Abstract
Rhodopsin (Rho) is a prototypical G protein-coupled receptor that changes from an inactive conformational state to a G protein-activating state as a consequence of its retinal chromophore isomerization, 11-cis-retinal → all-trans-retinal. The photoisomerized chromophore covalently linked to Lys296 by a Schiff base is subsequently hydrolyzed, but little is known about this reaction. Recent research indicates a significant role for tightly bound transmembrane water molecules in the Rho activation process. Atomic structures of Rho and hydroxyl radical footprinting reveal ordered waters within Rho transmembrane helices that are located close to highly conserved and functionally important receptor residues, forming a hydrogen bond network. Using 18O-labeled H2O, we now report that water from bulk solvent, but not tightly bound water, is involved in the hydrolytic release of chromophore upon Rho activation by light. Moreover, small molecules (and presumably, water) enter the Rho structure from the cytoplasmic side of the membrane. Thus, this work indicates two distinct origins of water vital for Rho function.
Keywords: Receptor Desensitization, Receptor Recycling, Receptor Structure-Function, Receptors, Retinoid, Retinoid-binding Protein, Vision
Introduction
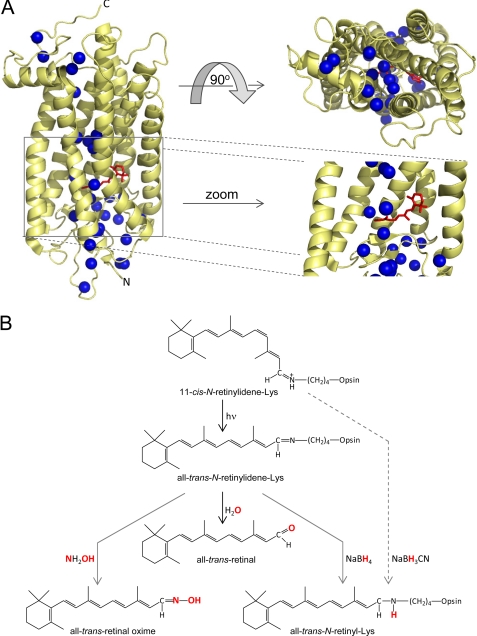
Rhodopsin (Rho),3 the visual pigment in retinal rod photoreceptors, is a G protein-coupled receptor responsible for dim light vision (1, 2). Rho is composed of its apoprotein, opsin, and a retinylidene chromophore in an 11-cis-conformation covalently linked by a Schiff base to Lys296 of the opsin (Fig. 1A). Photoactivation of Rho is initiated by isomerization of the retinylidene ligand to its all-trans-configuration (3), allowing the photoreceptor after deprotonation of the Schiff base to adopt the active Meta II state required for G protein (transducin) activation. The atomic structure of Rho led to the identification of crystallographically ordered water molecules adjacent to functionally important conserved protein residues (Fig. 1A) (4), supporting the conclusion that these waters are essential not just for structural stabilization of the receptor but also for the activation process. One of these tightly bound waters positioned close to the chromophore-binding pocket has been postulated to play a key role in the counterion switch between ground state (dark; Glu113) and activated states (Glu181) of Rho (5, 6). Moreover, Glu113 and Glu181 are also likely involved in the hydrolytic process via the carbinol ammonium ion and in a regeneration reaction between 11-cis-retinal and opsin. To form the Schiff base, Lys296 must be deprotonated, the carbonyl group must be polarized, and water must be accommodated within the chromophore-binding site (1). Hydroxyl radical footprinting revealed local conformational changes in the Rho structure following its photoactivation, presumably mediated by the dynamics of both ordered water molecules and the protein (7, 8). Moreover, protein footprinting combined with rapid H218O mixing methodology and deuterium-hydrogen exchange on C2 His residues indicated that these tightly bound internal waters do not exchange with bulk solvent in ground state Rho, Meta II, or opsin (7, 8). Thus, ordered waters function as noncovalent cofactors that actively participate in transmitting the activation signal from the retinylidene-binding pocket to the cytoplasmic face of Rho, where binding of transducin occurs. These individual waters are observed in high resolution structures of Rho as well as other G protein-coupled receptors, and a high level of conservation of polar residues in close proximity to these waters is also found in all G protein-coupled receptor sequences (9, 10).
FIGURE 1.
Water molecules present in the crystal structure of bovine Rho and the role of water and small molecules in retinylidene Schiff base cleavage and reduction. A, positions of selected waters (shown as blue spheres) in structures of bovine Rho (Protein Data Bank code 1U19). Several Rho-bound waters are located within the transmembrane helical bundle of the protein. The water network extends from the region of visual chromophore binding (red) to the N and C termini. A magnified view of the chromophore-binding site reveals the presence of oriented water molecules near the retinylidene Schiff base. B, schematic representation of retinylidene Schiff base chemical reactivity in the ground and photoactivated states of Rho. As a consequence of photoreceptor activation by light, the retinylidene-Lys covalent bond is hydrolyzed. In this process, a water molecule donates its oxygen atom to form a new at-RAL molecule. The activated state of Rho allows a small nucleophile (NH2OH) or reducing agents such as NaBH4 to penetrate inside the photoreceptor and chemically modify the Schiff base. Additionally, NaBH3CN was shown to be effective in reducing retinylidene-Lys bound in the ground state of Rho (38).
Rho does not react with hydroxylamine (NH2OH) in the dark, but changes in the Rho structure upon photoactivation allowing this small nucleophile to penetrate inside the protein and accelerate chromophore hydrolysis (Fig. 1B) (11). Molecular dynamic simulations and 1H magnetic spinning NMR indicate a dramatic increase in interior Rho hydration after 11-cis-retinylidene isomerization (12). This large increase in intratransmembrane region hydration is characteristic of the Meta I state and appears to be directly related to the Schiff base counterion interaction that occurs immediately after breaking the Glu113-Schiff base salt bridge. Water influx is not accompanied by a significant change in the overall structure of this receptor, but most likely, it plays a functional role in the transition of dark state Rho to its Meta I intermediate (13).
As a consequence of photoactivation and relaxation processes in opsin, the chromophore-Schiff base linkage is hydrolyzed, releasing all-trans-retinal (at-RAL) (Fig. 1B), but it is unclear if ordered water or bulk water is responsible for retinylidene Schiff base hydrolysis. Although a role of extracellular water in this process had been suggested (14), we addressed this issue by using 18O and 15N labeling methodologies combined with modern mass spectrometry techniques. This approach allowed the experimental determination of which side of the photoreceptor NH2OH (and presumably, bulk water) enters the chromophore-binding pocket upon Rho photoactivation.
EXPERIMENTAL PROCEDURES
Materials
Fresh bovine eyes were obtained from a local slaughterhouse (Mahan's Packing, Bristolville, OH). WT and Lrat-deficient (Lrat−/−) mice (15) were housed in the animal facility at the School of Medicine of Case Western Reserve University, and all animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committees. Manipulations in the dark were performed under dim red light transmitted through a filter (transmittance > 560 nm; No. 1 Safelight, Eastman Kodak). at-RAL was obtained from TRC (Toronto, Canada), and H218O (97 atom %) and 15NH2OH (98 atom %) was purchased from Sigma.
Mouse Retina and Bovine Rod Outer Segment (ROS) Isolation and Rho Purification
Retinas were removed from 24-h dark-adapted WT and Lrat−/− mouse eyeballs through an incision in the cornea and immediately immersed in 10 mm Bistris propane, pH 7.4, and 100 mm NaCl. Retinas were then washed twice with the same buffer, followed by centrifugation at 1000 × g to remove contaminants. Bovine ROS membranes were prepared from frozen retinas under dim red light according to the procedure of Papermaster (16). Isolated ROS membranes were washed five times with hypotonic buffer (5 mm Bistris propane, pH 7.5) to dispose of membrane-associated proteins. Washed ROS were stored at −80 °C or used immediately for Rho extraction. Rho was solubilized in n-nonylglucopyranoside and purified from ROS by ZnCl2/opsin precipitation (17). ZnCl2 was removed by dialysis in the presence of 0.5% n-nonylglucopyranoside. Rho concentrations were determined with a Cary 50 UV-visible spectrophotometer (Varian, Palo Alto, CA) and quantified by absorption at 500 nm using the absorption coefficient ϵ = 40,600 m−1 cm−1 (18).
Preparation of Bovine Rho Proteoliposomes
Proteoliposomes were prepared by the procedure described by Niu et al. (19). A solution of 1% asolectin (commercially available soybean lipids, Sigma) in buffer composed of 10 mm MES, pH 5.8, 100 mm NaCl, and 1% n-nonylglucopyranoside was mixed with purified Rho. The molar ratio of phospholipids to Rho was 250:1. This solution was dialyzed against buffer composed of 10 mm MES, pH 5.8, and 100 mm NaCl in the dark at 4 °C for 48 h with four changes of buffer totaling 8 liters. The proteoliposome suspension was pelleted by centrifugation at 100,000 × g for 1 h at 4 °C. To prepare vesicles with enclosed NH2OH, the above procedure was performed with buffers containing 50 mm NH2OH. Pelleted proteoliposomes were washed with 10 mm MES, pH 5.8, and 100 mm NaCl and centrifuged again at 100,000 × g for 1 h at 4 °C. Proteoliposomes were resuspended in the same buffer to achieve a final Rho concentration of ∼2 mg/ml. The efficiency of Rho incorporation into lipid vesicles was determined by measuring the absorption spectra of the supernatant and pellet recorded after the first ultracentrifugation in a Cary 50 UV-visible spectrophotometer.
Orientation of Rho in Proteoliposomes
A proteolysis assay with Asp-N endoprotease, which specifically cleaves Rho between Gly329 and Asp330 in the C terminus (20), was used to determine the orientation of Rho in asolectin vesicles. A total of 20 μl of proteoliposomes containing 30 μg of Rho in buffer composed of 10 mm Bistris propane, pH 7.4, and 100 mm NaCl was subjected to proteolysis. The sample was incubated at room temperature for 20 h, and an aliquot containing 5 μg of Rho was run on 10% SDS-polyacrylamide gel to resolve the cleaved fragments of Rho. The efficiency of digestion was determined by densitometric analysis with ImageJ software.
Chromophore Hydrolysis and Extraction
Bovine ROS membranes or mouse retinas were washed twice with 0.5 ml of buffer containing 10 mm Bistris propane, pH 7.0, and 100 mm NaCl prepared in either H216O or H218O and resuspended in the same buffer. The final concentration of Rho in ROS was 1.5 mg/ml. Each experimental tissue sample from mice contained the equivalent of two retinas. To initiate chromophore hydrolysis, 50 μl of Rho in ROS membranes was illuminated with a 150-watt bulb for 5 min, during which time the samples were placed on ice to prevent overheating. Retinoids were then immediately extracted with 0.3 ml of hexane. The organic phase was separated by centrifugation at 16,000 × g for 1 min, and retinoid composition was analyzed promptly by LC/MS (see below). In experiments with proteoliposomes, 30 μg of Rho incorporated into liposomes and resuspended in 0.2 ml of 10 mm MES, pH 5.8, and 100 mm NaCl was illuminated with a 150-watt bulb for 5 min. Both NH2OH-loaded and “empty” proteoliposomes were exposed to light in the presence or absence of 10 mm NH2OH. Depending on the experiment, the sample extraction procedure involved addition of either 0.3 ml of hexane or 0.2 ml of methanol prior to the hexane, followed by vigorous shaking for 2 min and centrifugation at 16,000 × g for 1 min. To control the time during which at-RAL reacted with NH2OH and to minimize derivatization upon extraction, samples were supplemented with 40 mm formaldehyde just prior to hexane addition. The efficiency of rhodopsin photoactivation in the above-described light conditions was ∼98% as deduced from the 11-cis/all-trans-retinal oxime ratio after retinoid extraction in the presence of hydroxylamine and methanol.
LC/MS of Retinoids
Retinoid composition was analyzed with an Agilent 1100 series HPLC system attached to an LXQ mass spectrometer (Thermo Scientific). Retinoids extracted with hexane were injected onto a normal phase HPLC column (5-μm ZORBAX SIL, 4.6 × 250 mm, Agilent Technology) equilibrated with 10% ethyl acetate in hexane. Retinals were separated by isocratic elution with the equilibration solvent at a flow rate of 1.4 ml/min. The eluate was directed into the mass spectrometer through a diode array detector, followed by an atmospheric pressure chemical ionization source working in the positive mode. Data were recorded and analyzed with Xcalibur 2.0.7 software (Thermo Scientific). Elution times for at-RAL and its oximes were determined based on their characteristic UV-visible spectra with absorbance maxima at 368 and 357 nm, respectively. The ratio between at-RAL and at-[18O]RAL present in a sample was calculated based on areas under m/z 285.3 [M + H]+ and 287.3 [M + H]+ peaks determined from extracted ion chromatograms that corresponded to the labeled and unlabeled retinoid.
Oxygen Exchange in at-RAL
at-[18O]RAL was synthesized according to a previously published procedure, extracted with hexane, and stored under argon at −80 °C (21). To monitor oxygen back-exchange, 300 pmol of at-[18O]RAL (70% enriched) delivered in 1 μl of N,N-dimethylformamide was incubated in 0.2 ml of 10 mm Bistris propane, pH 7.0, and 100 mm NaCl containing 1% BSA and added to liposomes composed of soybean lipid extract (250 μm), homogenized retinas isolated from Lrat−/− mice (two retinas/sample), or 50% methanol. Samples were vigorously vortexed and incubated under conditions identical to those used for Rho bleaching for various time periods as indicated under “Results.” at-RAL was then extracted with 0.4 ml of hexane. The isotopic composition of at-RAL was examined by MS. Analogous experiments were performed in buffers composed of H218O in which the rate of 16O exchange in at-RAL was recorded.
RESULTS
Carbonyl Oxygen Exchange in at-RAL
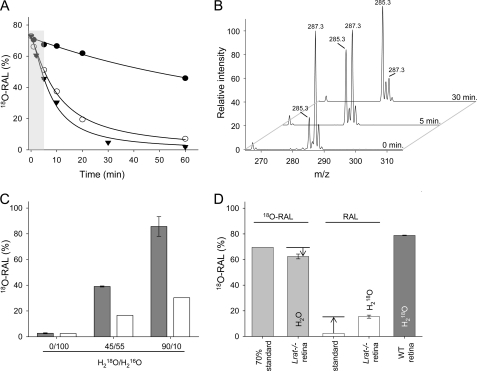
Aldehydes readily exchange their carbonyl oxygen with the oxygen atom of water (22). This replacement proceeds via a hydration-dehydration mechanism that depends on the electrophilicity of the carbonyl carbon and is accelerated by both acidic and basic conditions (23). This characteristic of aldehydes, which can lead to loss of the initial oxygen label, represents the main obstacle to interpreting experimental studies involving the mechanism of aldehyde formation (24). To counteract the potential effect of oxygen back-exchange, the rates of 18O isotope loss from synthetic at-[18O]RAL were studied under aqueous conditions in the presence of additives that included methanol, BSA, or lipids. Interestingly, progression of oxygen exchange, monitored by a decline of the m/z 287.3 signal in the mass spectrum corresponding to at-[18O]RAL ([M + H]+), strongly depended on the buffer composition and was much faster in the presence of proteins or lipids (Fig. 2, A and B). This phenomenon can be explained by transient formation of Schiff base adducts of at-[18O]RAL with available primary amino groups present in proteins or phospholipids. Reversibility of this reaction in the presence of water (hydrolysis) facilitates exchange of the 18O label with bulk solvent. Therefore, samples containing protein and lipids should be employed as appropriate controls for oxygen back-exchange in studies of at-RAL formation upon Rho photoactivation in ROS.
FIGURE 2.
Incorporation of 18O into at-RAL liberated from photoactivated Rho. A, exchange of 18O in synthetic at-[18O]RAL upon incubation in an aqueous solution containing 50% methanol (●), 1% BSA (○), or liposomes (▾). The 5-min time frame used for these experiments is indicated by the gray background. B, representative mass spectra indicate changes in the isotopic composition of at-[18O]RAL upon incubation in 10 mm Bistris propane buffer, pH 7.0, in 70% H218O with 100 mm NaCl and 1% BSA. The peak at m/z 285.3 corresponds to at-RAL ([M + H]+). The 2-Da shift in mass of at-[18O]RAL is indicated by the ion at m/z 287.3 ([M + H]+). C, incorporation of 18O into at-RAL released from photobleached Rho. Purified bovine ROS washed and resuspended in buffers containing various ratios of H218O were extracted with hexane, and the retinoid composition was examined by MS. The pool of analyzed at-RAL was significantly enriched in 18O. The ratio between at-RAL and at-[18O]RAL closely reflects the water composition present in the experimental samples (gray bars). The theoretical percentage of at-[18O]RAL that could be produced from unlabeled at-RAL due to oxygen back-exchange into buffers present in the samples is shown (white bars). D, retinal composition and oxygen back-exchange in isolated mouse retina. at-RAL or at-[18O]RAL (300 pmol) was added to homogenized retinas isolated from Lrat−/− mice and washed with buffers containing H218O or H2O. Samples were incubated on ice for 5 min and extracted with hexane. Oxygen back-exchange did not exceed 15% in the examined samples (light gray and white bars). WT mouse retinas washed with 90% H218O-containing buffer and exposed to light under similar experimental conditions revealed the presence of at-RAL highly enriched in 18O (dark gray bar). All data represent averaged values of three independent experiments, each performed in duplicate.
Origin of the Oxygen Atom in Newly Formed at-RAL Released from Light-activated Rho
To investigate the potential role of internal structural water molecules in retinylidene Schiff base hydrolysis, we washed isolated bovine ROS with buffers composed of water isotopically labeled with 18O. Because the tightly bound water of Rho does not exchange with bulk solvent in the ground state, unlabeled water molecules are preserved within the transmembrane helical bundle (7). Prepared experimental materials were exposed to light for 5 min and directly extracted with hexane. The amount of extracted at-RAL represented ∼10% of the total at-RAL found in a methanol-treated sample (protein denaturing conditions) and corresponded closely to the amount predicted by Meta II decay kinetics (25). Photoactivation of Rho in the presence of H218O followed by rapid organic extraction revealed a pool of at-RAL that was highly enriched in the 18O isotope (Fig. 2C). The isotopic composition of oxygen in at-RAL closely imitated that in the bulk water solvent and was much higher than the isotopic content of unlabeled at-RAL exposed to H218O predicted from oxygen exchange rates in control experiments (Fig. 2A). Thus, the contribution of unlabeled internal protein-bound water molecules to retinylidene Schiff base hydrolysis appears to be insignificant. Because experimental conditions might influence the rate of oxygen back-exchange, we examined retinas isolated from Lrat−/− mice to approximate the environment present in a biological sample. Although the lipid and protein composition and the retinal morphology are preserved in these animals, the retinoid pool is dramatically reduced due to metabolic blockade of the uptake, storage, and production of visual chromophore (15). Thus, the isotopic composition of externally added at-RAL cannot be diluted by internal retinoids. Experimentally determined oxygen back-exchange in retinas isolated from Lrat−/− mice that were supplemented with synthetic at-RAL was comparable to previous observations made in the presence of soybean lipids and indicated an ∼12% loss of label during the 5-min incubation (Fig. 2D). Consistent with the data obtained from bovine ROS, at-RAL liberated from WT mouse retinas resuspended in buffer containing H218O and exposed to light contained predominantly 18O (Fig. 2D). The above observations indicate that external water molecules penetrate the Rho photoreceptor upon light activation and contribute extensively to retinylidene Schiff base hydrolysis.
Light Activation of Rho Allows Small Molecules to Reach the Chromophore-binding Pocket from the Cytoplasmic Side
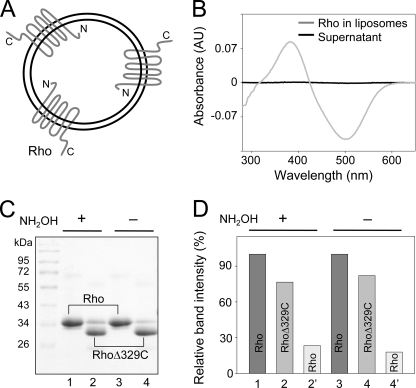
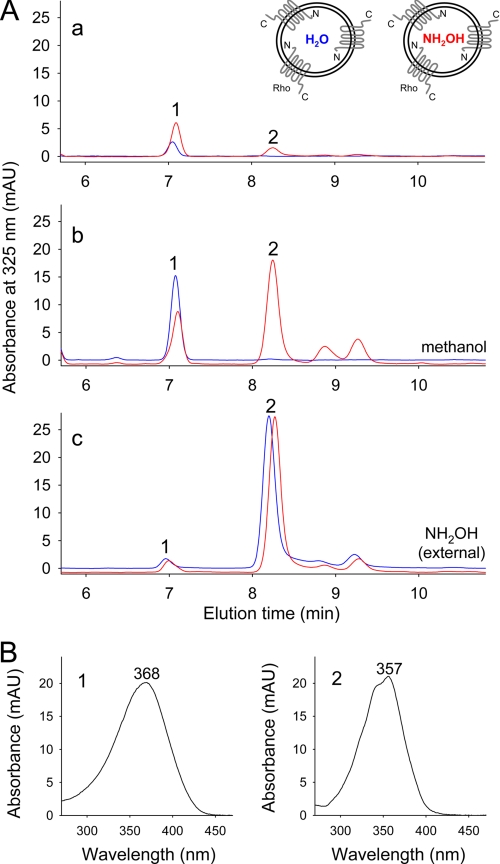
The N and C termini of Rho are oriented on opposite sides of the lipid bilayer and differ in terms of function (1). To investigate the potential asymmetrical role of these termini in the transduction of water molecules into the chromophore-binding pocket, we prepared proteoliposomes that contained preferentially oriented Rho with N termini located inside the liposomal lumen (Fig. 3A). The functional integrity of the preparation was examined spectrophotometrically by recording Meta II state formation in response to a light stimulus (Fig. 3B). The orientation of photoreceptors inserted into liposomes, as determined by proteolytic digestion with Asp-N protease, indicated that ∼85% of the protein was appropriately and uniformly oriented. Moreover, at-RAL released from rhodopsin incorporated into proteoliposomes was enriched in 18O to the same extent as was observed in the experiments on bovine ROS (data not shown). Notably, photoreceptor orientation in the liposomes was preserved during proteoliposome washes (Fig. 3, C and D). Therefore, it was possible to deplete the external NH2OH concentration without changing the lumenal pool of solvent and generate proteoliposomes loaded with NH2OH (Fig. 3A). Under our experimental conditions, Rho exposed to light is rapidly converted into the Meta II state, which is relatively stable at acidic pH. Moreover, hydrolysis of the retinylidene Schiff base in Meta II can be accelerated by added nucleophiles such as NH2OH. Consequently, extraction of proteoliposomes under nondenaturing conditions in the absence of NH2OH revealed the presence of only small amounts of at-RAL. Interestingly, the presence of NH2OH inside the liposomes did not provoke massive at-RAL oxime formation under these conditions (Fig. 4A, panel a). The small amount of oximes seen in chromatograms represented <10% of the total extractable at-RAL oximes and may have been derived from the small fraction of Rho molecules with a reversed orientation. However, significant amounts of at-RAL oximes were detected after liberation of NH2OH trapped inside the vesicles by addition of either methanol or detergent (10 mm diheptanoylphosphatidylcholine) following light exposure (Fig. 4A, panel b, data for methanol treatment shown). In this case, the extent of at-RAL derivatization depended on the amount of NH2OH released from inside the vesicles. The striking difference between these two groups of samples indicates that NH2OH was indeed trapped within the liposomal lumen and did not diffuse across the lipid membrane, at least within the time frame of this experiment. In contrast, the presence of NH2OH added externally to proteoliposomes led to the rapid breakdown of the retinylidene Schiff base, resulting in quantitative formation of at-RAL oximes in both types of proteoliposomes (Fig. 4A, panel c). Thus, NH2OH molecules involved in Schiff base decomposition came from outside the vesicles. Considering the orientation of this protein in liposomes, we conclude that small molecules enter the chromophore-binding pocket of activated Rho from its C-terminal side.
FIGURE 3.
Characterization of Rho proteoliposomes. A, schematic representation of Rho orientation within proteoliposomes. B, functional characterization of incorporated Rho. Spectrophotometric analyses of changes in absorption spectra observed after proteoliposomes were exposed to light indicate Meta II state formation. The difference spectrum was calculated by subtracting the spectrum recorded after and before light exposure. AU, absorbance units. C, determination of Rho molecular orientation by proteolytic digestion with Asp-N endoprotease, which specifically cleaves the C terminus of Rho. Lanes 1 and 3 represent washed intact proteoliposomes prepared in the presence or absence of NH2OH. Lanes 2 and 4 (protease-treated samples) reveal dominant protein bands corresponding to the Rho proteolytic fragment. D, densitometric quantification of protein bands shown in C indicate that at least 85% of Rho molecules preferentially adopt an orientation in which the N termini face the lumen of proteoliposomes.
FIGURE 4.
Side of NH2OH entry into light-activated Rho. Two types of Rho-containing proteoliposomes (prepared in either the absence or presence of NH2OH) were exposed to light while they were maintained in NH2OH-free buffers. Extracted retinoids were analyzed by HPLC. A, chromatograms show HPLC separation of at-RAL (peak 1) and its oxime (syn) (peak 2). Red traces correspond to proteoliposomes loaded with NH2OH, and blue traces represent data obtained from the empty vesicles. Panel a, extraction with hexane; panel b, disruption of proteoliposomes with methanol prior to extraction; panel c, incubation with NH2OH added after light exposure and 5 min prior to extraction. B, identification of detected retinoids. Peaks corresponding to at-RAL (left panel) and its oxime (syn) (right panel) were assigned based on the characteristic shapes and absorbance maxima of their UV-visible spectra. mAU, milliabsorbance units.
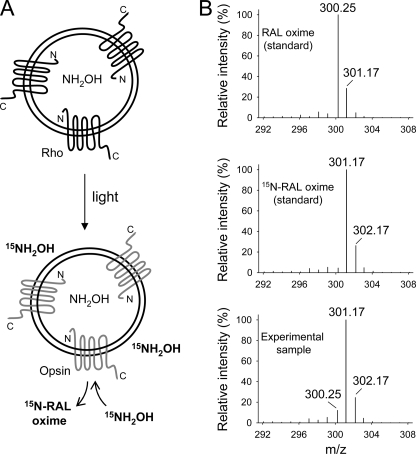
For improved discrimination between internal and external pools of NH2OH, proteoliposomes that contained NH2OH inside the vesicles were exposed to light in the presence of 10 mm 15N isotope-labeled reagent (15NH2OH) added externally during the bleaching procedure (Fig. 5A). Similar to previous experiments and to avoid potential cross-contamination during extraction, the excess of unreacted NH2OH was depleted by addition of 40 mm formaldehyde after the incubation period but just prior to adding the organic solvent. In this case, the isotopic composition of at-RAL oximes examined by MS revealed a predominant peak at m/z 301.17 ([M + H]+) corresponding to [15N]RAL oxime (Fig. 5B). The contribution of the m/z 300.25 peak corresponding to unlabeled at-RAL oxime in the mass spectrum of extracted retinoid was slightly higher than that in the spectrum of the synthetic [15N]RAL standard. However, it did not exceed 10% of the m/z 301.17 ion intensity. Again, this result could be a consequence of a small pool of Rho molecules with a reversed membrane orientation (Fig. 3). Together, these data indicate that only externally added 15NH2OH contributed to retinylidene Schiff base cleavage.
FIGURE 5.
Isotopic composition of at-RAL oximes. Proteoliposomes containing NH2OH were exposed to light in the presence of 15NH2OH added to the surrounding buffer. After extraction, the isotopic composition of at-RAL oxime (syn) liberated from Rho was examined by MS. A, schematic representation of the experimental setup. B, isotope distribution of the at-RAL oxime ([M + H]+) charged state. Upper and middle panels, unlabeled and 15N isotope-containing synthetic standards, respectively; lower panel, isotopic composition of at-RAL oxime (syn) extracted from proteoliposomes exposed to light. Robust incorporation of the 15N isotope into at-RAL oxime released from photoactivated Rho indicates that 15NH2OH derived from the C-terminal side of Rho is solely involved in retinylidene Schiff base cleavage.
DISCUSSION
Photoisomerization of the chromophore bound to Rho leads to activation of this G protein-coupled receptor. Consequently, the retinylidene Schiff base is hydrolyzed, followed by dissociation of at-RAL from the chromophore-binding pocket. We investigated this process in more detail and arrived at two significant conclusions: first, bulk solvent provides the source of water molecules utilized for chromophore hydrolysis following Rho activation, and second, a flux of small molecules (and presumably, water) enters into the retinal-binding pocket from the cytoplasmic side of this photoreceptor upon attainment of the activated state.
Increased intramembranous accessibility to external small molecules and water is required for Rho activation to advance beyond the Meta I state (26, 27). Moreover, the retinylidene chromophore becomes accessible to reagents such as NH2OH or NaBH4 only upon attainment of the Meta II state (11, 28). Results obtained by NMR and hydrogen-deuterium exchange also suggest that the hydration state of Rho increases upon photoactivation (12, 29). The process of retinylidene hydrolysis is complex, involving multiple protonation/deprotonation and proton transfer events. It most likely occurs in dark state Rho by employing the internal water network to mediate cleavage of the Schiff base linkage (30), but due to structural constraints provided by this state of the receptor, 11-cis-retinal release is hindered, and the Schiff base reforms. Subtle structural relaxation at the retinal-binding site rather than major conformational changes upon photoactivation can be envisioned as the driving force for this protein's transitions to new thermodynamically stable subconfigurations (31). The concomitant influx of water molecules upon photoactivation appears to remodel the network of internal water molecules relative to the ground state. This increase in hydration elevates the rate of deuterium exchange with the protonated Schiff base by several orders of magnitude (32). Such rapid proton exchange is enabled by an effective increase in water concentration near the retinal Schiff base. In D2O isotopic effect studies, slower rates of Schiff base hydrolysis were measured during Meta II decay, providing evidence that external D2O rather than internal water was involved in this process (30). Furthermore, exchange of the carbonyl oxygen of at-RAL released from Rho was visualized by FTIR spectroscopy (14). This evidence, along with our observations of 18O incorporation into at-RAL, indicates a role for bulk solvent in the transition from Meta II to opsin and hydrolyzed dissociated at-RAL.
Interestingly, increased hydration per se does not promote rapid replacement of tightly bound intertransmembrane waters as judged by radiolytic footprinting observations (7). Because the hydrolysis reaction requires a protonated Schiff base for efficacy, it is tempting to speculate that ordered water activated by the Glu113 side chain contributes to the protonation event, whereas the abundance of bulk solvent molecules ensures rapid and spontaneous hydrolysis of the retinylidene-Lys296 linkage. Thus, transient reprotonation of the Schiff base should be the rate-limiting step in overall Meta II decay, and the hydrogen bond network surrounding the chromophore-binding site would play an essential role in this process.
Ordered water molecules lie in a specific channel connecting the N- and C-terminal sides of Rho, which most likely acts as a conduit for the activation signal through the membrane. Increased hydration of Rho upon light exposure poses the question as to from which side of the membrane this incoming bulk water originates. To investigate this problem, we prepared proteoliposomes containing Rho preferentially oriented with its N termini facing the liposomal lumen, filled with unlabeled NH2OH, and its C termini, located on the liposome exterior, exposed to solvent enriched in 15N-labeled NH2OH. This experiment revealed that the NH2OH involved in Schiff base breakdown arose exclusively from the extravesicular space. Why would small polar molecules and water take such a long detour deep into the retinal-binding pocket if the chromophore is located closer to the lumen of the disk membrane and N terminus of Rho? The answer may relate to the fact that transmembrane helices III and IV, located on the extracellular side of Rho, are connected by an antiparallel β-sheet that forms a “plug,” completely masking the retinal-binding pocket and retinal from extracellular bulk solvent (33). This plug also hinders chromophore release from dark state Rho. Mutations in this region have been shown to perturb photoreceptor function, leading to retinitis pigmentosa (34). Minor structural changes in the same location have been identified by FTIR spectroscopy after Rho photoactivation. Therefore, it appears that changes in the N-terminal face of Rho that occur after light activation are not large enough to allow massive water influx from the extracellular space. Marked changes do occur at the cytoplasmic surface of Rho, opening its structure to the entry of bulk water. In fact, crystallographic structures of opsin and opsin complexed with the C-terminal peptide of Gαt display formation of a solvent-accessible cavity at the cytoplasmic site (35). Moreover, none of these structures reveal any openings on the extracellular side of this photoreceptor. However, in opsin, transmembrane bundles transiently open into the hydrophobic membrane region through two holes located between helices I and VII and helices V and VI (35). A connecting channel between these openings might provide the means for retinal passage after Schiff base hydrolysis, confirming earlier predictions based on random acceleration molecular dynamics (36, 37).
Acknowledgments
We thank Drs. Leslie T. Webster, Jr. and David Lodowski for critical comments on the drafts of the manuscript and Dr. K. Gawrisch (National Institutes of Health) for helpful advice during the course of this study.
This work was supported, in whole or in part, by National Institutes of Health Grants EY008061 and GM079191.
- Rho
- rhodopsin
- at-RAL
- all-trans-retinal
- ROS
- rod outer segment(s)
- Bistris propane
- 1,3-bis[tris(hydroxymethyl)methylamino]propane.
REFERENCES
- 1. Palczewski K. (2006) Annu. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Filipek S., Stenkamp R. E., Teller D. C., Palczewski K. (2003) Annu. Rev. Physiol. 65, 851–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshizawa T., Wald G. (1963) Nature 197, 1279–1286 [DOI] [PubMed] [Google Scholar]
- 4. Okada T., Fujiyoshi Y., Silow M., Navarro J., Landau E. M., Shichida Y. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5982–5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lüdeke S., Beck M., Yan E. C., Sakmar T. P., Siebert F., Vogel R. (2005) J. Mol. Biol. 353, 345–356 [DOI] [PubMed] [Google Scholar]
- 6. Sandberg M. N., Amora T. L., Ramos L. S., Chen M. H., Knox B. E., Birge R. R. (2011) J. Am. Chem. Soc. 133, 2808–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Angel T. E., Gupta S., Jastrzebska B., Palczewski K., Chance M. R. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14367–14372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lodowski D. T., Palczewski K., Miyagi M. (2010) Biochemistry 49, 9425–9427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mirzadegan T., Benkö G., Filipek S., Palczewski K. (2003) Biochemistry 42, 2759–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Angel T. E., Chance M. R., Palczewski K. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8555–8560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bownds D., Wald G. (1965) Nature 205, 254–257 [DOI] [PubMed] [Google Scholar]
- 12. Grossfield A., Pitman M. C., Feller S. E., Soubias O., Gawrisch K. (2008) J. Mol. Biol. 381, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szundi I., Ruprecht J. J., Epps J., Villa C., Swartz T. E., Lewis J. W., Schertler G. F., Kliger D. S. (2006) Biochemistry 45, 4974–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper A., Dixon S. F., Nutley M. A., Robb J. L. (1987) J. Am. Chem. Soc. 109, 7254–7263 [Google Scholar]
- 15. Batten M. L., Imanishi Y., Maeda T., Tu D. C., Moise A. R., Bronson D., Possin D., Van Gelder R. N., Baehr W., Palczewski K. (2004) J. Biol. Chem. 279, 10422–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papermaster D. S. (1982) Methods Enzymol. 81, 48–52 [DOI] [PubMed] [Google Scholar]
- 17. Okada T., Le Trong I., Fox B. A., Behnke C. A., Stenkamp R. E., Palczewski K. (2000) J. Struct. Biol. 130, 73–80 [DOI] [PubMed] [Google Scholar]
- 18. Wald G. (1968) Science 162, 230–239 [DOI] [PubMed] [Google Scholar]
- 19. Niu L., Kim J. M., Khorana H. G. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 13409–13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palczewski K., Buczyłko J., Imami N. R., McDowell J. H., Hargrave P. A. (1991) J. Biol. Chem. 266, 15334–15339 [PubMed] [Google Scholar]
- 21. McBee J. K., Kuksa V., Alvarez R., de Lera A. R., Prezhdo O., Haeseleer F., Sokal I., Palczewski K. (2000) Biochemistry 39, 11370–11380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Byrn M., Calvin M. (1966) J. Am. Chem. Soc. 88, 1916–1922 [Google Scholar]
- 23. Greenzaid P., Luz Z., Samuel D. (1967) J. Am. Chem. Soc. 89, 756–759 [Google Scholar]
- 24. Schmidt H., Kurtzer R., Eisenreich W., Schwab W. (2006) J. Biol. Chem. 281, 9845–9851 [DOI] [PubMed] [Google Scholar]
- 25. Jastrzebska B., Maeda T., Zhu L., Fotiadis D., Filipek S., Engel A., Stenkamp R. E., Palczewski K. (2004) J. Biol. Chem. 279, 54663–54675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wald G., Durell J., St George C. C. (1950) Science 111, 179–181 [DOI] [PubMed] [Google Scholar]
- 27. Rafferty C. N., Shichi H. (1981) Photochem. Photobiol. 33, 229–234 [DOI] [PubMed] [Google Scholar]
- 28. Akhtar M., Blosse P. T., Dewhurst P. B. (1968) Biochem. J. 110, 693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rath P., DeGrip W. J., Rothschild K. J. (1998) Biophys. J. 74, 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janz J. M., Farrens D. L. (2004) J. Biol. Chem. 279, 55886–55894 [DOI] [PubMed] [Google Scholar]
- 31. Salom D., Lodowski D. T., Stenkamp R. E., Le Trong I., Golczak M., Jastrzebska B., Harris T., Ballesteros J. A., Palczewski K. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16123–16128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deng H., Huang L., Callender R., Ebrey T. (1994) Biophys. J. 66, 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 34. Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 6481–6485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park J. H., Scheerer P., Hofmann K. P., Choe H. W., Ernst O. P. (2008) Nature 454, 183–187 [DOI] [PubMed] [Google Scholar]
- 36. Hildebrand P. W., Scheerer P., Park J. H., Choe H. W., Piechnick R., Ernst O. P., Hofmann K. P., Heck M. (2009) PLoS ONE 4, e4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang T., Duan Y. (2007) J. Am. Chem. Soc. 129, 6970–6971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fager R. S., Gentilcore P. C., Abrahamson E. W. (1978) Vision Res. 18, 483–488 [DOI] [PubMed] [Google Scholar]