. For example, Arimoclomol delays disease progression in ALS mice by increasing the levels of HSP70 and HSP90 in motor neurons expressing a disease-causing mutant of the human Cu/Zn superoxide dismutase 1 (SOD1G93A) (45), whereas BGP-15 confers protection against obesity-induced insulin resistance, apparently by increasing the level of HSP72 in skeletal muscles of leptin-deficient (ob/ob) mice (46). Curiously, we found that NG-094 decreased polyQ-dependent overexpression of sHSPs and HSP40 to some degree. It is possible that by stimulating stress-induced activation of a number of HSP genes, NG-094 effectively delayed the accumulation of toxic Q35-YFP protein entities, which in turn led to a reduction in the chronic polyQ-dependent expression of HSPs. Noticeably, because endogenous HSP70s appear to be critical components of the cellular defense against toxic polyQ proteins (25, 69), even a small increase of HSP70 levels by NG-094 could strongly reduce the concentration of toxic Q35-YFP protein species.
We cannot exclude that NG-094 also reduced polyQ-dependent expression of HSPs by stimulating the activation of other cellular defense mechanisms that suppress polyQ proteotoxicity. However, our finding that HSF-1 was essential for NG-094-dependent amelioration of polyQ-mediated animal paralysis strongly supports the view that the drug predominantly conferred cytoprotection by potentiating the expression of stress-inducible HSPs. Overexpression of HSF-1 has been reported to improve the capacity of C. elegans to maintain proteostasis (43) and to extend the nematode life span (48). Yet, our data showed that NG-094 did not prolong the life span of Q0 animals, suggesting that the chaperone-dependent protective action of NG-094 is not associated with an overexpression of HSF-1. On the other hand, the co-inducing effect of hydroxylamines on HSP expression is mediated, at least in part, via a prolonged activation of HSP genes by HSF-1 (70). It is therefore conceivable that NG-094 conferred cytoprotection by facilitating and prolonging transcriptionally active HSF-1/DNA interactions, which in non-heat-shocked Q35 animals are triggered by toxic Q35-YFP conformers. Prolongation of the activation of HSP genes by NG-094 might also explain why, after a mild HS and several hours of recovery, we could detect, by Western blot analyses, NG-094-dependent amplification of heat-induced HSP expression, whereas qPCR analyses performed on animals collected immediately after the HS showed no amplification of heat-induced HSP expression.
We observed that NG-094-treated Q35 animals displayed an improved motility alongside with a reduced number of Q35-YFP aggregates. This is consistent with the earlier finding of a correlation between the motility defect and the accumulation of polyQ aggregates in C. elegans expressing intermediate-length polyQ expansions in body wall muscles (20). However, although the deposition of aggregated polyQ proteins in inclusion bodies is a hallmark of polyQ diseases, it seems that inclusion bodies are not the main cause of pathogenesis. Actually, there is a growing body of evidence that misfolded monomeric species and low molecular weight oligomeric misfolded species, also called protofibrils, are the most cytotoxic forms of the disease-causing proteins that disrupt proteostasis and initiate pathogenesis (2, 12). It is tempting to speculate that by potentiating the expression of HSPs, NG-094 reduced the number of small toxic aggregates, thereby also reducing the number of less toxic larger aggregates in Q35 animals. Earlier studies have shown that changes in the severity of polyQ diseases and other protein conformational diseases related with changes in the expression of HSPs are not necessarily accompanied by changes in the number of large aggregates, but by modifications of the biochemical properties of the aggregates associated with changes in the physical and biochemical properties of the disease-causing proteins that modulate their toxicity (12, 25). NG-094 may thus also have conferred cytoprotection by facilitating the conversion of toxic Q35-YFP intermediates into nontoxic or less toxic conformers.
Although NG-094 led to a rather modest delay of polyQ-mediated acceleration of aging in Q35 animals, it would be interesting to test the therapeutic effects of NG-094 in mammalian models of polyQ diseases and other late onset neurodegenerative disorders. Indeed, NG-094 might have stronger positive effects on longevity in mammals than in C. elegans, as suggested by the finding that arimoclomol significantly increased survival in a mouse model of ALS (45). Moreover, even if NG-094-like compounds would not significantly increase longevity in humans, they may significantly improve the quality of life by delaying the age of onset and/or decreasing the impact of neurodegenerative diseases associated with protein misfolding (4). Overall, our data provide good evidence that treatment with specific hydroxylamine derivatives could be a novel therapeutic approach to combat polyQ diseases and protein conformational disorders in general.
Supplementary Material
Acknowledgments
We thank Dr. R. I. Morimoto and Dr. A. Ben-Zvi (Northwestern University, Evanston, IL) for providing the Q35 and Q0 C. elegans strains and advice on motility assay, Dr. F. Müller (University of Fribourg, Switzerland) for information on C. elegans manipulation, and Dr. C. D. Link (Colorado University) for providing the HSP16.2 antibody. We thank Dr. Maria Peter and Dr. Ibolya Horvath for the determination of the concentration of NG-094 in animal tissues and for helpful comments on the manuscript. We thank the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources, for providing some of the strains used in this study and N-Gene Research Laboratories, Inc. (Budapest, Hungary) for providing NG-094.
This work was supported in part from Grants 3100A0-109290 and NRP57, both from the Swiss National Science Foundation and from the Hungarian National Scientific Research Foundation, OTKA NN 76716.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Table S1.
- HSP
- heat-shock protein
- HD
- Huntington disease
- HS
- heat shock
- HSF
- heat-shock factor
- ILS
- insulin/insulin-like growth factor 1 signaling
- NGM
- nematode growth medium
- polyQ
- polyglutamine
- qPCR
- quantitative PCR
- sHSP
- small HSP.
REFERENCES
- 1. Soto C. (2003) Nat. Rev. Neurosci. 4, 49–60 [DOI] [PubMed] [Google Scholar]
- 2. Ross C. A., Poirier M. A. (2005) Nat. Rev. Mol. Cell Biol. 6, 891–898 [DOI] [PubMed] [Google Scholar]
- 3. Finkbeiner S., Cuervo A. M., Morimoto R. I., Muchowski P. J. (2006) J. Neurosci. 26, 10349–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balch W. E., Morimoto R. I., Dillin A., Kelly J. W. (2008) Science 319, 916–919 [DOI] [PubMed] [Google Scholar]
- 5. Morimoto R. I. (2008) Genes Dev. 22, 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powers E. T., Morimoto R. I., Dillin A., Kelly J. W., Balch W. E. (2009) Annu. Rev. Biochem. 78, 959–991 [DOI] [PubMed] [Google Scholar]
- 7. Hinault M. P., Ben-Zvi A., Goloubinoff P. (2006) J. Mol. Neurosci. 30, 249–265 [DOI] [PubMed] [Google Scholar]
- 8. Finka A., Mattoo R. U., Goloubinoff P. (2011) Cell Stress Chaperones 16, 15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartl F. U., Hayer-Hartl M. (2002) Science 295, 1852–1858 [DOI] [PubMed] [Google Scholar]
- 10. Bukau B., Weissman J., Horwich A. (2006) Cell 125, 443–451 [DOI] [PubMed] [Google Scholar]
- 11. Muchowski P. J. (2002) Neuron 35, 9–12 [DOI] [PubMed] [Google Scholar]
- 12. Muchowski P. J., Wacker J. L. (2005) Nat. Rev. Neurosci. 6, 11–22 [DOI] [PubMed] [Google Scholar]
- 13. Calderwood S. K., Murshid A., Prince T. (2009) Gerontology 55, 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Macario A. J., Conway de Macario E. (2005) New Engl. J. Med. 353, 1489–1501 [DOI] [PubMed] [Google Scholar]
- 15. Ross C. A. (2002) Neuron 35, 819–822 [DOI] [PubMed] [Google Scholar]
- 16. Zoghbi H. Y., Orr H. T. (2000) Annu. Rev. Neurosci. 23, 217–247 [DOI] [PubMed] [Google Scholar]
- 17. Orr H. T., Zoghbi H. Y. (2007) Annu. Rev. Neurosci. 30, 575–621 [DOI] [PubMed] [Google Scholar]
- 18. Perutz M. F. (1999) Trends Biochem. Sci. 24, 58–63 [DOI] [PubMed] [Google Scholar]
- 19. Poirier M. A., Jiang H., Ross C. A. (2005) Hum. Mol. Genet. 14, 765–774 [DOI] [PubMed] [Google Scholar]
- 20. Morley J. F., Brignull H. R., Weyers J. J., Morimoto R. I. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10417–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker F. O. (2007) Lancet 369, 218–228 [DOI] [PubMed] [Google Scholar]
- 22. Wexler N. S., Lorimer J., Porter J., Gomez F., Moskowitz C., Shackell E., Marder K., Penchaszadeh G., Roberts S. A., Gayán J., Brocklebank D., Cherny S. S., Cardon L. R., Gray J., Dlouhy S. R., Wiktorski S., Hodes M. E., Conneally P. M., Penney J. B., Gusella J., Cha J. H., Irizarry M., Rosas D., Hersch S., Hollingsworth Z., MacDonald M., Young A. B., Andresen J. M., Housman D. E., De Young M. M., Bonilla E., Stillings T., Negrette A., Snodgrass S. R., Martinez-Jaurrieta M. D., Ramos-Arroyo M. A., Bickham J., Ramos J. S., Marshall F., Shoulson I., Rey G. J., Feigin A., Arnheim N., Acevedo-Cruz A., Acosta L., Alvir J., Fischbeck K., Thompson L. M., Young A., Dure L., O'Brien C. J., Paulsen J., Brickman A., Krch D., Peery S., Hogarth P., Higgins D. S., Jr., Landwehrmeyer B. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3498–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beal M. F., Ferrante R. J. (2004) Nat. Rev. Neurosci. 5, 373–384 [DOI] [PubMed] [Google Scholar]
- 24. Morimoto R. I., Santoro M. G. (1998) Nat. Biotechnol. 16, 833–838 [DOI] [PubMed] [Google Scholar]
- 25. Wacker J. L., Huang S. Y., Steele A. D., Aron R., Lotz G. P., Nguyen Q., Giorgini F., Roberson E. D., Lindquist S., Masliah E., Muchowski P. J. (2009) J. Neurosci. 29, 9104–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagai Y., Fujikake N., Popiel H. A., Wada K. (2010) Curr. Pharm. Biotechnol. 11, 188–197 [DOI] [PubMed] [Google Scholar]
- 27. Cummings C. J., Sun Y., Opal P., Antalffy B., Mestril R., Orr H. T., Dillmann W. H., Zoghbi H. Y. (2001) Hum. Mol. Genet. 10, 1511–1518 [DOI] [PubMed] [Google Scholar]
- 28. Adachi H., Katsuno M., Minamiyama M., Sang C., Pagoulatos G., Angelidis C., Kusakabe M., Yoshiki A., Kobayashi Y., Doyu M., Sobue G. (2003) J. Neurosci. 23, 2203–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Westerheide S. D., Morimoto R. I. (2005) J. Biol. Chem. 280, 33097–33100 [DOI] [PubMed] [Google Scholar]
- 30. Vígh L., Literáti P. N., Horváth I., Török Z., Balogh G., Glatz A., Kovács E., Boros I., Ferdinándy P., Farkas B., Jaszlits L., Jednákovits A., Korányi L., Maresca B. (1997) Nat. Med. 3, 1150–1154 [DOI] [PubMed] [Google Scholar]
- 31. Vígh L., Horváth I., Maresca B., Harwood J. L. (2007) Trends Biochem. Sci. 32, 357–363 [DOI] [PubMed] [Google Scholar]
- 32. Sõti C., Nagy E., Giricz Z., Vígh L., Csermely P., Ferdinandy P. (2005) Br. J. Pharmacol. 146, 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prahlad V., Morimoto R. I. (2009) Trends Cell Biol. 19, 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brenner S. (1974) Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Link C. D., Cypser J. R., Johnson C. J., Johnson T. E. (1999) Cell Stress Chaperones 4, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henderson S. T., Johnson T. E. (2001) Curr. Biol. 11, 1975–1980 [DOI] [PubMed] [Google Scholar]
- 37. McKay S. J., Johnsen R., Khattra J., Asano J., Baillie D. L., Chan S., Dube N., Fang L., Goszczynski B., Ha E., Halfnight E., Hollebakken R., Huang P., Hung K., Jensen V., Jones S. J., Kai H., Li D., Mah A., Marra M., McGhee J., Newbury R., Pouzyrev A., Riddle D. L., Sonnhammer E., Tian H., Tu. D., Tyson J. R., Vatcher G., Warner A., Wong K., Zhao Z., Moerman D. G. (2003) Cold Spring Harbor Symp. Quant. Biol. 68, 159–169 [DOI] [PubMed] [Google Scholar]
- 38. Gidalevitz T., Ben-Zvi A., Ho K. H., Brignull H. R., Morimoto R. I. (2006) Science 311, 1471–1474 [DOI] [PubMed] [Google Scholar]
- 39. Dillin A., Crawford D. K., Kenyon C. (2002) Science 298, 830–834 [DOI] [PubMed] [Google Scholar]
- 40. Kamath R. S., Fraser R. G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., Welchman D. P., Zipperlen P., Ahringer J. (2003) Nature 421, 231–237 [DOI] [PubMed] [Google Scholar]
- 41. Timmons L., Court D. L., Fire A. (2001) Gene 263, 103–112 [DOI] [PubMed] [Google Scholar]
- 42. Morley J. F., Morimoto R. I. (2004) Mol. Biol. Cell 15, 657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ben-Zvi A., Miller E. A., Morimoto R. I. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14914–14919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nollen E. A., Garcia S. M., van Haaften G., Kim S., Chavez A., Morimoto R. I., Plasterk R. H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6403–6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kieran D., Kalmar B., Dick J. R., Riddoch-Contreras J., Burnstock G., Greensmith L. (2004) Nat. Med. 10, 402–405 [DOI] [PubMed] [Google Scholar]
- 46. Chung J., Nguyen A. K., Henstridge D. C., Holmes A. G., Chan M. H., Mesa J. L., Lancaster G. I., Southgate R. J., Bruce C. R., Duffy S. J., Horvath I., Mestril R., Watt M. J., Hooper P. L., Kingwell B. A., Vigh L., Hevener A., Febbraio M. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1739–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalmar B., Novoselov S., Gray A., Cheetham M. E., Margulis B., Greensmith L. (2008) J. Neurochem. 107, 339–350 [DOI] [PubMed] [Google Scholar]
- 48. Hsu A. L., Murphy C. T., Kenyon C. (2003) Science 300, 1142–1145 [DOI] [PubMed] [Google Scholar]
- 49. Cohen E., Dillin A. (2008) Nat. Rev. Neurosci. 9, 759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cohen E., Bieschke J., Perciavalle R. M., Kelly J. W., Dillin A. (2006) Science 313, 1604–1610 [DOI] [PubMed] [Google Scholar]
- 51. Zuccato C., Valenza M., Cattaneo E. (2010) Physiol. Rev. 90, 905–981 [DOI] [PubMed] [Google Scholar]
- 52. Sharma S. K., De los Rios P., Christen P., Lustig A., Goloubinoff P. (2010) Nat. Chem. Biol. 6, 914–920 [DOI] [PubMed] [Google Scholar]
- 53. Satyal S. H., Schmidt E., Kitagawa K., Sondheimer N., Lindquist S., Kramer J. M., Morimoto R. I. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5750–5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Török Z., Tsevtkova N. M., Balogh G., Horváth I., Nagy E., Pénzes Z., Hargitai J., Bensaude O., Csermely P., Crowe J. H., Maresca B., Vigh L. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3131–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Escribá P. V. (2006) Trends Mol. Med. 12, 34–43 [DOI] [PubMed] [Google Scholar]
- 56. Hageman J., Rujano M. A., van Waarde M. A., Kakkar V., Dirks R. P., Govorukhina N., Oosterveld-Hut H. M., Lubsen N. H., Kampinga H. H. (2010) Mol. Cell 37, 355–369 [DOI] [PubMed] [Google Scholar]
- 57. Hinault M. P., Cuendet A. F., Mattoo R. U., Mensi M., Dietler G., Lashuel H. A., Goloubinoff P. (2010) J. Biol. Chem. 285, 38173–38182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Walker G. A., Lithgow G. J. (2003) Aging Cell 2, 131–139 [DOI] [PubMed] [Google Scholar]
- 59. Fonte V., Kipp D. R., Yerg J., 3rd, Merin D., Forrestal M., Wagner E., Roberts C. M., Link C. D. (2008) J. Biol. Chem. 283, 784–791 [DOI] [PubMed] [Google Scholar]
- 60. Walker G. A., White T. M., McColl G., Jenkins N. L., Babich S., Candido E. P., Johnson T. E., Lithgow G. J. (2001) J. Gerontol. A56, B281–B287 [DOI] [PubMed] [Google Scholar]
- 61. Veinger L., Diamant S., Buchner J., Goloubinoff P. (1998) J. Biol. Chem. 273, 11032–11037 [DOI] [PubMed] [Google Scholar]
- 62. Haslbeck M., Franzmann T., Weinfurtner D., Buchner J. (2005) Nat. Struct. Mol. Biol. 12, 842–846 [DOI] [PubMed] [Google Scholar]
- 63. Hansson O., Nylandsted J., Castilho R. F., Leist M., Jäättelä M., Brundin P. (2003) Brain Res. 970, 47–57 [DOI] [PubMed] [Google Scholar]
- 64. Hay D. G., Sathasivam K., Tobaben S., Stahl B., Marber M., Mestril R., Mahal A., Smith D. L., Woodman B., Bates G. P. (2004) Hum. Mol. Genet. 13, 1389–1405 [DOI] [PubMed] [Google Scholar]
- 65. Zourlidou A., Gidalevitz T., Kristiansen M., Landles C., Woodman B., Wells D. J., Latchman D. S., de Belleroche J., Tabrizi S. J., Morimoto R. I., Bates G. P. (2007) Hum. Mol. Genet. 16, 1078–1090 [DOI] [PubMed] [Google Scholar]
- 66. Katsuno M., Sang C., Adachi H., Minamiyama M., Waza M., Tanaka F., Doyu M., Sobue G. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 16801–16806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tokui K., Adachi H., Waza M., Katsuno M., Minamiyama M., Doi H., Tanaka K., Hamazaki J., Murata S., Tanaka F., Sobue G. (2009) Hum. Mol. Genet. 18, 898–910 [DOI] [PubMed] [Google Scholar]
- 68. Herbst M., Wanker E. E. (2007) Neurodegener. Dis. 4, 254–260 [DOI] [PubMed] [Google Scholar]
- 69. Lotz G. P., Legleiter J., Aron R., Mitchell E. J., Huang S. Y., Ng C., Glabe C., Thompson L. M., Muchowski P. J. (2010) J. Biol. Chem. 285, 38183–38193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hargitai J., Lewis H., Boros I., Rácz T., Fiser A., Kurucz I., Benjamin I., Vígh L., Pénzes Z., Csermely P., Latchman D. S. (2003) Biochem. Biophys. Res. Commun. 307, 689–695 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.