Abstract
Autosomal dominant polycystic kidney disease (ADPKD), the most common inherited cause of kidney failure, is caused by mutations in either PKD1 (85%) or PKD2 (15%). The PKD2 protein, polycystin-2 (PC2 or TRPP2), is a member of the transient receptor potential (TRP) superfamily and functions as a nonselective calcium channel. PC2 has been found to form oligomers in native tissues, suggesting that similar to other TRP channels, it may form functional homo- or heterotetramers with other TRP subunits. We have recently demonstrated that the homodimerization of PC2 is mediated by both N-terminal and C-terminal domains, and it is known that PC2 can heterodimerize with PC1, TRPC1, and TRPV4. In this paper, we report that a single cysteine residue, Cys632, mutated in a known PKD2 pedigree, constitutes the third dimerization domain for PC2. PC2 truncation mutants lacking both N and C termini could still dimerize under nonreducing conditions. Mutation of Cys632 alone abolished dimerization in these mutants, indicating that it was the critical residue mediating disulfide bond formation between PC2 monomers. Co-expression of C632A PC2 mutants with wild-type PC2 channels reduced ATP-sensitive endoplasmic reticulum Ca2+ release in HEK293 cells. The combination of C632A and mutations disrupting the C-terminal coiled-coil domain (Val846, Ile853, Ile860, Leu867 or 4M) nearly abolished dimer formation and ATP-dependent Ca2+ release. However, unlike the 4M PC2 mutant, a C632A mutant could still heterodimerize with polycystin-1 (PC1). Our results indicate that PC2 homodimerization is regulated by three distinct domains and that these events regulate formation of the tetrameric PC2 channel.
Keywords: Calcium, Human Genetics, Kidney, Mutant, TRP Channels, ADPKD, TRPP2, Polycystic Kidney Disease, Polycystin-2
Introduction
Autosomal dominant polycystic kidney disease (ADPKD)3 accounts for ∼10% of patients on renal replacement therapy and is therefore an important cause of end-stage renal failure worldwide. It is the most common inherited human renal disease and results from germ line mutations in either PKD1 or PKD2 (1). The cardinal feature of the ADPKD kidney is the presence of multiple fluid-filled cysts. However, cysts can arise in other epithelial structures such as the liver and pancreas. Variable expression of noncystic manifestations such as cardiac valve abnormalities, diverticular disease, and intracranial aneurysms have been described in this condition (2).
Mutations in PKD2 account for 15% of all patients with ADPKD. The PKD2 protein, polycystin-2 (PC2), is a type II membrane protein with the properties of a high conductance nonselective Ca2+-permeable cation channel (3). PC2 (or TRPP2) has been included in the TRP (transient receptor potential) superfamily of channels due to its sequence homology to other TRP channels (4, 5). Its major site of action within the cell has been debated with evidence of ciliary, basolateral, and ER locations reported. In primary cilia, PC2 forms a flow-activated mechanosensitive channel complex in association with PC1 and/or TRPV4 (6, 7). At the basolateral membrane, PC2 (with PC1) could be implicated in cell-cell or cell-matrix adhesion, including possibly in mechanotransduction (8, 9). PC2 has also been shown to function as an ER Ca2+ release channel, in association with inositol trisphosphate and ryanodine receptors in different studies (10–12).
The finding of PC2 oligomers in native tissues indicated that similar to other TRP channels, PC2 channel assembly was likely to involve homodimerization and heterodimerization (8). PC2 interacts with PC1 in vitro and in vivo to form a stable heterodimeric complex (8). Interactions between the two proteins may regulate their trafficking, and there is evidence for reciprocal activation or inhibition of activity in different experimental systems (13–15). PC2 is also likely to function independently of PC1 in determining left-right asymmetry at the embryonic node (16). Interactions between PC2 and two other TRP channel subunits, TRPC1 and TRPV4, have also been reported (5, 7). The physiological function of PC2-TRPC1 channels is unknown, although it has distinct properties from homomeric PC2 and TRPC1 channels (17). A role in cutaneous thermosensation has been reported for PC2-TRPV4 channels (7).
In recent papers, we have described two distinct domains involved in PC2 homodimerization (18, 19). The C-terminal coiled-coil domain (CC2) specifically mediates C-terminal homodimerization, and this event is critical for PC1 recognition and binding (19). PC2 CC2 mutants were unable to interact with PC1 but were still able to dimerize via an N-terminal dimerization domain to form tetrameric ATP-sensitive ER Ca2+ release channels (19). In this paper, we report a third dimerization domain for PC2. Mutation at a single cysteine residue, Cys632, abolishes disulfide bond-dependent dimerization and impairs PC2 channel function.
EXPERIMENTAL PROCEDURES
Materials
All chemicals were purchased from Sigma unless otherwise stated.
Generation of PKD2 Plasmids
Unless otherwise stated, the PKD2 plasmids used in this paper have been reported previously (18–21). C-terminal Pk-tagged full-length PKD2 truncations were subcloned into pcDNA3 by PCR and ligation using the restriction sites XhoI and XbaI. Three truncation clones were generated: clone 1 had PKD2(224–968), clone 2 had PKD2(469–968), and clone 3 had PKD2(224–679). Site-specific mutations were introduced into full-length PKD2 and its truncations using the QuikChange Site-directed Mutagenesis protocol (Stratagene) as described previously. All changes were verified by nucleotide sequencing.
Immunoblotting and Immunoprecipitation
HEK293 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). Transient transfection was carried out on cells cultured to 60–80% confluence using GeneJuice transfection reagent (Novagen) according to the manufacturer's instructions. Immunoblotting and immunoprecipitation (IP) were performed as described previously using epitope-specific antibodies (8). 10 μg of total protein was loaded per lane, and IP was performed from 500 μg of total protein. Samples were separated on a 5% SDS-polyacrylamide gel under reducing and nonreducing conditions. The PC2 antibody, p30, generated to the C-terminal 258 amino acids of human PC2, and the PC1 mAb (7e12) have been reported previously (20, 22). The goat polyclonal PKD2 antibody G20 (sc-10376) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Oxidation of Cysteine Residues and Disulfide Bond Formation
HEK293 cells were transfected with the pcDNA3-Pk-PC2(224–679) plasmid and lysates prepared after 48 h. To specifically oxidize cysteine residues, performic acid was freshly prepared by mixing 1 volume of 30% H2O2 with 9 volumes of 100% formic acid and incubated at room temperature for 1 h (23). Performic acid was then added to HEK293 lysates at a 1:80 ratio (v/v) and neutralized with 5 m NaOH at a similar ratio prior to loading in nonreducing buffer. In parallel, cell lysates were treated with the reducing agents DTT (2 mm) or β-mercaptoethanol (5%), respectively, for 30 min to disrupt disulfide bonds. 15 μg of protein of each sample was resolved on 7.5% SDS-PAGE prior to immunodetection with an anti-Pk tag antibody.
Transfection of HEK293T Cells and Calcium Imaging
HEK293T cells were cultured as described previously (19). Briefly, HEK293T cells were maintained in DMEM-HEPES (GlutaMAXTM-l, 4,500 mg/liter glucose, 25 mm HEPES buffer) supplemented with 10% decomplemented fetal bovine serum, 1% MEM nonessential amino acids, and 1% sodium pyruvate. Cells at 60% confluence were transfected by Jet PEI (Polyplus transfection) or Lipofectamine 2000 (Invitrogen) in 0.7-cm2 wells coated with poly-l-lysine (5 μg/ml) or in 100-mm dishes. Coverslips with HEK293T cells were loaded with fura-PE3/AM (2 μm) for 30 min (37 °C, 5% CO2) in culture medium. Glass coverslips were then individually inserted in a specific chamber (Harvard Apparatus) and placed on the stage of an inverted epifluorescence microscope (Olympus IX71) equipped with a X40, UApo/340–1.15 W water-immersion objective. Fura-PE3 was alternately excited at 345 and 380 nm, and ratios of the resulting images (345/380) were produced every 5 s. The source of excitation light was a xenon arc lamp, and excitation wavelength was selected by a fast excitation filter wheel (Illumination Systems MT20; Olympus). Digital images were sampled at 12-bit resolution by a fast-scan, cooled charge-coupled device (B/W CCD) digital camera (Orca-ER, Hamamatsu). All images were background-subtracted and controlled by Cell-R software (Olympus).
The 340/380 ratios of emitted fura-PE3/AM fluorescence were calibrated using the standard equation of Grynkiewicz (24), [Ca2+] = Kd × β× ((R − Rmin)/(Rmax − R)), where R is the fluorescence ratio recorded at the two excitation wavelengths (F340 and F380), Kd represents the apparent dissociation constant of fura-PE3/AM for Ca2+ (266 nm), Rmin and Rmax are the fluorescence ratios under Ca2+-free and Ca2+-saturating (5 mm) conditions and β = F380, zero Ca2+/F380, saturating Ca2+ (24). The calibration was performed by perfusing HEK293T cells with 5 μm ionomycin during 15 min before bath perfusion of solutions containing no calcium (0 mm Ca2+ and 5 mm EGTA) or 5 mm Ca2+ (with no EGTA added). Data calibration was then confirmed by measuring the emitted fura-PE3/AM fluorescence in the presence of known Ca2+ concentrations ranging from 0 to 39 μm (data not shown) (Molecular Probes).
RESULTS
Identification of a Third Dimerization Domain for PC2
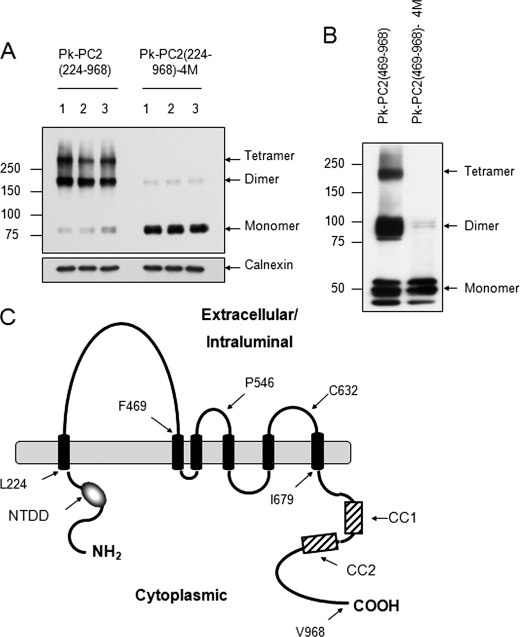
We recently reported the existence of two separate N- and C-terminal dimerization domains for PC2 important for channel assembly and activity (18, 19). To clarify the relative role of each domain for PC2 self-association, we initially deleted the entire N terminus from full-length PC2 (Pk-PC2(224–968)) (Fig. 1A). Surprisingly, this showed a very similar pattern to wild-type PC2 with prominent dimer and tetramer formation. The introduction of four substitution mutations (Val846, Ile853, Ile860, Leu867 or 4M) in the C-terminal CC2 has been shown to specifically disrupt C-terminal dimerization and abolish PC2 binding to PC1 (19). As shown in Fig. 1A, CC2 mutations also markedly reduced PC2 oligomerization to a predominant monomeric form, as seen with wild-type PC2. Deletion of the N-terminal domain did not completely abolish dimer formation in this protein. Furthermore, the combined deletion of both N- and C-terminal domains (Pk-PC2 (224–679)) permitted the formation of dimers under nonreducing conditions (Fig. 2B). These results indicated the existence of a third dimerization domain within the transmembrane region of PC2 between amino acids 224 and 679.
FIGURE 1.
Evidence of a third dimerization domain for PC2. A, expression pattern of epitope-tagged mutant PC2 on nonreducing SDS-PAGE. The truncation mutant PC2(224–968), which lacks the N terminus (1–223), is still able to form homodimers and tetramers. Mutation of CC2 (4M) in this construct results in a predominant monomeric pattern with faint but detectable dimers. Calnexin was used as an endogenous control for loading. B, deletion of the N terminus and first extracellular loop (469–968) shows a migration pattern similar to that of PC2(224–968) under nonreducing conditions. Mutation of CC2 (4M) results in a predominant monomeric pattern with faint dimers. C, diagram of PC2 showing its likely topology and key residues. The N-terminal dimerization domain (NTDD), coiled coil domains 1 (CC1) and 2 (CC2) within the C terminus have been described previously.
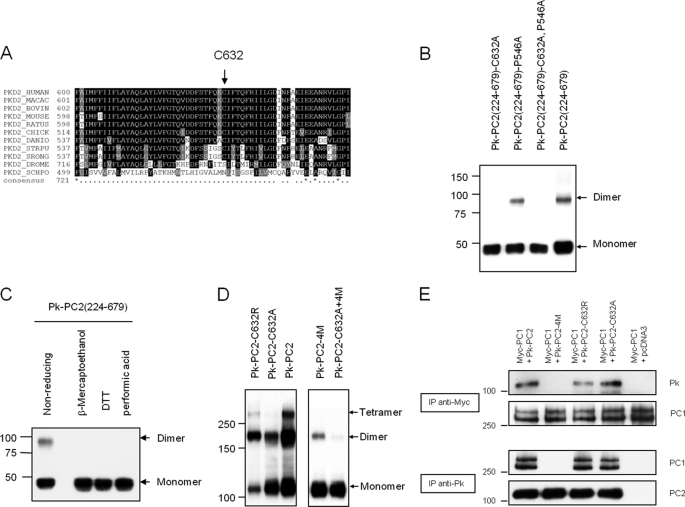
FIGURE 2.
A conserved cysteine residue in the third extracellular loop mediates PC2 dimerization. A, sequence alignment of Cys632 showing its complete evolutionary conservation from human to Caenorhabditis elegans PC2. B, expression pattern of epitope-tagged mutant PC2 on nonreducing SDS-PAGE. Deletion of both N- and C-terminal domains, PC2(224–679), does not abolish dimer formation. Mutation at Cys632 but not Pro546 abolishes the formation of dimer formation in the absence of the two known dimerization domains. C, cell lysates of PC2(224–679) resolved on SDS-PAGE. Lysates were either incubated under nonreducing conditions, reducing agents (β-mercaptoethanol, DTT), or performic acid prior to loading. Immunoblotting was with anti-Pk tag antibody. D, expression pattern of epitope-tagged full-length PC2 on nonreducing SDS-PAGE. Wild-type PC2 migrates most prominently with dimeric and tetrameric species. The C632A mutation abolished tetramer bands but with an equal ratio of monomers and dimers. The double mutation (C632A+4M) almost completely abolished dimer formation. The pattern observed with C632R was intermediate between wild type and C632A. E, immunoprecipitation assays with epitope-tagged full-length PC1 and PC2. Mutation at C632A or C632R did not affect the PC1-PC2 interaction, whereas CC2 mutations (4M) abolished PC1-PC2 interactions.
Deletion of the First Extracellular Loop Does Not Abolish Dimer Formation
In view of the sensitivity of PC2 dimerization to reducing agents, we hypothesized that cysteine residues within the putative extracellular loops of plasma membrane located PC2 (or the intraluminal loops for ER localized PC2) were most likely to be mediating PC2 dimerization through the formation of disulfide bonds. The first loop (amino acids 245–468) contains three cysteine residues at cysteines 331, 344, and 437.
We initially deleted the entire N-terminal region including the first extracellular loop (amino acids 1–468) in combination with a deletion of the C-terminal domain (amino acids 679–968). However, this mutant protein was highly unstable when expressed in HEK293 cells (data not shown). We therefore revised our strategy to generate a deletion construct omitting the N-terminal and first loop deletion (Pk-PKD2(469–968)) only. As shown in Fig. 1B, deletion of the first loop did not alter dimer or tetramer formation. Once again, introduction of CC2 mutations (4M) markedly reduced but did not abolish dimer formation under nonreducing conditions though tetramers were not visualized (Fig. 1B). These results indicate that the first loop is not involved in mediating homodimerization.
A Single Cysteine Residue in the Third Extracellular Loop Constitutes the Third Dimerization Domain
We next sought to investigate the role of other cysteine residues in either the second (amino acids 527–550) or third (amino acids 620–658) extracellular loops. There is a single cysteine residue in the third loop (Cys632) which has been reported to be mutated in a PKD2 pedigree (C632R) (25). Of relevance, this residue is highly conserved between human and worm PC2 protein (Fig. 2A). It also lies within the predicted pore forming region although no functional assays had been previously performed.
We generated a site-specific C632A mutation in a construct with both N- and C-terminal domains deleted (Pk-PKD2(224–679)). Deletion of both domains had no effect, but the C632A mutation completely abolished dimer formation in the absence of both N- and C-terminal domains under nonreducing conditions (Fig. 2B). In contrast, mutation of an irrelevant residue (P546A) in the second extracellular loop had no effect (Fig. 2B). Incubation of the Pk-PKD2(224–679)) protein with oxidizing or reducing agents confirmed that dimer formation was mediated by disulfide bonds between two cysteine residues (Fig. 2C).
Introduction of the C632A mutation into full-length PC2 shifted the ratio between monomers and dimers to the monomeric form with no tetramers seen (Fig. 2D). Under the same conditions, wild-type PC2 showed prominent dimers and tetramers (Fig. 2D). However, a C632R mutant had an oligomerization pattern intermediate between wild type and C632A. The reason for the difference between the C632A and C632R mutants is unclear. CC2 mutations (4M) had a more marked effect than C632A on dimer formation. Dimer formation was almost completely abolished in the combined mutant (C632A+4M), although a faint dimer band presumably due to the N-terminal dimerization domain could still be visualized on prolonged exposure (Fig. 2D).
Mutations of Cys632 Do Not Alter Formation of the PC1-PC2 Complex
Mutations of CC2 (4M) have been shown to abolish PC1 recognition and formation of a functional PC1-PC2 receptor-ion channel complex (19). We examined whether a C632A or C632R mutation would affect PC1 binding. As shown in Fig. 2D, PC1 bound normally to PC2 C632A and C632R mutants but not to PC2–4M in co-IP assays. These results confirm that binding to PC1 is specifically determined by C-terminal dimerization through the CC2 (Fig. 2E). It seems likely that the role of Cys632-mediated dimerization is in the assembly of tetrameric PC2 channels rather than the heteromeric PC1-PC2 channel complex.
Mutations of Cys632 Reduce ATP-sensitive Ca2+ Release from ER-located PC2 Channels
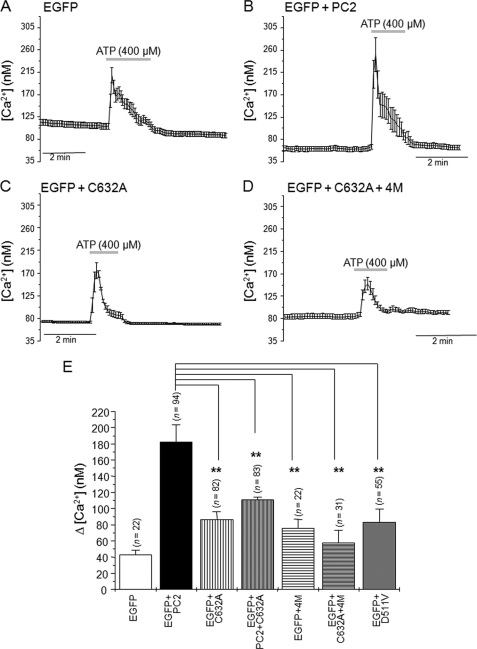
We have recently shown that PC2 channels can function as receptor-operated ER located homomeric Ca2+ release channels independently of PC1 (19). Based on these findings, we compared Ca2+ release from intracellular stores in response to ATP (400 μm) in cells expressing wild-type PC2 (PC2wt) and cells expressing PC2-C632A, PC2wt+PC2-C632A, PC2–4M, and PC2-C632A+4M mutants (Fig. 3). Compared with cells expressing wild-type PC2 (Δ[Ca2+], 182.06 ± 21.18, n = 94), ATP-mediated Ca2+ transients had significantly (p < 0.01) smaller amplitudes in cells expressing PC2-C632A (Δ[Ca2+], 86.66 ± 9.20, n = 82), PC2wt+PC2-C632A (Δ[Ca2+], 111.39 ± 4.60, n = 83), PC2–4M (Δ[Ca2+], 76.04 ± 10.74, n = 22), and PC2-C632A+4M (Δ[Ca2+], 57.72 ± 15.68, n = 31). We confirmed that Ca2+ release was mediated by ER PC2 by expressing the channel-dead mutant PC2-D511V. In these cells, the ATP-induced Ca2+ rise (Δ[Ca2+], 83.37 ± 16.25, n = 55) was not significantly different from ATP responses obtained in cells expressing EGFP alone (Δ[Ca2+], 43.24 ± 5.68, n = 22). These data indicate that residue Cys632 has a key role in the formation of functional ER tetrameric PC2 channels.
FIGURE 3.
The Cys632 mutation abrogates ATP-dependent PC2 Ca2+ release channel activity. A–D, averaged calcium transients in response to ATP application (400 μm) in HEK293T cells expressing EGFP (A), EGFP + PC2 (B), EGFP + PC2-C632A (C), and EGFP + PC2-C632A+4M (D). The gray horizontal bars indicate duration of ATP application. E, histogram showing the average rise in [Ca2+]i induced by ATP in cells expressing EGFP (0.5 μg of cDNA), EGFP + PC2 (0.5 μg of cDNA), EGFP + PC2-C632A, EGFP + PC2 + PC2-C632A, EGFP + PC2–4M, EGFP + PC2-C632A+4 M and EGFP + PC2-D511V. Bars represent mean ± S.E. *, p < 0.05; **, p < 0.01.
DISCUSSION
Mutations in PKD1 and PKD2 give rise to ADPKD whose clinical phenotype is characterized by the formation of fluid-filled cysts in the kidney and other organs as well as the presence of noncystic manifestations such as vascular aneurysms and cardiac valve defects. PC1 and PC2 have been shown to interact via their C-terminal domains (26, 27), and a native PC1-PC2 complex has been identified (8). It seems likely therefore that signals resulting from the interaction between both proteins are critical to the maintenance of normal kidney structure and function.
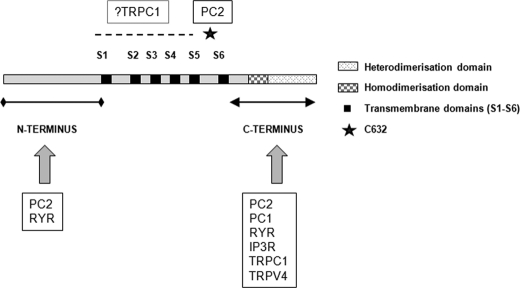
PC2 is known to form oligomers in native cells and tissues (8), and there is evidence that PC2 can function independently of PC1 at the embryonic node in the determination of body axis patterning (16). It is unclear whether this PC2-specific function is mediated by PC2 ciliary complexes or by PC2-ER complexes because there is evidence for the involvement of both in different studies (16, 19, 28). Unusually for TRP channels, PC2 has also been reported to interact with subunits from other TRP subfamilies such as TRPC1 and TRPV4 which co-localize to primary cilia (7, 17). PC2 interacts via its C terminus with the C termini of TRPC1 and TRPV4 (Fig. 4). In addition, PC2 and TRPC1 reportedly interact through their transmembrane regions, although it is unclear whether this interaction is direct (5). The physiological significance of these ciliary and/or plasma membrane complexes in kidney function is not yet clear. Mice deficient in TRPC1 and TRPV4 do not develop kidney cysts.
FIGURE 4.
Diagrammatic representation of PC2 homophilic and heterophilic interaction domains. The various interaction domains between PC2 and a number of key calcium channel proteins are shown. Apart from PC2, PC1, and inositol trisphosphate receptor (IP3R), the key residues mediating other interactions have not been fully defined. RYR, ryanodine receptor.
Functional interactions between PC2 and other major ER Ca2+ release channels such as the inositol trisphosphate receptor and the type II ryanodine receptor (in cardiac tissue) have also been reported (11, 12, 29). PC2 binds via its C terminus to both receptors, although possibly to the ryanodine receptor in its open state (Fig. 4). An additional N-terminal binding site for PC2 to the ryanodine receptor has been described (12). Again, the functional significance of these ER complexes is uncertain. In addition, the precise stoichiometry of the various PC2 complexes has not been resolved.
In this paper, we report that PC2 homodimerization is regulated by a third dimerization domain. In recent studies, we reported the existence of a new N-terminal dimerization domain (18) and with others, a previously unrecognized C-terminal CC2 for PC2 (19, 30, 31). Although there is disagreement as to whether the latter leads to dimerization or trimerization of the PC2 C terminus, oligomerization of PC2 appears to be an absolute requirement for PC1 binding (19, 30). The specificity of CC2 in mediating PC2 dimerization for PC1 recognition is confirmed by the lack of effect of the Cys632 mutants in this study.
Previously, we showed that PC2 mutants unable to undergo C-terminal dimerization (PC2–4M) were still able to form ER-based Ca2+ release channels and could partially rescue the LR defect seen in zebrafish embryos though not the cystic pronephric kidney (19). These results indicated that although C-terminal dimerization is specific to the formation of PC1-PC2 heteromers, other upstream dimerization regions (including the N-terminal domain) are critical to the formation of homomeric PC2 complexes. Our results demonstrate that PC2-C632A mutants have reduced ER PC2-mediated Ca2+ release similar to the PC2–4M mutants and predict that they will be similarly functionally effective in rescuing the laterality defect.
In conclusion, we demonstrate that PC2 homodimerization is regulated by three distinct domains and that these events regulate formation of the tetrameric PC2 channel (Fig. 5). Our results define a single cysteine residue, Cys632, known to be mutated in a PKD2 pedigree, as a key functional residue regulating PC2 channel activity (25). Cys632 lies in a putative third extracellular loop (for plasma membrane channel) or third intraluminal loop (for the ER channel) which constitutes part of the predicted channel pore region for PC2. Our studies indicate that it mediates disulfide bonding between PC2 monomers to form homodimers critical for the formation of tetrameric channels (Fig. 5). Co-expression of a C632A mutant suppressed the effect of wild-type PC2 in ER Ca2+ release assays, proving the functional importance of this interaction. Of interest, a combined mutant (C632A+4M) retaining the N-terminal domain had almost absent ATP-sensitive PC2-dependent Ca2+ release. In a previous study, we found that expression of the N-terminal dimerization domain as a surface-anchored protein in mammalian cells could inhibit plasma membrane PC2 channel activity in mIMCD3 cells (18). These results imply that the N-terminal dimerization domain is not critical for the function of ER PC2 channels but is required for the function of surface PC2 channels. In LLCPK-1 cells, surface PC2 can be activated via membrane depolarization or EGF stimulation. This effect appears to be mediated by the unblocking of PC2 by mDia-1 through its activation by RhoA (32). It seems likely that distinct regulatory pathways, possibly through altering key protein-protein interactions, regulate the function of the ER and surface PC2 channels.
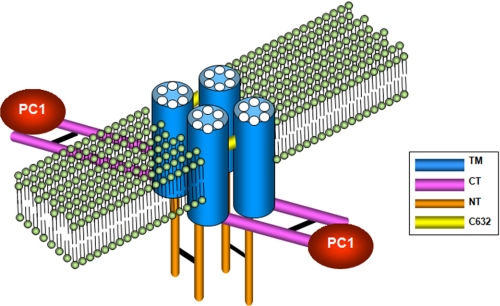
FIGURE 5.
Model of PC2 in a lipid bilayer showing the three dimerization domains. PC2 is displayed as a homotetramer with the transmembrane domains (TM) embedded in a lipid bilayer. C-terminal (CT) homodimerization mediated by the C-terminal CC2 (Ser835-Ala873) is indicated by a black line. CC2-mediated dimerization has a specific role in PC1 recognition and formation of a PC1-PC2 heteromeric complex. N-terminal (NT) dimerization mediated by the N-terminal dimerization domain (Gly199-Glu207) is indicated by a black line. Disulfide bonding between PC2 monomers mediated by Cys632 is indicated by a yellow line.
This work was funded by Wellcome Trust Grant GR071201 (to A. C. M. O.) and a Sheffield Kidney Research Foundation (to A. C. M. O.).
- ADPKD
- autosomal dominant polycystic kidney disease
- CC2
- coiled-coil domain 2
- ER
- endoplasmic reticulum
- PC2
- polycystin-2
- TRP
- transient receptor potential.
REFERENCES
- 1. Calvet J. P., Grantham J. J. (2001) Semin. Nephrol. 21, 107–123 [DOI] [PubMed] [Google Scholar]
- 2. Wilson P. D. (2004) N. Engl. J. Med. 350, 151–164 [DOI] [PubMed] [Google Scholar]
- 3. Mochizuki T., Wu G., Hayashi T., Xenophontos S. L., Veldhuisen B., Saris J. J., Reynolds D. M., Cai Y., Gabow P. A., Pierides A., Kimberling W. J., Breuning M. H., Deltas C. C., Peters D. J., Somlo S. (1996) Science 272, 1339–1342 [DOI] [PubMed] [Google Scholar]
- 4. Montell C., Birnbaumer L., Flockerzi V., Bindels R. J., Bruford E. A., Caterina M. J., Clapham D. E., Harteneck C., Heller S., Julius D., Kojima I., Mori Y., Penner R., Prawitt D., Scharenberg A. M., Schultz G., Shimizu N., Zhu M. X. (2002) Mol. Cell 9, 229–231 [DOI] [PubMed] [Google Scholar]
- 5. Tsiokas L., Arnould T., Zhu C., Kim E., Walz G., Sukhatme V. P. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3934–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. (2003) Nat. Genet. 33, 129–137 [DOI] [PubMed] [Google Scholar]
- 7. Köttgen M., Buchholz B., Garcia-Gonzalez M. A., Kotsis F., Fu X., Doerken M., Boehlke C., Steffl D., Tauber R., Wegierski T., Nitschke R., Suzuki M., Kramer-Zucker A., Germino G. G., Watnick T., Prenen J., Nilius B., Kuehn E. W., Walz G. (2008) J. Cell Biol. 182, 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newby L. J., Streets A. J., Zhao Y., Harris P. C., Ward C. J., Ong A. C. (2002) J. Biol. Chem. 277, 20763–20773 [DOI] [PubMed] [Google Scholar]
- 9. Streets A. J., Newby L. J., O'Hare M. J., Bukanov N. O., Ibraghimov-Beskrovnaya O., Ong A. C. (2003) J. Am. Soc. Nephrol. 14, 1804–1815 [DOI] [PubMed] [Google Scholar]
- 10. Koulen P., Cai Y., Geng L., Maeda Y., Nishimura S., Witzgall R., Ehrlich B. E., Somlo S. (2002) Nat. Cell Biol. 4, 191–197 [DOI] [PubMed] [Google Scholar]
- 11. Li Y., Wright J. M., Qian F., Germino G. G., Guggino W. B. (2005) J. Biol. Chem. 280, 41298–41306 [DOI] [PubMed] [Google Scholar]
- 12. Anyatonwu G. I., Estrada M., Tian X., Somlo S., Ehrlich B. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6454–6459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanaoka K., Qian F., Boletta A., Bhunia A. K., Piontek K., Tsiokas L., Sukhatme V. P., Guggino W. B., Germino G. G. (2000) Nature 408, 990–994 [DOI] [PubMed] [Google Scholar]
- 14. Xu G. M., González-Perrett S., Essafi M., Timpanaro G. A., Montalbetti N., Arnaout M. A., Cantiello H. F. (2003) J. Biol. Chem. 278, 1457–1462 [DOI] [PubMed] [Google Scholar]
- 15. Delmas P., Nauli S. M., Li X., Coste B., Osorio N., Crest M., Brown D. A., Zhou J. (2004) FASEB J. 18, 740–742 [DOI] [PubMed] [Google Scholar]
- 16. McGrath J., Somlo S., Makova S., Tian X., Brueckner M. (2003) Cell 114, 61–73 [DOI] [PubMed] [Google Scholar]
- 17. Bai C. X., Giamarchi A., Rodat-Despoix L., Padilla F., Downs T., Tsiokas L., Delmas P. (2008) EMBO Rep. 9, 472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng S., Okenka G. M., Bai C. X., Streets A. J., Newby L. J., DeChant B. T., Tsiokas L., Obara T., Ong A. C. (2008) J. Biol. Chem. 283, 28471–28479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giamarchi A., Feng S., Rodat-Despoix L., Xu Y., Bubenshchikova E., Newby L. J., Hao J., Gaudioso C., Crest M., Lupas A. N., Honoré E., Williamson M. P., Obara T., Ong A. C., Delmas P. (2010) EMBO J. 29, 1176–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ong A. C., Ward C. J., Butler R. J., Biddolph S., Bowker C., Torra R., Pei Y., Harris P. C. (1999) Am. J. Pathol. 154, 1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Streets A. J., Moon D. J., Kane M. E., Obara T., Ong A. C. (2006) Hum. Mol. Genet. 15, 1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ong A. C., Harris P. C., Davies D. R., Pritchard L., Rossetti S., Biddolph S., Vaux D. J., Migone N., Ward C. J. (1999) Kidney Int. 56, 1324–1333 [DOI] [PubMed] [Google Scholar]
- 23. Hirs C. H. W. (1967) in Methods in Enzymology (Colowick S. P., Kaplan N. O. eds) vol. 11, pp. 198–199, Academic Press, New York [Google Scholar]
- 24. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 25. Magistroni R., He N., Wang K., Andrew R., Johnson A., Gabow P., Dicks E., Parfrey P., Torra R., San-Millan J. L., Coto E., Van Dijk M., Breuning M., Peters D., Bogdanova N., Ligabue G., Albertazzi A., Hateboer N., Demetriou K., Pierides A., Deltas C., St. George-Hyslop P., Ravine D., Pei Y. (2003) J. Am. Soc. Nephrol. 14, 1164–1174 [DOI] [PubMed] [Google Scholar]
- 26. Tsiokas L., Kim E., Arnould T., Sukhatme V. P., Walz G. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qian F., Germino F. J., Cai Y., Zhang X., Somlo S., Germino G. G. (1997) Nat. Genet. 16, 179–183 [DOI] [PubMed] [Google Scholar]
- 28. Fu X., Wang Y., Schetle N., Gao H., Pütz M., von Gersdorff G., Walz G., Kramer-Zucker A. G. (2008) J. Am. Soc. Nephrol. 19, 1342–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sammels E., Devogelaere B., Mekahli D., Bultynck G., Missiaen L., Parys J. B., Cai Y., Somlo S., De Smedt H. (2010) J. Biol. Chem. 285, 18794–18805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu Y., Ulbrich M. H., Li M. H., Buraei Z., Chen X. Z., Ong A. C., Tong L., Isacoff E. Y., Yang J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 11558–11563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Celiæ A., Petri E. T., Demeler B., Ehrlich B. E., Boggon T. J. (2008) J. Biol. Chem. 283, 28305–28312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bai C. X., Kim S., Li W. P., Streets A. J., Ong A. C., Tsiokas L. (2008) EMBO J. 27, 1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]