Abstract
HoxA10 is a homeodomain transcription factor that influences a number of developmental processes, including hematopoiesis. During definitive hematopoiesis, expression of HoxA10 is maximal in committed myeloid progenitor cells and decreases as differentiation proceeds. Aberrantly increased expression of HoxA10 was found in bone marrow cells in a poor prognosis subset of human acute myeloid leukemia (AML). Consistent with this, AML developed in mice transplanted with HoxA10-overexpressing bone marrow. However, relatively few target genes have been identified that explain the role of HoxA10 in leukemogenesis. In the current study, we identified CDX4 as a HoxA10 target gene. Cdx4 is a homeodomain transcription factor that was also implicated in myeloid leukemogenesis. Although relatively few Cdx4 target genes have been identified, Cdx4 was known to influence HOX gene transcription. We identified a HoxA10-binding cis element in the CDX4 promoter that activated transcription. We also identified a Cdx4-binding cis element that activated the HOXA10 promoter. Therefore, increased Cdx4 expression in HoxA10-overexpressing cells augmented transcription of the endogenous HOXA10 gene. Increased endogenous HoxA10 in these cells induced additional CDX4 transcription. We found that Cdx4 influenced transcription of HoxA10 target genes in a HoxA10-dependent manner. Similarly, HoxA10 influenced transcription of HOX genes in a Cdx4-dependent manner. We previously found that HoxA10-overexpressing myeloid progenitors were hypersensitive to a variety of cytokines. In the current studies, we found that Cdx4 knockdown decreased cytokine hypersensitivity of HoxA10-overexpressing cells. Therefore, these studies identified a positive feedback relationship between HoxA10 and Cdx4, which potentially amplified the contribution of either transcription factor to the pathogenesis of AML.
Keywords: Gene Expression, Gene Transcription, Homeobox, Leukemia, Myeloid Cell
Introduction
Hox proteins are homeodomain (HD)2 transcription factors that are highly conserved from Drosophila to humans. The human and murine HOX genes are arranged in four groups (A–D) on four different chromosomes with 9 and 11 genes in each group (1). HOX gene transcription is tightly regulated during embryogenesis with activation occurring from 5′ to 3′ cranial to caudal (1). HOX gene transcription is also tightly regulated during definitive hematopoiesis with the 5′-most genes active in hematopoietic stem cells (HSC) and 3′ genes in differentiating progenitors (2). Therefore, HOX1 to -4 genes are actively transcribed in HSC, and HOX7 to -11 (also referred to as posterior or ABD HOX genes) are transcribed in committed and differentiating progenitors.
The spectrum of Hox activity during hematopoiesis has been characterized by studies in human disease and murine models. In such studies, overexpression of HoxB3 or HoxB4 in murine bone marrow cells was associated with HSC expansion in vitro and in vivo. However, leukemia did not develop in mice transplanted with HoxB3- or HoxB4-overexpressing bone marrow (3, 4). In contrast, overexpression of either HoxA9 or HoxA10 in murine bone marrow expanded the committed myeloid progenitor population in vitro (granulocyte/monocyte progenitors; GMP) and resulted in development of a myeloproliferative disorder in vivo (5–9). Additional studies suggested that HoxA10 blocked myeloid differentiation and that HoxA9 promoted the choice of myeloid over lymphoid differentiation (10, 11). Consistent with these observations, the myeloproliferative disorder in HoxA10-overexpressing mice progressed to AML over time. However, the myeloproliferative disorder due to HoxA9 overexpression only progressed to AML in the presence of co-overexpression of Meis1, a proto-oncogene and frequent Hox DNA-binding partner (5, 12).
In addition to these murine models, correlative studies of human AML also implicated Hox proteins in leukemogenesis. These studies identified a statistically significant increase in expression of HoxB3, HoxB4, and HoxA9–HoxA11 in CD34+ bone marrow cells from human subjects with treatment-refractory, poor prognosis AML (13–15). Increased Hox expression was associated with a number of recurring chromosomal translocations, including those involving 11q23 (the location of the MLL gene) (16–19). Increased Hox expression was also found in a subset of cytogenetically normal AML with poor prognosis.
These studies suggested that dysregulated Hox expression was a potential contributor to the pathogenesis of leukemia. Because Hox proteins are transcription factors, this effect would be attributable to altered expression of key target genes. To address this issue, we have been identifying HoxA10 target genes. In myeloid progenitor cells, we found that HoxA10 repressed several genes encoding phagocyte effector proteins (20–22). Decreased HoxA10-mediated repression of these genes during myelopoiesis contributed to phagocyte functional competence and phenotypic differentiation. We also identified DUSP4 as a HoxA10 target gene (23). DUSP4 encodes Mkp2 (mitogen-activated protein kinase phosphatase 2), an inhibitor of C-terminal Jun kinase (JNK). This target gene suggested a mechanism by which HoxA10 overexpression in leukemic myeloid progenitor cells impaired JNK-induced apoptosis. In other studies, we found that HoxA10 overexpression increased β3 integrin expression in myeloid progenitor cells (24). This may facilitate leukemia cell expansion via interaction of αvβ3 with bone marrow stroma.
In the current study, we identified CDX4 as a HoxA10 target gene. Cdx4 is a HD transcription factor that plays an important role in hematopoiesis, in part by influencing HOX gene transcription (25). In studies of zebrafish, knockdown of Cdx4 homologue disrupted hematopoiesis and impaired expression of specific HOX genes (25). Dysregulation expression of Cdx4 in murine bone marrow also altered HOX gene expression, including expression of the ABD HOX genes (25). Mice transplanted with Cdx4-overexpressing bone marrow developed a myeloproliferative disorder, which progressed to AML over time (26). In other studies, overexpression of Cdx4 in murine embryonic stem cells facilitated embryoid body and hematopoietic progenitor development and was associated with increased ABD HOX expression (27). Embryoid bodies engineered to co-overexpress Cdx4 and HoxB4 provided complete long term hematopoiesis in lethally irradiated mice, although neither protein had this function alone (28). Additionally, increased Cdx4 expression was documented in bone marrow cells from human subjects with AML (26).
These studies suggested that there is a relationship between Hox and Cdx proteins that influences myeloid development and leukemogenesis. In this report, we explored regulation of CDX4 transcription by HoxA10 and regulation of HOXA10 transcription by Cdx4. Our studies identified a positive feedback loop between HoxA10 and Cdx4. We also found that mutually reinforcing overexpression of these genes may contribute to the pathogenesis of AML by amplifying dysregulation of other HoxA10 and Cdx4 target genes.
MATERIALS AND METHODS
Plasmids
Protein Expression Vectors
The cDNA representing the major transcript of human HoxA10 was obtained from C. Largman (University of California, San Francisco, CA) (29, 30). The cDNA for human Cdx4 was obtained by reverse transcription and PCR from U937 cells. The sequence of this cDNA was compared with the sequence in GenBankTM. Both were subcloned into the pSRα vector for expression in mammalian cell lines and the MSCV vector for generation of retrovirus (per manufacturer's instructions; Stratagene, La Jolla, CA).
shRNA Expression Vectors
HoxA10- and Cdx4-specific shRNAs and scrambled control sequences were designed with the assistance of the Promega Web site. Double-stranded oligonucleotides representing the complementary sequences separated by a hairpin loop were subcloned into the pLKO.1puro vector (a gift from Dr. Kathy Rundell, Northwestern University, Chicago). Several sequences were tested, and the most efficient sequences were used in combination. Negative controls for these experiments were matched shRNAs with rearranged sequences (bp swapping or scrambled controls).
CDX4 and HOXA10 Reporter Vectors
Various sequences from the CDX4 or HOXA10 5′-flanks were amplified from U937 chromatin by PCR. The fragments were sequenced to ensure identity with the ENSEMBL data base. CDX4 5′-flank sequences were subcloned into the pGL3-E reporter vector, and HOXA10 5′-flank sequences were subcloned into the pGL3-basic reporter vector (Promega, Madison, WI). Additional constructs were generated in the pGL3 promoter vector with three copies of the −139 to −150 bp (HoxA10-binding) sequence from the CDX4 promoter (or a non-Hox-binding mutant sequence) and with the −124 to −140 bp (Cdx4-binding) sequence from the HOXA10 promoter (or a non-Cdx-binding mutant sequence).
Oligonucleotides
Oligonucleotides were custom synthesized by MWG Biotech (Piedmont, NC). Oligonucleotides used for EMSA were as follows: −139 to −150 bp of CDX4 promoter (wild type 5′-TAAGAAACGTGGGATGGAGTA-3′ and Hox-binding mutant 5′-TAAGAAACGTGGGGCAGAGTA-3′), −124 to −140 bp of the HOXA10 promoter (wild type 5′-AGATTACATATTTATATCAAT-3′ and Cdx-binding mutant 5′-AGATTACATACCCATATCAAT-3′), −588 to −612 bp CDX4 promoter (5′-ATTGGCATCTACTAGCAAGTCTATGTCTTT-3′), the −1064 to −1092 bp CDX4 5′-flank (5′-GAGTAAGGGAAAGCTCTTTTCATT-3′), a HoxA10-binding site from the CYBB promoter (5′-CCACTATGTTTAATTGTGACTGGATCATTTA-3′), and the proximal HoxA10-binding site from the DUSP4 promoter (wild type 5′-GCTCTGTGATTAATTCTCACTAACAAGACA-3′ and HoxA10-binding mutant 5′-GCTCTGTTCGGAATTCTCACTAACAAGACA-3′).
Oligonucleotides used for real-time PCR to quantify mRNA expression were as follows (listed 5′ to 3′): Cdx4 (forward, CGAGAAGACTGGAGCGTGTA; reverse, CTGTAGTCGGTCGAGCAGAA), HoxA10 (forward, ACACTGGAGCTGGAGAAGGA; reverse, TCACTTGTCTGTCCGTGAGG), HoxA9 (forward, TGGTTCCAGAACCGCAGGATGAAA; reverse, GCCCAAATGGCATCACTCGTCTTT), HoxB3 (forward, TCAACCACCTTTCCCATCACCCTT; reverse, CACAGGTGTGTTAATTTGGGCGCT), HoxB4 (forward, ACCTCGACACCCGCTAACAAATGA; reverse, AATGGGCACGAAAGATGAGGGAGA), gp91PHOX (forward, CAGCCTGCCTGAATTTCAACT; reverse, TTTCGACAGACTGGCAAGAGAAT), Mkp2 (forward, CGACATCTGCCTGCTCAAAG; reverse, GAATTCTGGGTACTCGGAGGAAA), Tgfβ2 (forward, CGAACCCAAAGGGTACAATG; reverse, TAAGCTCAGGACCCTGCTGT), β3 integrin (forward, TGGACAAGCCTGTGTCACCATACA; reverse, TTGTAGCCAAACATGGGCAAGCAG).
Myeloid Cell Line Culture
The human myelomonocytic leukemia cell line U937 (32) was obtained from Andrew Kraft (Hollings Cancer Center, University of South Carolina, Charleston, SC). Cells were maintained as described (33).
Primary Murine Bone Marrow Studies
Animal studies were performed according to a protocol approved by the Animal Care and Use Committees of Northwestern University and Jesse Brown Veterans Affairs Medical Center.
Bone Marrow Harvest and Culture
Bone marrow mononuclear cells were obtained from the femurs of WT or HoxA10−/− C57/BL6 mice (34). Sca1+ cells were separated using the Miltenyi magnetic bead system (Miltenyi Biotechnology, Auburn, CA). Bi-potential myeloid progenitor cells were cultured (2 × 105 cells/ml) for 48 h in DMEM supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, 10 ng/ml murine GM-CSF (R & D Systems Inc., Minneapolis, MN), and 10 ng/ml murine recombinant IL-3 (R & D Systems Inc.). Cells were either maintained in GM-CSF + IL-3 for 48 h or differentiated over 48 h in 10 ng/ml G-CSF (granulocyte) or 10 ng/ml of murine M-CSF (monocyte).
Bone Marrow Retroviral Transduction
Retrovirus (∼107 pfu/ml) was generated with the HoxA10/MSCV or control MSCV plasmid using the Phoenix cell packaging line according to the manufacturer's instructions (Stratagene, La Jolla, CA). Bone marrow mononuclear cells were cultured for 24 h in 10 ng/ml IL-3, 10 ng/ml GM-CSF, and 100 ng/ml SCF. Cells were transduced by incubation with retroviral supernatant in the presence of Polybrene (6 μg/ml) as described (10). Transduced cells were selected for 48 h in puromycin and differentiated with M-CSF or G-CSF (20 ng/ml).
Quantitative Real-time PCR
RNA was isolated using TRIzol reagent (Invitrogen) and tested for integrity by denaturing gel electrophoresis. Primers were designed with Applied Biosystems software, and real-time PCR was performed using SYBR Green according to the “standard curve” method. Results were normalized to 18 S (for mRNA) or input chromatin (for co-precipitated chromatin).
Chromatin Immunoprecipitation and Gene Discovery
U937 cells were cultured with or without IFNγ for 48 h. Cells were briefly treated with formaldehyde to generate DNA-protein cross-links. For sequencing studies, cell lysates were sonicated to generate chromatin fragments with an average size of 2.0 kb (35). Lysates underwent two rounds of immunoprecipitation with a custom generated antibody to a specific HoxA10 peptide, as described (23). Precipitated chromatin was recovered, and several batches of immunoprecipitated chromatin were combined for each experiment (35). The chromatin was blunt ended with Klenow enzyme, BamHI linkers were added, and BamHI-digested chromatin was subcloned into Bluescript plasmid. Individual clones were sequenced by standard, non-high throughput methods. Sequences representing individual clones were analyzed by a GenBankTM search to identify matches with 5′-flanks of potential target genes.
In additional studies, chromatin was co-precipitated from U937 lysates with antibody to HoxA10 or Cdx4 or with control preimmune serum. Lysates were sonicated to generate chromatin fragments of 500 bp to 1.0 kb. This precipitated chromatin was analyzed by PCR using primer sets to amplify various sequences in the 5′-flank or first exon. In other studies, cell lysates were sonicated to generate chromatin fragments of ∼200 bp, and the chromatin was used in real-time PCR experiments.
Myeloid Cell Line Transfections and Assays
Stable Transfectants
U937 cells were transfected by electroporation with equal amounts of HoxA10 or Cdx4 expression vector or empty vector control (using pcDNAamp) plus a vector with a neomycin phosphotransferase cassette (pSRα) (30 μg each). Stable pools of transfected cells were selected in G418 (0.5 mg/ml), and aliquots of cells were tested for HoxA10 and Cdx4 expression by Western blot and real-time PCR.
Other cells were transfected by electroporation with a lentiviral vector (pLKO.1puro) for expression of HoxA10- or Cdx4-specific shRNAs or scrambled control shRNAs. Stable pools of transfected cells were selected in puromycin (1.2 μg/ml) and tested for HoxA10 or Cdx4 expression by Western blot and real time PCR. In some experiments, U937 cells were stably co-transfected with a HoxA10 expression vector or empty vector control, plus a vector to express either Cdx4-specific shRNAs or scrambled control shRNAs. Co-transfectants were selected in both G418 and puromycin.
CDX4 and HOXA10 Reporter Assays
To identify HoxA10-binding cis elements in the CDX4 promoter, U937 cells were co-transfected with a luciferase reporter vector (pGL3-E) containing various CDX4 5′-flank sequences (1.4-kb (1.369-kb), 834-bp, 539-bp, 240-bp, 159-bp, or 121-bp CDX4-pGL3-E) or pGL3-E control (30 μg) and a vector to overexpress HoxA10 (or empty vector control) (50 μg). In other experiments, cells were co-transfected with a luciferase reporter vector (pGL3-basic) with 1.5 kb, 760 bp, 140 bp, and 85 bp of the HOXA10 5′-flank or pGL3-basic control (30 μg) and a vector to overexpress Cdx4 or empty expression vector (50 μg). In additional experiments, cells were co-transfected with a minimal promoter/luciferase reporter vector with multiple copies of either the HoxA10-binding cis element from the CDX4 promoter (−139 to −150 bp wild type or non-Hox-binding mutant) or the Cdx4-binding cis element from the HOXA10 promoter (−124 to −140 bp wild type or non-Cdx4-binding mutant) (30 μg) and vectors to overexpress various combinations of HoxA10, Cdx4, HoxA10-specific shRNAs, Cdx4-specific shRNAs, or relevant controls (empty vectors or vector with scrambled shRNA sequences) (50 μg). Cells were also transfected with a β-galactosidase reporter vector to control for transfection efficiency (CMV/β-Gal).
Western Blots
U937 or murine bone marrow cells were lysed by boiling in 2× SDS sample buffer. Lysate proteins (50 μg) were separated by SDS-PAGE and transferred to nitrocellulose. Western blots were serially probed with antibodies to HoxA10, Cdx4, and GAPDH or tubulin. Each experiment was repeated at least three times with different lysate proteins. A representative blot is shown.
In Vitro DNA-binding Assays
Isolation of Nuclear Proteins
Nuclear proteins were extracted from U937 cells by the method of Dignam (36) with protease inhibitors, as described (20).
Electrophoretic Mobility Shift Assays
Oligonucleotides probes were prepared, and EMSAs were performed, as described (23, 24). For binding reactions with a HoxA10 antibody, disruption of the complex (not supershift) was anticipated, based on the location of the epitopes used for antibody production. Binding competition studies were performed with double-stranded synthetic oligonucleotides. These oligonucleotides were used in dose titration experiments to determine the efficiency of binding competition. Data are presented for assays with a 200-fold molar excess of the competitor (standard for such studies; see also Refs. 23 and 24).
For all experiments, at least three batches of nuclear proteins were tested in at least two independent experiments. A representative study is shown. Integrity of the nuclear proteins and equality of protein loading was determined in EMSA with a probe representing a classical CCAAT box from the α-globin gene promoter.
DNA Affinity Purification Assays (DAPA)
Nuclear proteins (300 μg) were incubated with biotin-labeled double-stranded oligonucleotide probe representing the −130 to −159 bp CDX4 promoter sequence in DAPA buffer (25 mm HEPES (pH 7.6), 60 mm KCl, 5 mm MgCl2, 7.5% glycerol, 0.1 mm EDTA, 1 mm DTT, and 0.25% Triton X-100). DNA-protein complexes were precipitated with 50 μl of a 50% slurry of neutravidin-coated agarose beads (Pierce). Proteins bound to the beads were eluted, separated by SDS-PAGE (10% acrylamide), and transferred to nitrocellulose. Western blots were probed with an antibody to HoxA10 obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Proliferation Assays
U937 cells that were stably transfected with a vector to overexpress HoxA10 or empty vector control and Cdx4-specific shRNAs or scrambled shRNA control in various combinations were deprived of fetal calf serum for 24 h and then treated with a dose titration of fetal calf serum (0.1–10%). Cell proliferation was determined by incorporation of [3H]thymidine (for the last 8 h of incubation) according to standard techniques.
Genomic Sequence Analysis
Conserved genomic sequences and consensus sequences for HoxA10 protein-DNA binding were identified using VISTA software (Genomics Division of the Lawrence Berkley National Laboratory (Berkley, CA)) (37–39).
Statistical Analysis
Statistical significance was determined by Student's t test and analysis of variance, using SigmaPlot and SigmaStat software. In all of the graphs, error bars represent S.E.
RESULTS
Identifying CDX4 as a HoxA10 Target Gene
To identify HoxA10 target genes, we performed chromatin immunoprecipitation (ChIP) using a HoxA10 antibody and U937 cells. U937 is a myeloid leukemia cell line that represents a committed myeloid progenitor. U937 cells undergo monocyte differentiation when treated with interferon γ (IFNγ), tumor necrosis factor α, or phorbol myristate acetate and granulocyte differentiation with retinoic acid (32). Chromatin that co-precipitated with HoxA10 was subcloned into a plasmid vector, and individual clones were sequenced. Putative target genes were identified by a GenBankTM search (as in our previous studies (40)). Clones with inserts of ∼100 bp or larger were further considered. Analysis of 35 clones identified 21 sequences from the 5′ regions of known genes, five telomeric repeated sequences, and four sequences that were not in the 5′-regions of genes encoding known proteins. Another five clones were not in or adjacent to any genes. This is similar to the experience of other investigators with this “low throughput” approach.3 Even with limited sampling, we identified genes that fell into a few broad categories (Table 1). Most putative target genes were involved in cell proliferation and survival, consistent with the role of HoxA10 in progenitor expansion.
TABLE 1.
HoxA10 target genes (low throughput screen)
| HD transcription factors | Proliferation/signal transduction | Cytokines/Receptors |
|---|---|---|
| CDX4 | PRKAR2A | SELL |
| PBX2 | PLCB1 | TGFBR3 |
| MEIS1 | NUDT6 | FGF2 |
| CUX1 | MAPK6PS1 |
| Apoptosis | Inflammation | Other |
|---|---|---|
| PDCD5 | IL11 | UBE2S |
| TAF2 | TBXAS1 | RANBP17 |
| BRSK1 | ATPGV1H | ZNF45 |
We also identified another HoxA10 target gene category of interest: genes encoding homeodomain transcription factors. In this group, CDX4 was of potential significance because Cdx4 regulates HOX genes, including HOXA10. This suggested the possibility of cross-regulation between Hox proteins and transcription factors that regulate the “Hox code.” The CDX4 sequence identified by ChIP encompassed −855 to −905 bp of the 5′-flank (relative to the first bp of the Cdx4 mRNA, which was considered +1; referred to as the transcription start site for convenience) (Fig. 1A). Because lysates were sonicated to generate chromatin fragments of ∼2.0 kb prior to ChIP, this suggested a possible HoxA10-binding site within the proximal 3 kb of the CDX4 5′-flank.
FIGURE 1.
Identification of CDX4 as a HoxA10 target gene. A, comparison between the proximal 5′-flanks of the human and murine CDX4 gene identified a number of conserved sequences for Hox binding. The 5′-flanks of the human and murine CDX4 genes were compared for conserved sequences (indicated in gray) and Hox/Pbx DNA-binding consensus sequences. Locations of the ChIP chromatin sequence and the truncations used for reporter assays are indicated. B, the proximal 5′-flank co-immunoprecipitated with HoxA10 from U937 cells. ChIP was performed with U937 cells and an antibody to HoxA10 or preimmune serum (as a negative control). Chromatin was amplified by PCR using primers flanking various sequences in the CDX4 5′-flank. Non-precipitated (input) chromatin was a positive control. IP, immunoprecipitation.
To identify Hox-DNA binding consensus sequences, we analyzed the proximal 3.0 kb of 5′-flank from the human and murine CDX4 genes using VISTA software (Fig. 1A). Because Hox proteins generally bind DNA as a heterodimer with Pbx or Meis proteins, this region was interrogated for consensus sequences for Hox/Pbx or Hox/Meis binding. VISTA analysis determined that the proximal ∼1 kb of CDX4 5′-flank was highly conserved between mice and humans. The VISTA consensus sequence algorithm identified a number of conserved sequences with homology to Hox/Pbx binding sites but no potential Hox/Meis sites. Eight of the identified potential Hox/Pbx sequences appeared to have significant homology with the published consensus sequence (Table 2).
TABLE 2.
Hox/Pbx consensus sequences
An optional base in the consensus is underlined. Italic type represents an extra base in the sequence.
| Sequence | CDX4 promoter location |
|---|---|
| ATGATTNATN | Consensus |
| ATGATT ATT | CYBB-proximal |
| ATGTTTAATT | CYBB-distal |
| GTGATTAATT | DUSP4-proximal |
| CTGATTTAAT | DUSP4-distal |
| ATGGCTTAGG | −24 to −34 |
| ATCCTGAAGT | −33 to −44 |
| ATGGAGTAGC | −135 to −146 |
| ATTTTGAATG | −294 to −305 |
| ATGGCCCTTC | −308 to −318 |
| ATCGGTGAAA | −559 to −568 |
| ATGCCT ACA | −779 to −790 |
| ATGGGG ATA | −789 to −798 |
Independent chromatin immunoprecipitation experiments were performed to confirm the screening assay results. For these experiments, U937 cell lysates were sonicated to generate 1.0-kb chromatin fragments, and chromatin was amplified by PCR using CDX4-specific primers. Non-precipitated chromatin (referred to as input chromatin) was a positive control, and chromatin that precipitated with preimmune serum was a negative control for these studies. Three sets of primers were chosen spanning the proximal CDX4 5′-flank from 0 to −834 bp, based upon the location of potential Hox binding sites. Chromatin representing these CDX4 sequences specifically precipitated with HoxA10 (Fig. 1B); however, chromatin from the distal 5′-flank (−1.0 and −2.0 kb) and exon 1 did not (not shown). These studies localized the HoxA10-binding site to the proximal 1.0 kb of CDX4 5′-flank.
HoxA10 Activates a CDX4 Promoter Cis Element
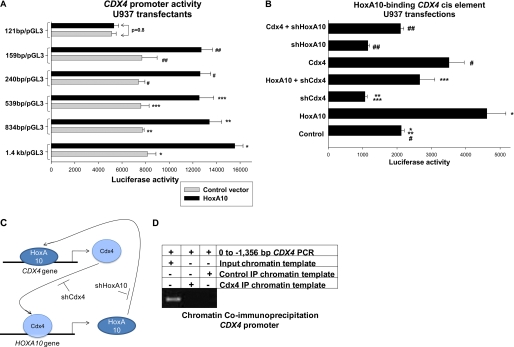
We next investigated the influence of HoxA10 on CDX4 promoter activity. To locate the putative HoxA10-binding cis element, a series of luciferase reporter constructs were made with truncations of the proximal CDX4 5′-flank (1346, 834, 539, 240, 159, or 121 bp). U937 cells were co-transfected with the reporter constructs (or empty reporter vector), and a vector to overexpress HoxA10 (or empty expression vector) and reporter activity was determined. We found that overexpression of HoxA10 significantly increased activity of the 1346-, 834-, 539-, 240-, and 159-bp CDX4 promoter constructs (Fig. 2A). HoxA10 overexpression resulted in an equivalent increase in reporter activity for each of these constructs (p = 0.5, n = 4). In contrast, HoxA10 overexpression did not alter reporter activity from the 121-bp CDX4 promoter construct (Fig. 2A). These results identified a potential HoxA10-binding cis element between −121 and −159 bp in the CDX4 promoter.
FIGURE 2.
HoxA10 activated a cis element in the proximal CDX4 promoter. A, overexpressed HoxA10 activated a cis element between −121 and −159 bp in the CDX4 promoter. U937 cells were co-transfected with a series of reporter constructs representing the 5′-flank of the CDX4 gene (or empty reporter vector) and a vector to overexpress HoxA10 (or empty expression vector). Reporter gene activity was determined. *,**, ***, #, and ##, statistically significant differences in reporter activity in HoxA10-overexpressing versus control transfectants (p < 0.01, n = 6). B, activation of the CDX4 cis element by overexpressed HoxA10 is impaired by knockdown of endogenous Cdx4. U937 cells were co-transfected with an artificial promoter/reporter vector with three copies of the −139 to −150 bp sequence of CDX4 promoter (or empty reporter vector), a vector to overexpress HoxA10 or Cdx4 (or empty vector control), and a vector to express a HoxA10- or Cdx4-specific shRNA (or bp-swapping, scrambled shRNA control). Reporter gene activity was determined. *, statistically significant differences in reporter activity in HoxA10-overexpressing versus control transfectants (p < 0.001, n = 6). **, significant difference in reporter expression with a Cdx4-specific shRNA (p < 0.0001, n = 6). ***, significant increase in reporter activity with overexpression of HoxA10 in transfectants with Cdx4 knockdown (p = 0.02, n = 6). #, statistically significant increase in reporter activity with Cdx4 overexpression (p < 0.0001, n = 6). ##, significant increase in reporter expression in transfectants with shRNA to HoxA10 due to Cdx4 overexpression (p < 0.0001, n = 6). C, cross-regulation of CDX4 and HOXA10 transcription by HoxA10 and Cdx4. Shown is a graphical representation of the hypothesis of these studies, that HoxA10 activates the CDX4 promoter and Cdx4 activates the HOXA10 promoter. In this schematic, shHoxA10 and shCdx4 represent HoxA10- and Cdx4-specific shRNAs, respectively. D, Cdx4 did not bind the CDX4 promoter in vivo. ChIP was performed with U937 cells and an antibody to Cdx4 or irrelevant control antibody (as a negative control). ChIP chromatin was amplified by PCR using primers to the proximal 1.4 kb of CDX4 5′-flank. Non-precipitated (input) chromatin was a positive control in these studies. IP, immunoprecipitation. Error bars, S.E.
Activity of the control luciferase reporter vector was less than 5% of the activity of CDX4 promoter constructs and was not influenced by HoxA10 overexpression. This minimal activity was subtracted as background.
VISTA sequence analysis identified a potential Hox/Pbx consensus sequence between −135 and −146 bp in the CDX4 promoter (Fig. 1A and Table 2). To investigate whether this region of the CDX4 5′-flank included a functional cis element that was activated by HoxA10, a luciferase reporter construct was made with three copies of the −130 to −159 bp sequence linked to a minimal promoter. To determine if the Hox/Pbx-like sequence was a functional cis element, a minimal promoter/reporter construct was also made with three copies of the −130 to −159 bp CDX4 sequence with a binding site mutation (ATGGAGTAGCCT changed to GCAGAGTAGCCT).
U937 cells were co-transfected with these constructs (or minimal promoter control vector) and a vector to overexpress HoxA10 (or empty expression vector) and analyzed for reporter activity. We found that overexpression of HoxA10 significantly increased activity of the construct with the wild type −130 to −159 bp sequence (Fig. 2B). However, reporter expression in transfectants with the mutant CDX4 cis element construct was not significantly different than transfectants with the minimal promoter control vector, with or without HoxA10 overexpression (not shown). Reporter activity in transfectants with the control construct was less than 10% that of the WT CDX4 cis element construct. This control vector activity was not influenced by overexpression of HoxA10 (or other proteins or shRNAs; see below) and was subtracted as background.
This finding suggested that HoxA10 overexpression activated a CDX4-cis element. Previous studies determined that overexpression of Cdx4 in bone marrow progenitor cells increased HoxA10 expression (25). Therefore, we hypothesized that an increase in endogenous Cdx4 in HoxA10-overexpressing cells would activate the endogenous HOXA10 gene. Increased endogenous HoxA10 would augment the total amount of HoxA10 protein in HoxA10-overexpressing cells, which would contribute to activation of both our CDX4 cis element construct and the endogenous CDX4 promoter. According to this hypothesis, blocking expression of endogenous Cdx4 would decrease the total amount of HoxA10 protein in HoxA10-overexpressing cells (due to an effect on endogenous HoxA10), which would decrease CDX4 cis element activity (graphically represented in Fig. 2C).
To investigate this hypothesis, U937 cells were co-transfected with the CDX4-cis element/minimal promoter construct (or control vector) and various combinations of vectors to express HoxA10, Cdx4-specific shRNAs, or relevant control vectors. We found that Cdx4 knockdown decreased activity of the CDX4 cis element (Fig. 2B), suggesting that Cdx4 influenced activity of its own promoter. Expression of Cdx4-specific shRNAs also impaired activation of the CDX4 cis element by overexpressed HoxA10, as per our hypothesis (Fig. 2B). We performed the reciprocal experiment and co-transfected U937 cells with the CDX4-cis element/minimal promoter construct and a Cdx4 expression vector with or without a vector to express HoxA10-specific shRNAs. We found that Cdx4 overexpression significantly increased activity of this CDX4 cis element (Fig. 2B). Knockdown of endogenous HoxA10 significantly decreased activity of the CDX4-cis element and partially blocked activation by overexpressed Cdx4 (Fig. 2B).
In Fig. 2B, the Control bar represents reporter activity of the CDX4-cis element/minimal promoter construct in transfection experiments with an empty expression vector. This control reporter activity was not significantly different from that in transfectants with vectors expressing base pair-swapping mutations of the HoxA10- or Cdx4-specific shRNAs used in these experiments (p = 0.7, n = 4). In other control experiments, neither the Cdx4- or HoxA10-specific shRNAs nor their scrambled control shRNAs influenced activity of a reporter vector containing a non-HoxA10-binding cis element from the PTPN13 gene (not shown).
These results supported the hypothesis that overexpressed HoxA10 increased expression of endogenous Cdx4, which in turn increased endogenous HoxA10, which further activated the CDX4 cis element. However, another possible explanation was that Cdx4 regulated its own promoter through interaction with this cis element. To investigate this possibility, we performed chromatin immunoprecipitation using U937 cells and an antibody to Cdx4. Chromatin was amplified by PCR using primers to the CDX4 5′-flank, as above. Non-precipitated chromatin (input chromatin) was a positive control in these experiments, and chromatin that precipitated with an irrelevant antibody was a negative control. We found that Cdx4 did not interact with the proximal CDX4 5′-flank in vivo (Fig. 2D). A positive control for this Cdx4 antibody in ChIP experiments is found below.
HoxA10 Binds a CDX4 Promoter Element
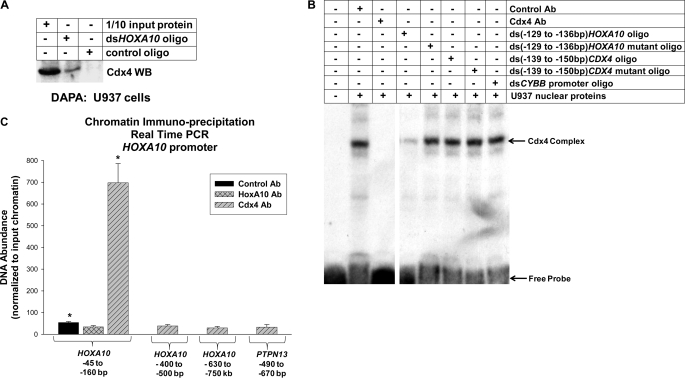
These studies identified a CDX4 cis element that was influenced by HoxA10 but did not demonstrate that the cis element was a HoxA10-binding site. We investigated this using a DAPA. For these studies, nuclear proteins from U937 cells were incubated with a biotin-labeled, double-stranded synthetic oligonucleotide probe representing the −130 to −159 bp CDX4 promoter sequence or a non-HoxA10-binding oligonucleotide probe (as a negative control). The probes were purified by affinity to neutravidin beads, and co-purifying proteins were separated by SDS-gel electrophoresis and identified by Western blot. We found that HoxA10 specifically co-purified with the WT CDX4 cis element probe (Fig. 3A). Reprobing the blots with a Cdx4 antibody failed to identify Cdx4 interaction with these probes (not shown).
FIGURE 3.
HoxA10 bound a cis element in the CDX4 promoter in vitro and in vivo. A, HoxA10 bound a cis element in the CDX4 promoter by a DAPA. DAPA was performed using nuclear proteins from U937 cells and a biotin-labeled, double-stranded, synthetic oligonucleotide probe representing the −139 to −150 bp sequence of CDX4 (or irrelevant control oligonucleotide). Proteins that co-purified with the oligonucleotide probes were separated by SDS-PAGE, and Western blots were probed with antibody (Ab) to HoxA10. Non-precipitated (input) nuclear protein was a control in this experiment. B, HoxA10 bound a cis element in the CDX4 promoter by EMSA. EMSA was performed using nuclear proteins from U937 cells and a radiolabeled, double-stranded, synthetic oligonucleotide probe representing the −139 to −150 bp sequence of CDX4. Binding assays were incubated with either irrelevant control antibody or HoxA10 antibody or with double-stranded oligonucleotide competitors representing homologous −139 to −150 bp CDX4 sequence, −139 to −150 bp CDX4 sequence with ATG mutated to GCA, an irrelevant CDX4 sequence (−588 to −612 bp), a more distal irrelevant CDX4 sequence (−1064 to −1092 bp), a Hox/Pbx binding site from the CYBB gene, a Hox/Pbx binding site from the DUSP4 gene, the same CYBB sequence with a mutation in the Hox/Pbx binding site, or no oligonucleotide, as indicated. The arrow represents the HoxA10 DNA-bound complex. The asterisk represents a higher mobility complex which is cross-immunoreactive with HoxA10 and shares binding site specificity. C, HoxA10 bound a cis element in the CDX4 promoter in vivo. ChIP was performed with U937 cells and antibodies to HoxA10, HoxA9, or Cdx4 (or irrelevant control antibody). Co-precipitating chromatin was amplified by real-time PCR with primers that flank the −50 to −150 bp CDX4 promoter sequence (the functional cis element), the −750 to −850 bp CDX4 sequence (an irrelevant CDX4 region), the −1.3 to −1.4 kb CDX4 sequence (another irrelevant CDX4 region), or an irrelevant, non-Hox-binding sequence from the PTPN13 gene. ChIP results were normalized to PCR with non-precipitated, input chromatin to control results between experiments. Statistically significant difference in HoxA10 binding versus nonspecific binding to the CDX4 probe is indicated by an asterisk (p < 0.0001, n = 6). Error bars, S.E.
We also investigated in vitro HoxA10-binding to the CDX4 cis element by EMSA. For these studies, U937 nuclear proteins were incubated with a radiolabeled, double-stranded, synthetic oligonucleotide probe representing the −130 to −159 bp CDX4 promoter sequence, separated by non-denaturing electrophoresis, and DNA-protein complexes were identified by autoradiography (Fig. 3B). Some binding reactions were preincubated with an antibody to HoxA10 or an irrelevant control antibody. We found that a low mobility complex that bound to the CDX4 probe was cross-immunoreactive with HoxA10 (Fig. 3B, compare lanes 1 and 2). In additional studies, we found that this complex was not cross-immunoreactive with Cdx4 (not shown). Because HoxA9 and HoxA10 have redundant functions and possibly some common target genes, we also investigated HoxA9 binding to this cis element. The complex was also not cross-immunoreactive with HoxA9 (not shown).
To further investigate binding site specificity, additional EMSA were performed with an excess of various unlabeled, double-stranded oligonucleotides. We found that unlabeled oligonucleotide with the −130 to −159 bp CDX4 sequence efficiently competed for complex binding to the −130 to −159 bp probe (Fig. 3B, lane 3). However, an unlabeled oligonucleotide with mutation in the putative Hox/Pbx-binding sequence did not compete for complex binding to the probe (Fig. 3B, lane 4, ATGGAGTAGCCT to GCAGAGTAGCCT); nor did two oligonucleotides representing other regions of the CDX4 5′-flank (Fig. 3B, lanes 5 and 6). Protein binding to the −130 to −159 bp CDX4 probe was efficiently competed for by unlabeled probes representing Hox/Pbx binding sites from the CYBB and DUSP4 promoters (Fig. 3B, lanes 7 and 8). An irrelevant oligonucleotide from the CYBB promoter did not interfere with complex binding, which was similar to binding in assays without any competitor oligonucleotide (Fig. 3B, compare lanes 9 and 10).
In these experiments, there was also a higher mobility complex that was cross-immunoreactive with HoxA10 and exhibited the same binding site specificity (Fig. 3B, indicated by an asterisk). Hox proteins generally bind to DNA cis elements as multiprotein complexes. Based on studies of other HoxA10 target genes, we concluded that these two complexes represented HoxA10 binding to the CDX4 cis element probe as a monomer (or dimer) versus in a higher order complex with other proteins. We have observed this previously with Hox/Pbx binding sequences in the CYBB gene.
We also investigated in vivo HoxA10 and Cdx4 binding to this cis element by ChIP. For these experiments, cell lysates were sonicated to generate ∼200-bp chromatin fragments. Studies were performed with antibodies to HoxA10, Cdx4, and HoxA9, and chromatin was amplified by real-time PCR. Preimmune serum was a negative control for these studies, and non-precipitated (input) chromatin was used to normalize results between experiments. For these experiments, we used primer sets that flanked the putative HoxA10-binding cis element in the CDX4 promoter. As negative controls, chromatin was also amplified with sets of primers representing two irrelevant regions of the CDX4 5′-flank (−850 to −950 bp or −1.3 to −1.4 kb) or a non-HoxA10-binding cis element from the PTPN13 gene.
We found that the −50 to −150 bp sequence of the CDX4 cis element specifically co-precipitated from U937 cells with HoxA10 but not with Cdx4 or HoxA9 (Fig. 3B). Positive controls for Cdx4 ChIP are discussed below. The HoxA9 binding site from PIM1 was a positive control for HoxA9 DNA binding (not shown) (41). These results supported our hypothesis that HoxA10 interacted with this CDX4 promoter cis element in myeloid cells.
HoxA10 Influences Cdx4 Expression
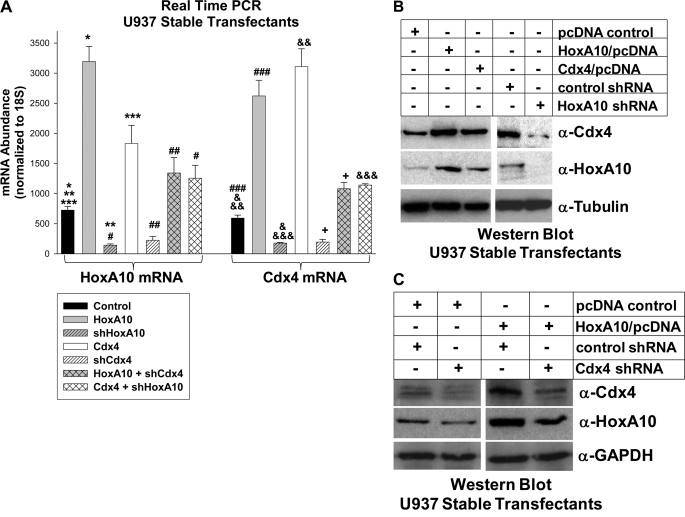
We next determined if increased CDX4 promoter activity in HoxA10-overexpressing cells increased expression of Cdx4 mRNA and protein. For these studies, U937 cells were stably transfected with various combinations of vectors to overexpress HoxA10 or Cdx4 (or empty vector control) and to express HoxA10-specific shRNAs, Cdx4-specific shRNAs, or bp-swapping, scrambled shRNA controls. Transfectants were analyzed for Cdx4 and HoxA10 expression by real-time PCR and Western blot.
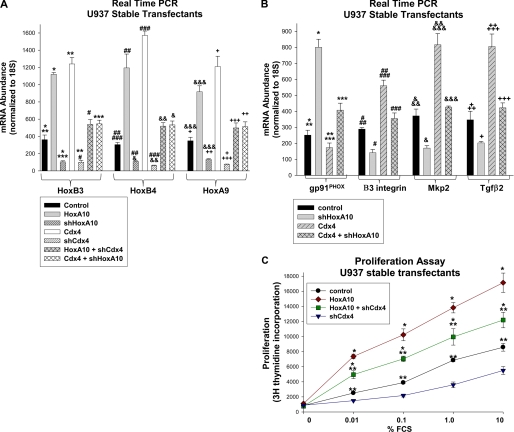
We found that HoxA10 overexpression significantly increased expression of Cdx4 mRNA (Fig. 4A, compare black and gray bars) and protein (Fig. 4B, top, compare the first two lanes). Conversely, HoxA10 knockdown decreased expression of Cdx4 mRNA (Fig. 4A, compare black and gray hashed bars) and protein (Fig. 4B, top, last two lanes).
FIGURE 4.
Cdx4 expression was increased by HoxA10 overexpression and decreased by HoxA10 knockdown in U937 myeloid leukemia cells. A, HoxA10 expression level influenced Cdx4 mRNA abundance in a dose-dependent manner. U937 cells were stably transfected with a vector to overexpress HoxA10 or Cdx4 (or empty expression vector) and a vector to express HoxA10-specific shRNAs, Cdx4-specific shRNAs, or scrambled shRNA controls. Expression of HoxA10 and Cdx4 mRNA was determined by real-time PCR. * and **, statistically significant differences in HoxA10 expression with HoxA10 overexpression or knockdown, respectively (p < 0.00001, n = 6). *** and #, statistically significant differences in HoxA10 expression with Cdx4 overexpression (p < 0.02, n = 6). ##, significant increase in HoxA10 expression in shCdx4-expressing cells with HoxA10 overexpression (p < 0.0001, n = 6). ### and &, significant differences in Cdx4 expression in cells with HoxA10 overexpression or knockdown (p < 0.0001, n = 6). && and &&&, significant increase in Cdx4 expression with Cdx4 overexpression (p < 0.0001, n = 6). +, significant increase in Cdx4 expression in cells with shHoxA10 upon Cdx4 overexpression (p < 0.0001, n = 6). B, HoxA10 expression level influenced Cdx4 protein abundance. U937 cells were stably co-transfected with a vector to overexpress HoxA10 or Cdx4 (or empty expression vector) and with a vector to express HoxA10-specific shRNAs or bp-swapping, scrambled shRNAs. Total cell lysates were separated by SDS-PAGE, and Western blots were serially probed with antibodies to Cdx4, HoxA10, and tubulin (as a loading control). C, HoxA10 overexpression partly rescued Cdx4 protein expression in cells with Cdx4 knockdown. U937 cells were stably co-transfected with a vector to overexpress HoxA10 (or expression vector control) and a vector to express Cdx4-specific shRNAs or scrambled shRNAs. Total cell lysates were separated by SDS-PAGE, and Western blots were serially probed with antibodies to Cdx4, HoxA10, and GAPDH (as a loading control). Error bars, S.E.
Because knockdown of endogenous Cdx4 impaired CDX4 cis element activation by overexpressed HoxA10, we verified that these changes were reflected in expression of Cdx4 mRNA and protein. Consistent with these data, HoxA10 overexpression partly rescued expression of Cdx4 mRNA in transfectants with Cdx4 knockdown (Fig. 4A, compare white and white hashed bars) and protein (Fig. 4C, top, last two lanes). Conversely, there was significantly less Cdx4 in Cdx4-overexpressing transfectants with HoxA10 knockdown (Fig. 4A, compare white and white cross-hashed bars).
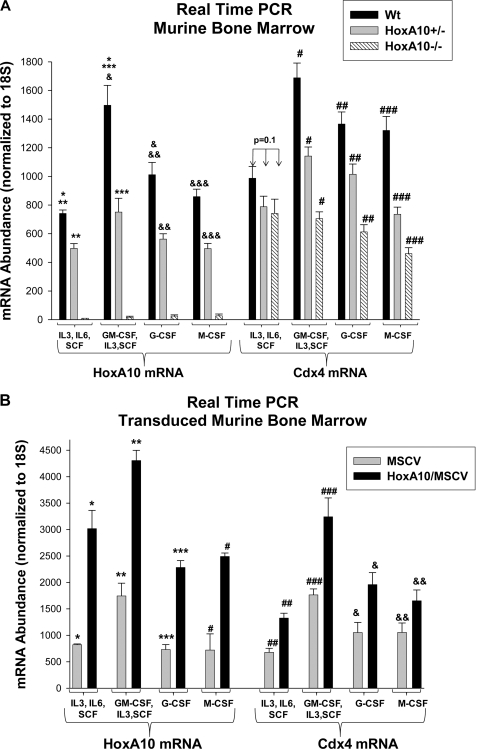
However, regulation of proto-oncogenes, such as HoxA10 and Cdx4, might be abnormal in U937 leukemia cells. Therefore, we also investigated the influence of HoxA10 on Cdx4 expression in primary murine bone marrow. For these studies, we used transgenic mice in which the HOXA10 gene was disrupted by homologous recombination (34). HoxA10−/− mice exhibit abnormalities in genitourinary development and fertility (34) but no gross abnormalities in hematopoiesis.4 Bone marrow was harvested from HoxA10−/−, HoxA10+/−, or WT control mice, and gene expression was analyzed by real-time PCR. Some cells were cultured in IL-3, IL-6, and SCF, and Sca1+ cells were isolated (immature progenitor population or HSC). Other cells were cultured in GM-CSF, IL-3, and SCF, and CD34+ cells were separated (committed myeloid progenitor population or GMP). Some cells in the latter group were differentiated to granulocytes and monocytes with G-CSF or M-CSF, respectively. We previously characterized these populations by flow cytometry and gene expression (10).
HoxA10 expression was significantly greater in WT GMP in comparison with HSC or differentiating cells (Fig. 5A, compare black bars), consistent with previous results (42). Expression of HoxA10 was ∼50% less in HoxA10+/− cells in comparison with WT cells (Fig. 5A, compare black and gray bars) and was not detected in HoxA10−/− cells (Fig. 5A). Cdx4 expression was also greater in the WT GMP population in comparison with HSC or differentiating cells (Fig. 5A, black bars), consistent with previous expression profiles (27, 28). In GMP and differentiating cells, HoxA10 knock-out decreased Cdx4 expression in a dose-dependent manner (Fig. 5A, compare black bars with gray bars and white hashed bars). In contrast, there was no difference in Cdx4 mRNA expression in WT, HoxA10+/−, or HoxA10−/− HSC, suggesting that Cdx4 expression was HoxA10-independent in this cell population (Fig. 5A).
FIGURE 5.
Cdx4 expression was increased by HoxA10 overexpression and decreased by HoxA10 knock-out in primary murine bone marrow cells. A, HoxA10 knock-out decreased Cdx4 expression in myeloid progenitors and differentiating myeloid cells but not in immature progenitor cells. Bone marrow was isolated from WT, HoxA10+/−, and HoxA10−/− mice and cultured under various cytokine conditions. Immature progenitors were cultured in IL-3, IL-6, and SCF, and Sca1+ cells were isolated (HSC). Committed myeloid progenitors were cultured in GM-CSF, IL-3, and SCF, and CD34+ cells were isolated (GMP). Some of these cells were further differentiated to monocytes or granulocytes with M-CSF or G-CSF, respectively. Expression of HoxA10 or Cdx4 mRNA was determined by real-time PCR. *, statistically significant difference in HoxA10 mRNA expression in WT cells under HSC versus GMP conditions; &, statistically significant difference in HoxA10 mRNA expression in WT cells under GMP versus differentiating conditions. **, ***, &&, and &&&, statistically significant differences in HoxA10 expression in WT versus HoxA10+/− cells (p < 0.0001, n = 6). #, ##, and ###, statistically significant difference in Cdx4 mRNA expression in WT versus HoxA10+/− versus HoxA10−/− cells (p < 0.001, n = 6). B, HoxA10 overexpression increased Cdx4 expression in progenitor cells and differentiating myeloid cells. WT murine bone marrow cells were transduced with a retroviral vector to express HoxA10 or with empty control vector. Cells were cultured under the conditions described above. Expression of HoxA10 and Cdx4 mRNA was determined by real-time PCR. *, **, ***, and #, statistically significant increase in HoxA10 expression in HoxA10-overexpressing cells under the four cytokine conditions (p < 0.0001, n = 6). ##, ###, &, and &&, statistically significant increase in Cdx4 expression in HoxA10-overexpressing cells (p < 0.001, n = 6). Error bars, S.E.
We also investigated the impact on Cdx4 expression of overexpressing HoxA10 in primary bone marrow cells. For these studies, WT cells were transduced with a retroviral vector to express HoxA10 (or empty expression vector control) and cultured as above. We found that overexpression of HoxA10 significantly increased Cdx4 mRNA under all cytokine conditions (Fig. 5B, compare black and gray bars). These studies suggested that HoxA10 overexpression in AML might lead to Cdx4-overexpression, which might further deregulate HOX genes.
Identification of HOXA10 as a Cdx4 Target Gene in Myeloid Cells
These studies suggested the possibility that HOXA10 was a Cdx4 target gene. To investigate this, we used VISTA software to interrogate the human and murine HOXA10 5′-flank sequences for conserved Cdx4 DNA-binding consensus sequences. This analysis revealed a single, conserved consensus sequence (−129 to −136 bp) (Fig. 6A).
FIGURE 6.
Identification of HOXA10 as a Cdx4 target gene. A, comparison between the proximal 5′-flanks of the human and murine HOXA10 gene identified a conserved Cdx4 DNA-binding consensus sequence. The 5′-flanks of the human and murine HOXA10 genes were compared for conserved sequences (indicated in gray) and Cdx4-binding consensus sequences. B, Cdx4 bound to the HOXA10 promoter in vivo in myeloid cells. Chromatin immunoprecipitation was performed with U937 cells and an antibody to Cdx4 or HoxA10 or irrelevant control antibody. Co-precipitating chromatin was amplified by PCR using primers that flanked the proximal 1.0 kb of HOXA10 5′-flank. Non-precipitated (input) chromatin was a positive control in these studies. IP, immunoprecipitation.
To investigate the possibility that this consensus sequence was a genuine Cdx4 binding site, we performed chromatin immunoprecipitation studies using U937 cell lysates and an antibody to Cdx4. HoxA10 binding to its own promoter was investigated in ChIP with a HoxA10 antibody. Non-precipitated (input) chromatin was a positive control in this study, and ChIP with an irrelevant antibody was a negative control. Co-precipitating chromatin was amplified by PCR with primers flanking the proximal 1.0 kb of HOXA10 5′-flank. We found that the proximal HOXA10 5′-flank specifically co-precipitated with Cdx4 but not with HoxA10 (Fig. 6B). These results were consistent with the identification of HOXA10 as a target gene for Cdx4 but not for HoxA10.
Cdx4-activated HOXA10 Transcription
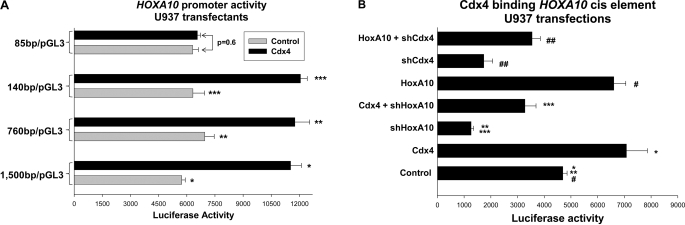
To determine if the HOXA10 promoter was activated by Cdx4, a series of reporter constructs were made with 1500, 760, 140, and 85 bp of the HOXA10 5′-flank (relative to the first bp of the cDNA, which was considered +1 and is referred to as the transcription start site for convenience). These constructs (or empty reporter vector) were co-transfected into U937 cells with a vector to overexpress Cdx4 (or empty expression vector). We found that Cdx4 overexpression significantly increased activity of constructs with 1500, 760, and 140 bp of HOXA10 5′-flank but did not alter activity of the 85-bp construct (Fig. 7A). We also found that Cdx4 overexpression resulted in an equivalent increase in activity of the 1500-, 760-, and 140-bp constructs (p = 0.4, n = 3). Expression of the empty control reporter vector was not influenced by Cdx4 overexpression, and this minimal activity was subtracted as background.
FIGURE 7.
Cdx4 activated the HOXA10 promoter in myeloid cells. A, Cdx4 activated a cis element between −85 and −140 bp in the HOXA10 promoter. U937 cells were co-transfected with a series of reporter constructs representing the 5′-flank of the HOXA10 gene (or empty reporter vector) and a vector to overexpress Cdx4 (or empty expression vector). Reporter activity was determined. *, **, and ***, statistically significant differences in reporter gene activity in Cdx4-overexpressing versus control transfectants (p < 0.0001, n = 4). B, activation of a HOXA10 cis element by overexpressed Cdx4 was impaired by knockdown of endogenous HoxA10. U937 cells were co-transfected with a reporter construct with three copies of the −129 to −136 bp sequence from the HOXA10 promoter linked to a minimal promoter (or empty reporter vector), a vector to overexpress HoxA10 or Cdx4 (or empty vector control), and a vector to express a HoxA10- or Cdx4-specific shRNA (or scrambled shRNA). Reporter gene activity was determined. *, statistically significant differences in reporter gene activity in Cdx4-overexpressing versus control transfectants (p = 0.01, n = 5). **, significant difference in reporter expression with a HoxA10 specific shRNA (p < 0.0001, n = 5). ***, significant increase in reporter activity with overexpression of Cdx4 in transfectants with HoxA10 knockdown (p = 0.004, n = 5). #, statistically significant increase in reporter activity with HoxA10-overexpression (p = 0.003, n = 5). ##, significant increase in reporter expression due to HoxA10 overexpression in transfectants with shRNA to Cdx4 (p = 0.001, n = 5). Error bars, S.E.
These reporter assays were consistent with location of the Cdx4-binding consensus sequence between −129 and −136 bp of the HOXA10 5′-flank because the consensus was included in the 140-bp construct but not the 85-bp construct. To determine if this consensus sequence functioned as a Cdx4-binding cis element, a reporter construct was made with three copies of the −124 to −140 bp HOXA10 sequence linked to a minimal promoter. A minimal promoter/reporter construct was also made with three copies of a mutant form of the Cdx-binding consensus (TATTTATA to TACCCATA). These constructs (or empty minimal promoter/reporter vector) were co-transfected into U937 cells with a vector to overexpress Cdx4 (or empty expression vector).
We found that Cdx4 overexpression significantly increased activity of the −124 to −140 bp HOXA10-containing construct in these transfectants (Fig. 7B). In contrast, reporter activity in transfectants with the mutant −124 to −140 bp construct was not significantly different from activity in transfectant control minimal promoter/reporter vector, with or without Cdx4 overexpression (not shown). Reporter activity in transfectants with control minimal promoter/reporter vector was less than 10% the activity of the HOXA10 cis element-containing construct. This activity was not influenced by Cdx4 overexpression and was subtracted as background.
Based on our studies of the CDX4 cis element, we anticipated that knockdown of endogenous HoxA10 would impair activation of the HOXA10 cis element by Cdx4. To test this hypothesis, U937 cells were co-transfected with the HOXA10-cis element/minimal promoter construct (or control vector) and various combinations of vectors to overexpress Cdx4 and to express HoxA10-specific shRNAs (or relevant control vectors). We found that HoxA10 knockdown significantly decreased HOXA10 cis element activity and partly blocked activation of this cis element by overexpressed Cdx4 (Fig. 7B).
In the reciprocal experiment, U937 cells were co-transfected with the HOXA10 cis element-containing reporter construct and various combinations of vectors to overexpress HoxA10 and to express Cdx4-specific shRNAs (or relevant control vectors). HoxA10 overexpression increased activity of the HOXA10 cis element, consistent with our hypothesis. Cdx4 knockdown decreased activity of the HOXA10 cis element, as anticipated, and also impaired activation of the cis element by overexpressed HoxA10 (Fig. 7B).
As discussed above, there was minimal reporter expression in cells transfected with control minimal promoter/reporter vector. This activity was not influenced by overexpression or knockdown of Cdx4 or HoxA10 and was subtracted as background.
In Fig. 7B, the Control lane represents transfection with the HOXA10 cis element/minimal promoter construct and empty expression vector. In other control experiments, there was no difference in the activity of this reporter construct in transfectants with empty expression vector versus control vectors with base pair-swapping mutants of Cdx4- or HoxA10-specific shRNAs. Therefore, the latter control studies were not shown.
Cdx4 Binds to a HOXA10 Promoter Element
These results were consistent with the hypothesis that Cdx4 bound to a HOXA10 cis element and activated transcription. An alternative explanation would be that Cdx4 influenced another transcription factor that interacted with this cis element. Also, results of reporter assays suggested that increased HoxA10 expression in Cdx4-overexpressing cells augmented activity of the endogenous CDX4 promoter, increasing total Cdx4 protein. An alternative explanation would be that HoxA10 bound to this HOXA10 promoter cis element and activated transcription.
We investigated in vitro interaction of Cdx4 with the HOXA10 cis element by a DAPA, similar to studies described above. In these studies, U937 nuclear proteins were incubated with a biotin-labeled, double-stranded oligonucleotide probe representing the −124 to −140 bp HOXA10 promoter sequence or a non-Cdx4-binding control oligonucleotide. Probes were purified by affinity to neutravidin beads, and interacting proteins were separated by SDS-gel electrophoresis and identified by Western blot. We found that Cdx4 specifically co-purified with the HOXA10 cis element probe (Fig. 8A). Reprobing the blots with a HoxA10 antibody failed to identify interaction with the HOXA10 probe (not shown).
FIGURE 8.
Cdx4 bound to the HOXA10 promoter. A, Cdx4 bound to the HOXA10 promoter by a DAPA. The DAPA was performed using nuclear proteins from U937 cells and a biotin-labeled, double-stranded, synthetic oligonucleotide probe representing the −129 to −136 bp sequence of HOXA10 (or irrelevant control oligonucleotide). Proteins that co-purified with the oligonucleotide probes were separated by SDS-PAGE, and Western blots were probed with antibody (Ab) to HoxA10. Non-precipitated (input) nuclear protein was a control in this experiment. B, Cdx4 bound to the HOXA10 promoter by EMSA. EMSA was performed using nuclear proteins from U937 cells and a radiolabeled, double-stranded, synthetic oligonucleotide probe representing the −129 to −136 bp sequence of HOXA10. Binding assays were incubated with an irrelevant control antibody or Cdx4 antibody or with double-stranded, unlabeled oligonucleotide competitors representing homologous −129 to −136 bp HOXA10 sequence, −129 to −136 bp HOXA10 sequence with mutation of the Cdx4-binding consensus, the −139 to −150 bp HoxA10-binding CDX4 sequence, a non-HoxA10-binding mutant form of the −139 to −150 bp CDX4 sequence, or an irrelevant oligonucleotide from the CYBB gene, as indicated. The arrow represents the Cdx4 DNA-bound complex. The first lane represents free probe in a reaction with no nuclear proteins or oligonucleotide competitors. C, Cdx4 bound a cis element in the HOXA10 promoter in vivo. Chromatin immunoprecipitation was performed with U937 cells and antibodies to Cdx4 or HoxA10 (or irrelevant control antibody). Co-precipitating chromatin was amplified by real-time PCR with primers that flank the −45 to −160 bp HOXA10 promoter sequence (the functional cis element), the −400 to −500 bp HOXA10 promoter sequence (an irrelevant region of HOXA10), the −630 to −750 bp HOXA10 promoter sequence (another irrelevant region of HOXA10), or an irrelevant, non-Cdx4-binding cis element from the PTPN13 gene. ChIP results were normalized to PCR with non-precipitated, input chromatin to control results between experiments. *, statistically significant difference in Cdx4 binding versus nonspecific binding to the HOXA10 probe (p = 0.0003, n = 6). Error bars, S.E.
We also used EMSA to study in vitro Cdx4 binding to the HOXA10 cis element. In these studies, U937 nuclear proteins were incubated with a radiolabeled, double-stranded synthetic oligonucleotide probe representing the −124 to −140 bp sequence of the HOXA10 promoter. Some binding reactions were incubated with an antibody to Cdx4 (or irrelevant control antibody). We found that a protein complex that was cross-immunoreactive with Cdx4 bound to the HOXA10 cis element probe (Fig. 8B, compare lanes 1 and 2). We also investigated binding specificity of this protein complex in a series of assays with excess, unlabeled oligonucleotide competitors. In these studies, we found that an unlabeled oligonucleotide with the −124 to −140 bp HOXA10 sequence competed for binding of the labeled probe, but an oligonucleotide with mutation of the Cdx4-binding consensus sequence did not (Fig. 8B, lanes 3 and 4). Unlabeled oligonucleotide competitor with the HoxA10-binding site from the CDX4 promoter did not compete for complex binding to the HOXA10 cis element probe; nor did the HoxA10-binding mutant version of this sequence (Fig. 8B, lanes 5 and 6) or an oligonucleotide with an irrelevant sequence from the CYBB promoter (Fig. 8B, lane 7).
To investigate in vivo Cdx4 interaction with the HOXA10 cis element, we performed additional ChIP studies. For these experiments, U937 cell lysates were sonicated to generate chromatin fragments of ∼200 bp, and chromatin immunoprecipitation was performed with antibodies to Cdx4 or HoxA10 (or irrelevant, control antibody). Non-precipitated (input) chromatin was a positive control used to normalize results between experiments.
Co-precipitating chromatin was analyzed by real-time PCR with primers that flanked the HOXA10 cis element (−45 to −160 bp). Primers flanking two irrelevant sequences in the HOXA10 5′-flank (−400 to −500 bp and −630 to −750 bp) and an irrelevant, non-Cdx-binding cis element from the PTPN13 promoter were negative controls in these studies. We found that the −45 to −160 bp HOXA10 promoter sequence specifically co-precipitated with Cdx4 but not with HoxA10 (Fig. 8C).
Cdx4 Expression Level Influences HoxA10 Expression
We next determined if increased HOXA10 promoter activity in Cdx4-overexpressing cells was associated with increased HoxA10 expression. For these studies, U937 cells were stably co-transfected with various combinations of vectors to overexpress Cdx4 or HoxA10 (or empty expression vector) and vectors to express Cdx4-specific shRNAs, HoxA10-specific shRNAs, or scrambled control shRNAs (described above). We found that Cdx4 overexpression increased expression of HoxA10 mRNA (Fig. 4A, compare black and white bars) and protein (Fig. 4B, compare first and third lanes, middle panel). Conversely, Cdx4 knockdown decreased expression of HoxA10 mRNA (Fig. 4A, compare black and white hashed bars) and protein (Fig. 4C, compare first and second lanes, middle panel).
Consistent with our hypothesis, Cdx4 knockdown decreased expression of HoxA10 mRNA in HoxA10-overexpressing cells (Fig. 4A, compare gray and gray cross-hashed bars) and protein (Fig. 4C, compare third and fourth lanes, middle panel), reflecting the contribution of endogenous HoxA10 to total HoxA10 in U937 cells transfected with a HoxA10 expression vector. Also, overexpressed Cdx4 partly rescued HoxA10 expression in cells with HoxA10-specific shRNAs (Fig. 4A, compare gray hashed bar with white cross-hashed bar).
Cdx4 Influences HoxA10 Target Gene Expression, and HoxA10 Influences Cdx4 Target Gene Expression
We next investigated the functional significance of CDX4 transcriptional regulation by HoxA10. Few genuine target genes for Cdx4 were previously identified. However, it was previously established that overexpression or knock-out of Cdx4 influenced expression of a group of HOX genes, including B3, B4, A9, and A10. Therefore, we analyzed Hox expression as a direct method of determining the impact of HoxA10 on events related to regulation of Cdx4. We approached this by stably transfecting U937 cells with various combinations of vectors to overexpress HoxA10 or Cdx4 (or with empty expression vector) and to express HoxA10-specific shRNAs, Cdx4-specific shRNAs, or scrambled control shRNAs. These transfectants were analyzed for expression of HoxB3, HoxB4, and HoxA9 by real-time PCR.
We found that expression of HoxB3, -B4, and -A9 was significantly increased by Cdx4 overexpression (Fig. 9A, compare black and white bars) and decreased by Cdx4 knockdown (Fig. 9A, compare black and white hashed bars), as anticipated. We also found that expression of HoxB3, -B4, and -A9 was significantly increased by HoxA10 overexpression (Fig. 9A, compare black and gray bars) and decreased by HoxA10 knockdown (Fig. 9A, compare black and gray hashed bars). Consistent with our hypothesis, Cdx4 overexpression rescued Hox expression in cells with HoxA10 knockdown (Fig. 9A, compare gray hashed bars with white cross-hashed bars), and HoxA10 overexpression rescued expression in cells with Cdx4 knockdown (Fig. 9A, compare white hashed bars with gray cross-hashed bars). Control experiments demonstrating expression of HoxA10 and Cdx4 in these transfectants were presented in Fig. 4A.
FIGURE 9.
Cdx4 influenced expression of HoxA10 target genes, and HoxA10 influenced expression of Cdx4 target genes in myeloid cells. A, HoxA10 overexpression influenced Cdx4 target gene expression. U937 cells were stably co-transfected with a vector to overexpress HoxA10 or Cdx4 (or empty expression vector) and a vector to express HoxA10- or Cdx4-specific shRNAs (or scrambled control shRNAs). Expression of HoxB3, HoxB4, and HoxA9 mRNA was determined by real-time PCR. *, ##, and &&&, statistically significant differences in expression of HoxB3, HoxB4, and HoxA9, respectively, with HoxA10 overexpression or knockdown in comparison with control (p < 0.00001, n = 6). **, ###, and +, statistically significant differences in HoxB3, HoxB4, and HoxA9 expression, respectively, in cells with Cdx4 overexpression or knockdown versus control cells (p < 0.0001, n = 6). ***, &, and ++, statistically significant increase in HoxB3, HoxB4, and HoxA9 mRNA, respectively, in cells with shHoxA10 upon overexpression of Cdx4 (p < 0.0001, n = 6). #, &&, and +++, statistically significant increase in HoxB3, HoxB4, and HoxA9, respectively, in cells with shCdx4 upon overexpression of HoxA10 (p < 0.0001, n = 6). B, Cdx4 overexpression influences HoxA10 target gene expression. The U937 stable transfectants described above were also analyzed for expression of gp91PHOX, β3 integrin, Mkp2, or Tgfβ2 by real-time PCR. * and **, statistically significant increase in gp91PHOX mRNA expression with HoxA10 knockdown and decrease with Cdx4 overexpression, respectively (p ≤ 0.03, n = 3). ***, increased gp91PHOX mRNA expression in Cdx4-overexpressing cells with co-expression of HoxA10-specific shRNA (p < 0.001, n = 6). # and ##, statistically significant decrease in β3 integrin expression with HoxA10 knockdown and increase with Cdx4 overexpression, respectively (p ≤ 0.02, n = 3). ###, decreased β3 integrin expression in Cdx4-overexpressing cells with co-expression of HoxA10-specific shRNA (p < 0.001, n = 3). & and &&, statistically significant decrease in Mkp2 expression with HoxA10 knockdown and increase with Cdx4 overexpression, respectively (p ≤ 0.004, n = 3). &&&, decreased Mkp2 expression in Cdx4-overexpressing cells with co-expression of HoxA10-specific shRNA (p < 0.001, n = 4). + and ++, statistically significant decrease in Tgfβ2 expression with HoxA10 knockdown and increase with Cdx4 overexpression, respectively (p ≤ 0.03, n = 4). +++, decreased Tgfβ2 expression in Cdx4-overexpressing cells with co-expression of HoxA10-specific shRNA (p = 0.004, n = 4). C, Cdx4 knockdown decreased cytokine hypersensitivity of HoxA10-overexpressing cells. U937 cells were stably co-transfected with vectors to overexpress HoxA10-specific (or empty vector control) and Cdx4-specific shRNAs (or scrambled control shRNAs). Cells were deprived of cytokines, and proliferation was determined by [3H]thymidine incorporation after stimulation with a dose titration of fetal calf serum. *, statistically significant differences in [3H]thymidine incorporation in HoxA10-overexpressing transfectants with versus without Cdx4 knockdown (p < 0.01, n = 6). **, statistically significant difference in proliferation in HoxA10 overexpressing transfectants with knockdown of Cdx4 versus control transfectants (p < 0.01, n = 6). Error bars, S.E.
We were also interested in determining if regulation of HOXA10 by Cdx4 had consequences for transcription of HoxA10 target genes. For these studies, we also used the same U937 stable transfectants that were described above. Transfectants were analyzed for expression of a group of previously identified HoxA10 target genes by real-time PCR. In these studies, we investigated one target gene that is repressed by HoxA10 (the CYBB gene that encodes gp91PHOX) (33). We found that HoxA10-knockdown increased gp91PHOX expression, as anticipated (Fig. 9B, compare black and gray bars). Cdx4-overexpression decreased gp91PHOX mRNA, and this effect was partly reversed by HoxA10 knockdown (Fig. 9B, compare gray hashed and gray cross-hashed bars).
We also investigated three genes that are activated by HoxA10: β3 integrin (encoded by ITGβ3), Mkp2 (encoded by DUSP4), and Tgfβ2 (encoded by TGFβ2) (23, 24, 43). We found that HoxA10 knockdown decreased expression of β3 integrin, Mkp2, and Tgfβ2, as anticipated (Fig. 9B, compare black and gray bars). Overexpression of Cdx4 increased expression of these messages, and this effect was impaired by knockdown of HoxA10 in Cdx4-overexpressing cells (Fig. 9B, compare gray and gray hashed bars).
These results were consistent with reinforcing roles of HoxA10 and Cdx4 on gene expression. This has implications for understanding the roles of these two HD transcription factors in regulating hematopoiesis and leukemogenesis.
Cdx4 Contributes to Cytokine Hypersensitivity in HoxA10-overexpressing Myeloid Cells
In previous studies, we found that overexpression of HoxA10 increased cytokine-stimulated proliferation of myeloid cells. Specifically, we found that the proliferative effect of a given dose of cytokine on myeloid cells was increased by HoxA10 overexpression (i.e. cytokine hypersensitivity) (43). To determine if increased Cdx4 expression in HoxA10-overexpressing cells contributed to cytokine hypersensitivity, additional experiments were performed. For these studies, U937 cells were stably transfected with various combinations of vectors to overexpress HoxA10 or with empty vector control and to express Cdx4-specific shRNAs or scrambled control shRNAs, as above. The transfectants were deprived of cytokines and stimulated with a dose titration of fetal calf serum (FCS; the source of cytokine in this study), and proliferation was determined by incorporation of [3H]thymidine.
We found that the proliferative response to a given dose of FCS was significantly greater in HoxA10-overexpressing cells in comparison with control cells, consistent with our previous results (Fig. 9C, compare black circles and red diamonds). Cdx4 knockdown decreased the proliferative response to cytokine stimulation in control cells (Fig. 9C, compare black circles with blue triangles) and blocked the HoxA10-induced increase in proliferation in HoxA10-overexpressing cells (Fig. 9C, compare red diamonds with green squares). These studies suggested that HoxA10-induced expression of Cdx4 contributed to hypersensitivity of HoxA10-overexpressing cells, most likely due to an influence of Cdx4 on expression of HOX genes, including HOXA10 itself.
DISCUSSION
HoxA10 and Cdx4 are HD transcription factors that are implicated in the pathogenesis of AML. These proteins were shown to influence progenitor expansion and differentiation in vitro and in vivo. Consistent with these common activities, previous studies indicated that Cdx4 influenced transcription of various HOX genes, including HOXA10 (25–27). In the current studies, we identified CDX4 as a HoxA10 target gene using a chromatin immunoprecipitation-based approach. We determined that HoxA10 activated a CDX4 promoter cis element. We also found that knockdown of endogenous Cdx4 decreased the activity of this CDX4 cis element in HoxA10-overexpressing cells.
Because Cdx4 did not bind to this cis element, we hypothesized that Cdx4 knockdown influenced CDX4 promoter activity by decreasing expression of endogenous HoxA10. Consistent with this hypothesis, we found that Cdx4 activated a newly identified cis element in the HOXA10 promoter. We found that activation of this cis element by overexpressed Cdx4 was impaired by knockdown of endogenous HoxA10. Because HoxA10 did not bind to the HOXA10 promoter, our data were consistent with HoxA10 influencing HOXA10 transcription via Cdx4.
Therefore, our studies identified a previously unrecognized, positive feedback loop between HoxA10 and Cdx4. Reciprocal activation of CDX4 and HOXA10 transcription by each other's gene product suggested that this feedback is interrupted in some manner during normal myelopoiesis. Our studies also suggested that aberrant overexpression of either protein disrupted this regulatory process. This would be consistent with the hypothesis that decreased expression of either HoxA10 or Cdx4 was essential for decreased expression of the other and also for progression of myelopoiesis.
The MLL (mixed lineage leukemia) gene is involved in a number of leukemia-associated translocations found in poor prognosis AML (referred to as 11q23-AML). Such leukemias are characterized by increased expression of HoxB3, HoxB4, and Abd HoxA proteins in CD34+ bone marrow cells (44). Additionally, the normal decrease in expression of Abd HoxA proteins fails to occur during CD34+ to CD34− differentiation in 11q23-AML. Mll activates HOX gene transcription in immature progenitors but not in differentiating myeloid cells. In contrast, leukemia-associated Mll fusion proteins induce sustained HOX gene activation.
MLL knock-out is characterized by decreased Hox expression, which can be rescued by Cdx4. Therefore, regulation of the HOX locus by Cdx4 and regulation of Cdx4 expression by Hox proteins is complex. It will be of interest to identify negative regulatory factors, in addition to Mll, that decrease HOX transcription during myelopoiesis. These studies are ongoing in the laboratory as are investigations of mechanisms involved in negative regulation of CDX4.
Although the role of HoxA10 overexpression in leukemogenesis is established, the HoxA10 target genes that mediate this effect are relatively unknown. In the current study, we report the results of a relatively labor-intensive method of gene discovery in which chromatin fragments that co-precipitated with HoxA10 were subcloned into a plasmid vector and individually sequenced. This approach identified individual target genes sequentially rather than simultaneously. The latter can be accomplished by using co-precipitating chromatin to screen CpG island microarrays, as we have done in other studies (23, 45), or by automated sequencing.
The goal of our studies was to identify functionally relevant HoxA10 target genes that were indicators of disease progression or were rational targets for therapeutic interventions in human AML. The significance of these studies lies in functional characterization of pathways relevant to myeloid leukemogenesis. Given the role of Cdx4 in hematopoiesis, the fact that HoxA10 activates CDX4 transcription is of consequence to understanding the propagation of gene expression abnormalities in AML.
CDX4 transcriptional regulation has not been studied in detail. Other investigators identified two β-catenin/LEF-binding cis elements in the murine CDX4 promoter (31). Those cis elements are partly conserved in human CDX4 and are distal to the HoxA10-binding site described in this report. It will be of interest to determine if CDX4 transcription involves cooperation between HoxA10 and β-catenin. These studies are being actively pursued in the laboratory.
Similarly, little has been published regarding regulation of the HOXA10 promoter. Although Cdx4 was previously known to influence HoxA10 expression, the mechanism for this had not been identified. In the current studies, we found that Cdx4 interacted with a conserved DNA-binding consensus sequence in the proximal HOXA10 promoter and activated transcription. The relationship between this cis element and possible Mll binding to the HOXA10 5′-flank will be the topic of future studies.
In studies with primary murine bone marrow cells, HoxA10 and Cdx4 expression was maximal in GMP and decreased during monocyte or granulocyte differentiation. Cdx4 expression in GMP or differentiating myeloid progenitors decreased with HoxA10 knock-out and increased with HoxA10 overexpression. In murine HSC, there was no difference in Cdx4 expression in WT versus HoxA10 knock-out cells, but Cdx4 expression was increased by HoxA10 overexpression. These results suggested that ectopic expression of HoxA10 in leukemia may induce differentiation stage-inappropriate gene transcription.
We found that increased endogenous Cdx4 in HoxA10-overexpressing cells activated the endogenous HOXA10 promoter. The consequent increase in endogenous HoxA10 increased total HoxA10, which augmented activation of the CDX4 cis element (in endogenous and trans CDX4 genes). Our conclusion was supported by the observation that Cdx4 did not itself bind to the CDX4 cis element. However, Cdx4 might influence the expression of other proteins, in addition to HoxA10, that regulate this cis element. For example, Cdx4 might decrease expression of a protein that interferes with HoxA10-induced transcription or increase expression of a protein that cooperates with HoxA10.
Similarly, we found that increased expression of endogenous HoxA10 in Cdx4-overexpressing cells contributed to activation of the endogenous CDX4 promoter, further increasing the total amount of Cdx4 in the cell and providing additional activation of the HOXA10 promoter. Although HoxA10 did not bind to the HOXA10 promoter, it might exert this influence via some Cdx4-interacting protein. These possibilities will be explored in future investigations.
Because Cdx4 influenced transcription of multiple ABD HOX genes, it was also possible that Hox proteins, in addition to HoxA10, regulated CDX4. The most likely candidate would be HoxA9, because these two Abd Hox proteins have redundant influences on expansion of committed myeloid progenitor cells. We investigated this possibility and found that HoxA9 neither bound to nor influenced activity of the HoxA10-binding CDX4 cis element identified in these studies. However, it remained possible that HoxA9 influenced CDX4 transcription via other cis elements. This will be the topic of additional investigations.
Although HoxB3 and HoxB4 are maximally expressed at an earlier stage of hematopoiesis relative to HoxA9 and HoxA10, these Hox proteins also induce cell expansion. In this case, the influence of HoxB3 and HoxB4 is on HSC. In the current studies, we found that Cdx4 expression in HSC was not altered by knock-out or haploinsufficiency of HoxA10. Because any influence of HoxB3 or HoxB4 on CDX4 transcription would be likely to be in HSC, this suggested a lack of redundancy with the effects of HoxA10 that we documented in these studies. The role of HoxB3 or HoxB4 in CDX4 transcription in HSC will be pursued in future studies.
In these studies, we found that HoxA10 influenced transcription of multiple HOX genes in a manner that required Cdx4. Similarly, we found that Cdx4 influenced some previously identified HoxA10 target genes in a HoxA10-dependent manner. Although each of these genes will need to be studied individually, these results suggested that regulation of CDX4 promoter activity by HoxA10 contributed to regulation of Cdx4 target genes and vice versa. Additionally, we found that cytokine hypersensitivity of HoxA10-overexpressing myeloid cells was decreased by Cdx4 knockdown, providing functional significance to these gene expression studies.
Therefore, cross-regulation of HoxA10 and Cdx4 and their target genes may impact the pathogenesis of poor prognosis AML. This hypothesis was also suggested by studies in an 11q23-myeloid leukemogenesis murine model. In these studies, development of AML in mice transplanted with bone marrow expressing an Mll fusion protein was delayed in a background of CDX4 knock-out (44). Considered together with the current studies, these results suggest that Cdx4 contributes to 11q23 leukemogenesis by amplifying the effects of Mll on the transcription of key HOX genes. This hypothesis is the topic of ongoing studies in the laboratory.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-HL87717 (to E. E.). This work was also supported by a Veterans Affairs Merit Review (to E. E.).
P. Farnham, personal communication.
L. Bei, W. Huang, H. Wang, C. Shah, E. Horvath, and E. Eklund, unpublished observations.
- HD
- homeodomain
- HSC
- hematopoietic stem cell(s)
- GMP
- granulocyte/monocyte progenitor(s)
- SCF
- stem cell factor
- DAPA
- DNA affinity purification assay(s).
REFERENCES
- 1. Acampora D., D'Esposito M., Faiella A., Pannese M., Migliaccio E., Morelli F., Stornaiuolo A., Nigro V., Simeone A., Boncinelli E. (1989) Nucleic Acids Res. 17, 10385–10402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sauvageau G., Lansdorp P. M., Eaves C. J., Hogge D. E., Dragowska W. H., Reid D. S., Largman C., Lawrence H. J., Humphries R. K. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 12223–12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thorsteinsdottir U., Kroon E., Jerome L., Blasi F., Sauvageau G. (2001) Mol. Cell Biol. 21, 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sauvageau G., Thorsteinsdottir U., Eaves C. J., Lawrence H. J., Largman C., Lansdorp P. M., Humphries R. K. (1995) Genes Dev. 9, 1753–1765 [DOI] [PubMed] [Google Scholar]
- 5. Calvo K. R., Sykes D. B., Pasillas M., Kamps M. P. (2000) Mol. Cell Biol. 20, 3274–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawrence H. J., Helgason C. D., Sauvageau G., Fong S., Izon D. J., Humphries R. K., Largman C. (1997) Blood 89, 1922–1930 [PubMed] [Google Scholar]
- 7. Buske C., Feuring-Buske M., Antonchuk J., Rosten P., Hogge D. E., Eaves C. J., Humphries R. K. (2001) Blood 97, 2286–2292 [DOI] [PubMed] [Google Scholar]
- 8. Björnsson J. M., Andersson E., Lundström P., Larsson N., Xu X., Repetowska E., Humphries R. K., Karlsson S. (2001) Blood 98, 3301–3308 [DOI] [PubMed] [Google Scholar]
- 9. Thorsteinsdottir U., Mamo A., Kroon E., Jerome L., Bijl J., Lawrence H. J., Humphries K., Sauvageau G. (2002) Blood 99, 121–129 [DOI] [PubMed] [Google Scholar]
- 10. Wang H., Lindsey S., Konieczna I., Bei L., Horvath E., Huang W., Saberwal G., Eklund E. A. (2009) J. Biol. Chem. 284, 2549–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golub T. R., Slonim D. K., Tamayo P., Huard C., Gaasenbeek M., Mesirov J. P., Coller H., Loh M. L., Downing J. R., Caligiuri M. A., Bloomfield C. D., Lander E. S. (1999) Science 286, 531–537 [DOI] [PubMed] [Google Scholar]
- 12. Kroon E., Krosl J., Thorsteinsdottir U., Baban S., Buchberg A. M., Sauvageau G. (1998) EMBO J. 17, 3714–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawagoe H., Humphries R. K., Blair A., Sutherland H. J., Hogge D. E. (1999) Leukemia 13, 687–698 [DOI] [PubMed] [Google Scholar]
- 14. Armstrong S. A., Staunton J. E., Silverman L. B., Pieters R., den Boer M. L., Minden M. D., Sallan S. E., Lander E. S., Golub T. R., Korsmeyer S. J. (2002) Nat. Genet. 30, 41–47 [DOI] [PubMed] [Google Scholar]
- 15. Camós M., Esteve J., Jares P., Colomer D., Rozman M., Villamor N., Costa D., Carrió A., Nomdedéu J., Montserrat E., Campo E. (2006) Cancer Res. 66, 6947–6954 [DOI] [PubMed] [Google Scholar]
- 16. Guenther M. G., Jenner R. G., Chevalier B., Nakamura T., Croce C. M., Canaani E., Young R. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 8603–8608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milne T. A., Briggs S. D., Brock H. W., Martin M. E., Gibbs D., Allis C. D., Hess J. L. (2002) Mol. Cell 10, 1107–1117 [DOI] [PubMed] [Google Scholar]
- 18. Ernst P., Mabon M., Davidson A. J., Zon L. I., Korsmeyer S. J. (2004) Curr. Biol. 14, 2063–2069 [DOI] [PubMed] [Google Scholar]
- 19. Horton S. J., Grier D. G., McGonigle G. J., Thompson A., Morrow M., De Silva I., Moulding D. A., Kioussis D., Lappin T. R., Brady H. J., Williams O. (2005) Cancer Res. 65, 9245–9252 [DOI] [PubMed] [Google Scholar]
- 20. Eklund E. A., Jalava A., Kakar R. (2000) J. Biol. Chem. 275, 20117–20126 [DOI] [PubMed] [Google Scholar]
- 21. Lindsey S., Zhu C., Lu Y. F., Eklund E. A. (2005) J. Immunol. 175, 5269–5279 [DOI] [PubMed] [Google Scholar]
- 22. Eklund E. A., Goldenberg I., Lu Y., Andrejic J., Kakar R. (2002) J. Biol. Chem. 277, 36878–36888 [DOI] [PubMed] [Google Scholar]
- 23. Wang H., Lu Y., Huang W., Papoutsakis E. T., Fuhrken P., Eklund E. A. (2007) J. Biol. Chem. 282, 16164–16176 [DOI] [PubMed] [Google Scholar]
- 24. Bei L., Lu Y., Bellis S. L., Zhou W., Horvath E., Eklund E. A. (2007) J. Biol. Chem. 282, 16846–16859 [DOI] [PubMed] [Google Scholar]
- 25. Davidson A. J., Ernst P., Wang Y., Dekens M. P., Kingsley P. D., Palis J., Korsmeyer S. J., Daley G. Q., Zon L. I. (2003) Nature 425, 300–306 [DOI] [PubMed] [Google Scholar]
- 26. Bansal D., Scholl C., Fröhling S., McDowell E., Lee B. H., Döhner K., Ernst P., Davidson A. J., Daley G. Q., Zon L. I., Gilliland D. G., Huntly B. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16924–16929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y., Yates F., Naveiras O., Ernst P., Daley G. Q. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 19081–19086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lengerke C., Grauer M., Niebuhr N. I., Riedt T., Kanz L., Park I. H., Daley G. Q. (2009) Ann. N.Y. Acad. Sci. 1176, 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lowney P., Corral J., Detmer K., LeBeau M. M., Deaven L., Lawrence H. J., Largman C. (1991) Nucleic Acids Res. 19, 3443–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. (1988) Mol. Cell Biol. 8, 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pilon N., Oh K., Sylvestre J. R., Bouchard N., Savory J., Lohnes D. (2006) Dev. Biol. 289, 55–63 [DOI] [PubMed] [Google Scholar]
- 32. Larrick J. W., Fischer D. G., Anderson S. J., Koren H. S. (1980) J. Immunol. 125, 6–12 [PubMed] [Google Scholar]
- 33. Eklund E. A., Jalava A., Kakar R. (1998) J. Biol. Chem. 273, 13957–13965 [DOI] [PubMed] [Google Scholar]
- 34. Satokata I., Benson G., Maas R. (1995) Nature 374, 460–463 [DOI] [PubMed] [Google Scholar]
- 35. Oberley M. J., Farnham P. J. (2003) Methods Enzymol. 371, 577–596 [DOI] [PubMed] [Google Scholar]
- 36. Dignam J. D., Lebovitz R. M., Roeder R. G. (1983) Nucleic Acids Res. 11, 1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dubchak I., Brudno M., Loots G. G., Pachter L., Mayor C., Rubin E. M., Frazer K. A. (2000) Genome Res. 10, 1304–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mayor C., Brudno M., Schwartz J. R., Poliakov A., Rubin E. M., Frazer K. A., Pachter L. S., Dubchak I. (2000) Bioinformatics 16, 1046–1047 [DOI] [PubMed] [Google Scholar]
- 39. Loots G. G., Ovcharenko I., Pachter L., Dubchak I., Rubin E. M. (2002) Genome Res. 12, 832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu C., Saberwal G., Lu Y., Platanias L. C., Eklund E. A. (2004) J. Biol. Chem. 279, 50874–50885 [DOI] [PubMed] [Google Scholar]
- 41. Hu Y. L., Passegué E., Fong S., Largman C., Lawrence H. J. (2007) Blood 109, 4732–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawrence H. J., Sauvageau G., Ahmadi N., Lopez A. R., LeBeau M. M., Link M., Humphries K., Largman C. (1995) Exp. Hematol. 23, 1160–1166 [PubMed] [Google Scholar]
- 43. Shah C. A., Wang H., Bei L., Platanias L. C., Eklund E. A. (2011) J. Biol. Chem. 286, 3161–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koo S., Huntly B., Wang Y., Chen J., Brumme K., Ball B., McKinney Freeman S., Yabuuchi A., Scholl C., Bansal D., Zon L., Froehling S., Daley G., Gilliland G., Mercher T. (2011) Haematologica, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang W., Zhu C., Wang H., Horvath E., Eklund E. A. (2008) J. Biol. Chem. 283, 7921–7935 [DOI] [PubMed] [Google Scholar]