Abstract
The ventromedial nucleus of the hypothalamus (VMH) is a major site for the control of female sexual behaviour by ovarian steroid hormones. This review will explore recent details that have emerged regarding the ovarian hormone-induced remodelling of neural circuits within the VMH in adult female rats, with the goal of refining the model of the VMH neural circuit. VMH neurones exhibit simple dendritic arbours, with a single long primary dendrite (LPD) and several short primary dendrites. We recently found that ovarian hormones have unanticipated differential effects on the length of the LPDs, suggesting an intricate synaptic reorganisation. LPDs extend into the lateral fibre plexus where they contact oxytocin-labelled terminals. Oestradiol treatment re-arranges this oxytocin innervation, in particular by withdrawing some of the LPDs and intensifying the oxytocin input to the remaining dendrites. These changes are reversed with concomitant progesterone treatment. Incorporating these new results, we have updated our working model of hormone-induced synaptic reorganisation in the VMH, emphasizing the re-balancing of local versus extrinsic connectivity. The new working model synthesizes the recent evidence for re-wiring with insights from electrophysiological and behavioural pharmacological studies that pertain to the roles of oxytocin and glutamate in VMH neural activity and mating behaviour.
Introduction to the Neuroendocrine Control of Lordosis
The ventromedial nucleus of the hypothalamus (VMH) is well established as a major site in the control of female sexual behaviour by ovarian steroid hormones in rats. The ovary sequentially releases oestradiol and progesterone during the oestrous cycle, and this temporal dynamic plays a critical role in the timing of mating behaviour. The essential function of these hormones has been demonstrated in experiments with ovariectomized rats given a regimen of hormone replacement. At modest doses, oestradiol acting alone can somewhat increase receptive behaviour in females. However, oestrogen action induces progesterone receptors in the VMH that, when also activated by the subsequent rise in progesterone levels, facilitate robust receptive and proceptive behaviours. The optimal dose of oestradiol may depend on the strain of rats, e.g., Sprague-Dawley versus Long Evans, and very high doses of oestradiol may trigger the production of brain-derived progesterone, allowing the high dose of oestradiol appear sufficient to promote maximal receptivity (1).
The centrality of the VMH in mediating hormonal effects on female sexual behaviour was demonstrated by lesion studies in which damage to the VMH eliminated the effect of oestradiol and/or oestradiol plus progesterone on female mating behaviour (2–4). In addition, behavioural experiments locally administered oestradiol and progesterone or selective receptor antagonists into the VMH (5–8). The relatively small size of this brain region and the readily diffusible nature of steroids are not well suited for the use of site-specific injections to determine possible sub-region involvement in hormone action. Despite the sustained interest of behavioural neuroscientists in the molecular, neurochemical, and neurophysiological consequences of hormone action in the VMH, our understanding of the changes in VMH function brought about by ovarian hormones that underlie such fundamental and biologically critical changes in female behaviour remains limited. This review will highlight recent insights into the ovarian hormone-induced remodelling of neural circuits within the VMH in adult female rats that provide insights into the dynamic neurological control of this crucial behaviour.
A few points of nomenclature should be made at the outset. First, it should be noted that the abbreviation “VMH” derives from several published rat brain atlases, in which the H distinguishes this nucleus from the ventromedial nucleus of the thalamus. However, some authors prefer the abbreviation “VMN,” and in some papers “VMH” refers to the entire ventromedial hypothalamus, including the arcuate nucleus. Given the lack of a universal abbreviation for this region, readers must attend to the definition being used in each report. Secondly, the borders of the VMH as defined by Nissl stain may not perfectly match the distribution of typical VMH markers, such as oestrogen receptors, in many species. Thus, a refined neurochemical definition of the VMH borders may emerge with future studies.
VMH Organisation and Cytoarchitecture
The VMH is a cell-dense region located near the third ventricle, slightly dorsal to the median eminence. A fibre plexus of dendrites and axons circumscribes the nucleus. The functional relevance of this later fibre plexus is supported by the reduction in female sexual behaviour observed after knife cuts in this region that sever connections between the VMH and other brain regions (4). The VMH is situated medial to a prominent fibre bundle en route to the median eminence (9). Like most hypothalamic nuclei, the VMH lacks any apparent laminar organisation; however, the majority of the 55,000 neurones in the rat VMH reside in one of two subdivisions: the ventrolateral (vlVMH) and the dorsomedial (dmVMH). As recently reviewed (10), these two major subdivisions are separated by a cell-poor area and have different axonal projection targets and afferents. These VMH subdivisions also have been associated with unique functions. The vlVMH expresses receptors needed for the regulation of sexual receptivity and is activated by mating behaviour, whereas the dmVMH expresses receptors associated with energy balance. This dichotomy may not be absolute, but connections between these subdivisions appear to be sparse (11).
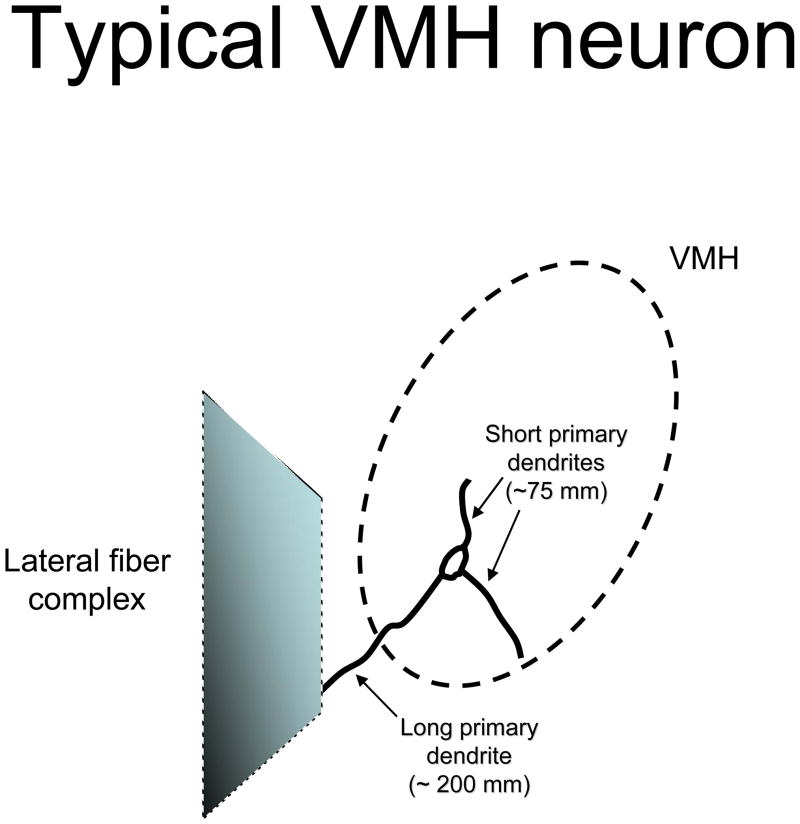
Neurones in both subdivisions of the VMH exhibit simple dendritic arbours. Typically, these neurones produce approximately three dendrites that extend directly from the soma, referred to as primary dendrites. Occasionally, there are secondary dendrites that ramify off one of the primary dendrites (12–14). A consistent feature of these neurones is that a single primary dendrite is quite long. This single long primary dendrite (LPD) is more than 100 μm longer than the other primary dendrites, which are classified by default as short primary dendrites (13). Figure 1 illustrates a typical VMH neurone.
Figure 1.
Illustration of the dendritic arbour of a typical VMH neurone. The VMH is demarcated by dashed line forming an oval. The lateral fibre complex is portrayed as a shaded trapezoid. The typical VMH neurone is shown with three primary dendrites, with the longest primary dendrite extending towards the lateral fibre complex. The short primary dendrites remain within the borders of the VMH, presumably to be innervated by local interneurones.
Actions of Oestradiol and Progesterone within the VMH
Oestradiol has a number of structural effects on neurones in the VMH. Hours after ovariectomized animals are treated with oestradiol (100 μg) the size of neuronal cell bodies is increased, based in part on the hypertrophy of the nucleus, rough endoplasmic reticulum, and Golgi apparatus (15). Golgi impregnation studies showed that the number of dendritic spines in the VMH correlates with reproductive behaviour across the oestrous cycle. In particular, the density of dendritic spines in VMH neurones is highest at the time when ovarian hormones are elevated (16). In ovariectomized rats, oestradiol treatment (10 μg) increases the number of spines on the dendrites of VMH neurones two-fold, as observed with a variety of methods (13, 16–19).
Keeping in mind that the dendritic tree of VMH neurones includes a single LPD that extends toward the lateral afferent fibre field, as well as several short primary dendrites (12, 13), our laboratory tested the hypothesis that these dendrite categories represent different functions in neuronal computation. In support of this notion, we found that oestradiol treatment (10 μg) markedly increased the number of dendritic spines specifically on the short primary dendrites. Thus, the oestradiol-induced spines in the VMH may signify additional local connectivity within the VMH induced by oestrogens to regulate reproductive behaviour. These changes occurred on the arbours of VMH neurones that did not express nuclear ER-α nor sent axonal projections to the periaqueductal gray (13, 20). Thus, oestradiol-induced spines occur on a specific subset of dendrites on a subset of neurones, and the mechanism appears not to depend on nuclear oestadiol action within the same cell.
At the same time that ovarian hormones promote the formation of dendritic spines on short primary dendrites, they dynamically regulate the length of the LPDs. In particular, oestradiol treatment causes a marked shortening of these dendrites, a process that is reversed with subsequent progesterone treatment. This regulation of dendrite extension was first observed with Golgi analysis, which allowed for straightforward measurement of dendrite length (14). A subsequent electron microscopy analysis of the VMH lateral fibre complex (VMH-lfc) verified that oestradiol treatment (10 μg) caused an attrition of dendritic profiles in that region (21). This pattern is consistent with findings reported from intact cycling rats (17). Thus, oestradiol acts to shorten the long primary dendrites, removing them from the VMH-lfc at the same time that the short primary dendrites manifest heightened excitatory input.
Although the structural effects of oestradiol have been widely studied, the effects of oestradiol combined with progesterone, which may be associated with a more substantial change in mating behaviour, have received somewhat less experimental consideration. A recent study from our laboratory provided evidence for a striking effect on structural plasticity in the VMH when oestradiol was given sequentially with progesterone. In particular, oestradiol alone (10 μg) versus oestradiol plus progesterone (500 μg) had opposite effects on the lengths of LPDs emanating from neurones in the vlVMH, based on Golgi impregnation analysis. Within four hours, progesterone treatment reversed the pronounced reduction of dendrite length induced by oestradiol administration alone (14). Electron microscopy analysis of the VMH-lfc validated the observation based on Golgi impregnation that oestradiol plus progesterone treatment reverses the effect of oestradiol alone on dendritic profiles in this region (21).
At first glance, this set of results may seem inconsistent with the often-described synergistic action of oestradiol and progesterone. In many cases, progesterone treatment augments the effects of oestradiol (22). However, disparate effects of oestradiol alone versus oestradiol plus progesterone have been described, for example, on mu-opioid receptor internalisation in the anterior hypothalamus (23). The unique effects of oestradiol alone versus oestradiol plus progesterone on the dendritic arbour of VMH neurones may reflect their disparate actions on female sexual behaviour. As mentioned above, at this dose oestradiol alone produces only a mild facilitation of female sexual behaviour (24, 25). For the full expression of the sexual behaviour, including proceptive behaviours, both ovarian hormones are required. These categorical effects of oestradiol alone versus oestradiol plus progesterone may be mediated in part by their non-linear effects on the synaptic organisation within the dendritic arbour of VMH neurones.
Implications of Dendrite Length Regulation
The unexpected dynamic regulation of the length of LPDs of VMH neurones by ovarian hormones requires an explanation in terms of synaptic re-organisation. It was previously observed that very few axonal afferents to the VMH directly protrude into the nucleus (9). Instead, the VMH-lfc contains important neuromodulators of sexual behaviour, including noradrenaline (26), gonadotrophin releasing hormone (27), serotonin (28), and oxytocin (OT) (29, 30). By modifying the length of the long primary dendrites, ovarian hormones potentially could alter both quantitative and qualitative aspects of extranuclear innervation received by VMH neurones.
In particular, oestradiol action would appear to cloister VMH dendrites away from the neuromodulators in the VMH-lfc, thereby restricting connections with inputs that promote sexual receptivity. This may initially seem at odds with the view that oestradiol is critical for priming reproductive behaviour. However, oestradiol may set the stage for mating behaviour by initiating molecular changes, such as the induction of progestin receptors, while simultaneously imposing a brake on mating behaviour through disengagement of the VMH dendrites and their extranuclear afferents.
Conversely, progesterone action, by re-extending the LPDs, would allow the reestablishment of contact with the neuromodulators present in this region. Currently, there is insufficient information about the balance of excitatory and inhibitory connections received by the major dendrites types and the cell bodies across hormonal conditions. The evidence available allows us to construct a working model. Ultrastructural analysis revealed that not all axonal boutons in the fibre plexus expressed vesicular glutamate transporter-2, a marker of glutamatergic presynaptic terminals (21). Thus, the re-extension of dendrites towards the fibre plexus also may bring them into contact with inhibitory neurotransmitters, decreasing activity in the VMH and thereby facilitating sexual receptivity. It has been reported that progesterone reverses the oestradiol-induced reduction of 3H-muscimol binding to GABAA receptors (31). Furthermore, infusions of GABA agonists into the VMH have been shown to promote female sexual behaviour (32). Conversely, infusions of the glutamate receptor agonists N-methyl-D-aspartic acid (NMDA) and kainate into the VMH decreased the expression of lordosis in ovariectomized animals treated with oestradiol and progesterone (33). These findings suggest that the facilitative effects of progesterone on female sexual behaviour are dependent on the hormone’s ability to dampen neuronal excitation in the VMH. By re-extending dendrites towards the fibre plexus, progesterone may reduce neuronal activity in the VMH as a means to promote the full expression of female sexual behaviour. Consistent with the behavioural pharmacology experiments that predicted a negative correlation between neural activity and lordosis behaviour, recent ex vivo multi-electrode recordings of VMH slices taken at difference phases of the oestrous cycle found that evoked excitatory postsynaptic potentials were negatively correlated with the expected lordosis response (34).
Taken together, ovarian hormones have complex effects on the synaptic input of VMH dendritic arbours. When only oestradiol levels are elevated, dendritic spines are induced on short primary dendrites, which may allow local interneurones to mutually increase excitability, a pattern associated with reduced lordosis behaviour. Thus, we propose that ER-α, expressed by local interneurones, mediates the increased number of spines on the short primary dendrites of other VMH neurones. At the same time, ovarian hormones regulate the connectivity of LPDs, which may be mediated by transmitter release or trophic factors governed by oestradiol receptors expressed in other brain regions, given that many brain areas that send afferents to the VMH also express oestradiol receptors (35),
Oxytocin: Behaviourally Relevant Connection to the LPDs
OT is a nine amino acid peptide (Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly) found in all placental mammals. OT is synthesized in magnocellular and parvocellular neurones of the paraventricular nucleus (PVN) and the supraoptic nucleus (SON), as well as nearby ectopic groups of neurones (36). Ovarian hormones increase messenger RNA and peptide levels of OT in the PVN and the SON (37, 38). The peptide is initially translated into a prohormone known as oxytocin-neurophysin. During axonal transport, peptidases cleave the active OT from its carrier neurophysin (39). In addition to a projection to the neurohypophysis, OT-labelled axonal fibres are found in various hypothalamic, brainstem, and limbic regions (40, 41), which suggests that central OT may modulate a variety of brain systems.
The receptor for OT (OTR) has been localized to a number of the regions that appear to be the target of these OT axons, such as the central nucleus of the amygdala and the dorsal motor nucleus of the vagus (42, 43). Pertinent here, the VMH is a region that expresses the OTR, and levels of this receptor are strikingly elevated by oestradiol alone and oestradiol plus progesterone treatment (44, 45). In fact, ovarian hormone treatment may be associated with a lateral migration of these receptors (46, 47). Unfortunately, while quantitative receptor autoradiography is well suited to measure receptor number, it is less useful for determining the cellular location of receptors. Future analysis of OTR distribution within the arbor of VMH neurons awaits the development of an OTR antibody. In addition, the mechanism of delivery of OT to these receptors in the VMH has not been established. It is noteworthy that the neurohypophysial tract travels laterally to the VMH (29, 30). It has been proposed that OT is released as an endocrine-like or bulk transmission signal to the VMH, based on dendritic release of OT in the PVN and SON or en passage release from these nearby axons (48, 49). This mechanism of diffuse delivery seems at odds with the notion that the cognate receptors exhibit directional migration.
An alternative explanation for the mode of delivery takes note of the LPDs extending laterally from the VMH towards the OT fibre tract. Although the majority of OT in the lateral fibre plexus may be en route to the pituitary, a subset of the axons may provide synaptic input to the LPDs. It is also possible that endocrine-type release provides tonic levels of OT in the VMH, whereas axonal delivery offers more phasic and circuit-specific effects. Thus, multiple modes of delivery are possible, each with unique functional significance.
To discover structural evidence for a synaptic connection between OT in the VMH-lfc and the LPDs, we employed immunoelectron microscopy (21). As mentioned above, this analysis complemented the Golgi experiment in demonstrating the differential effects of oestradiol alone and oestradiol plus progesterone on the presence of dendrites extending from the VMH into this region. Immunohistochemical staining demonstrated the presence of OT in synaptic terminals in this region. The identity of the cellular compartments containing OT labelling was verified as presynaptic terminals using synaptophysin and vesicular glutamate transporter 2 (vGlut2) double labelling. The presence of vGlut2 was consistent with the high density of small clear vesicles that apposed the assymetrical synaptic junction. Thus, OT appears to be a neuromodulator co-released from glutamatergic terminals in the VMH-lfc, the location where the lengths of LPDs are being regulated. This is consistent with other work that reported OT neurones in the PVN and SON also release glutamate (50, 51). The postsynaptic structures that received these OT-labelled terminals were dendrites, identified by structural markers, such as microtubules and their lack of vesicles or myelin sheathing, in addition to double labelling for microtubule-associated protein 2 (MAP2), a dendrite-specific protein. These findings provide structural evidence for a neurocrine, as opposed to an endocrine, mode of OT delivery to its receptors on VMH dendrites. This study also reinforces the model of the VMH-lfc as a nexus wherein LPDs extending from the VMH are positioned to receive inputs from extranuclear afferents, including the OT.
Possible Mechanisms that Regulate the Length of LPDs
Neuronal activity itself may be a critical mediator of the effects on LPDs by ovarian hormones, particularly that of oestradiol. Persistent stimulation of glutamate receptors leads to a decrease in levels of MAP2, but not actin, in rat hippocampal neurones (52), mediated by calcium-dependent protease calpain. Calpain reduces the levels of MAP2 but not other neural proteins (53). Changes in neuronal morphology, specifically dendrite length, due to alterations in synaptic input have been observed within three hours (54). If similar mechanisms operate in the VMH, one may reason that under conditions in which levels of oestradiol alone are elevated, there may be increased glutamatergic activity, as is associated with reduced lordosis behavior, which may decrease LPD length through the effect of calpain on MAP2. When progesterone levels rise sequentially, progesterone may dampen the neuronal excitability induced by oestradiol, in part by increased GABAA receptor expression (31), thereby restoring the levels of MAP2 and prompting the re-extension of the LPDs. Thus, the regulation of dendrite length by ovarian hormones may be secondary to changes in neural activity that control MAP2 levels. The changes in dendrite length, in turn, would gate the flow of extrinsic afferent information to the VMH, and those extrinsic connections may be required ultimately for reproductive behaviour to proceed.
While the mechanism proposed above emphasizes the role of neurotransmitters to initiate modifications to the dendritic tree, there may be complementary ways in which neural activity of local interneurones and extrinsic afferents may have these effects. For example, neurones may release growth factors that regulate dendrite length. In fact, the VMH is known to express brain-derived nerve growth factor (55). Alternatively, peptide neuromodulators may be co-released with classic neurotransmitters, which may activate signal transduction pathways postsynaptically that contribute to dendrite re-modelling and/or synaptic plasticity. Such peptide neuromodulators also may have presynaptic effects to regulate the release of classical neurotransmitters. As mentioned above, OT, with potent effects on mating behaviour, appears to be released as a neuromodulator from glutamatergic synapses in the lateral fibre plexus. An avenue of future studies will be to discover how OT and glutamate may collaborate in this region to regulate the morphology of dendrites ramifying from the VMH. Such actions of OT may explain its effects on VMH neural activity and lordosis behaviour.
Refined Working Model and Future Directions
In summary, this review has provided an overview of the synaptic re-organisation in the VMH induced by ovarian hormones, which involves differential effects on long and short dendrites. Based on our findings, we have updated our working model in which oestradiol transynaptically induces dendritic spines on the short primary dendrites (summarized in Figure 2). In this model, an early effect of ovarian hormones is to alter the balance of excitatory and inhibitory neurotransmission within the VMH, which has been implicated in other studies demonstrating rapid effects on neurotransmitters that mediate synaptic changes (56–58). The short primary dendrites may be selectively innervated by local estrogen receptor-containing interneurones, which are known to be glutamatergic. In contrast, ovarian hormones affect the LPDs by controlling their length, thus determining their availability for extranuclear innervation. This effect on dendrite length may also be transynaptic, in which case the targets of hormone action would be the brain regions that send axons to the fibre plexus that encircles the VMH. One type of input in the VMH-lfc is glutamate-OT, possibly arising from the parvocellular PVN. As oestradiol treatment reduces the number of dendrites extending into the fibre plexus, lordosis behaviour is limited. Thus, the change in excitability remodels the dendritic arbour, which in turn, may mediate the altered pattern of locally evoked excitatory postsynaptic potentials. It is this change in electrophysiological responsiveness that then determines the female’s ability to exhibit the lordosis response. Subsequent progesterone treatment is associated with restoring the presence of dendrites in the lateral fibre plexus and the maximal expression of lordosis.
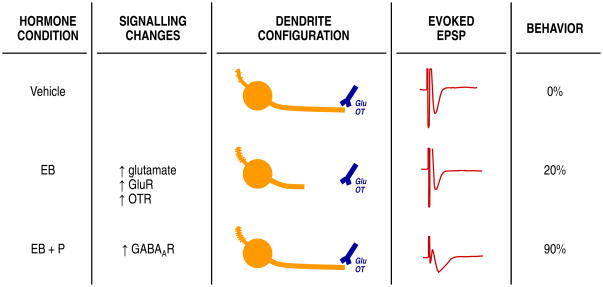
Figure 2.
A working model of ovarian hormone-induced synaptic reorganisation in the VMH. From left to right, the schematic shows the proposed progression from ovarian hormone action to changes in neurotransmitters and neuromodulators, such as GABA, glutamate and oxytocin (44, 59, 60). These hormone state-dependent profiles in neurotransmission and various signalling cascades produces wiring changes in the VMH, such oestradiol-induced spines on the short primary dendrites and retraction of long primary dendrites from the lateral fibre plexus (13, 14, 21). Each wiring pattern is associated with its own profile of evoked EPSPs, which may differ in their duration and magnitude (34). Finally, it is the evoked EPSPs that determine whether or not the VMH output will promote lordosis behaviour (25).
In synthesizing these neural circuitry findings with results from neurochemistry, electrophysiological and behavioural pharmacology studies, we have proposed a working model that underscores several key mechanistic questions for future research. The working model proposes that oestradiol works on a broader hypothalamic network to allow for disparate effects on short versus long primary dendrites. Several strategies may better define the possible distinct mechanisms of action on VMH dendrites, which may take advantage of possible differences in time courses, oestrogen receptor subtypes, neurotransmitters, neuromodulators and second messenger pathways. A limitation of this working model is that the proposed progression from hormones to neurochemistry, to synaptic organisation, to electrophysiological activity, and finally to behaviour is based on correlations across different studies, and demonstrating the direction of causality is an imperative future direction. Incorporating the physical changes in the VMH synaptic organisation with various molecular, neurochemical and electrophysiological changes will be an important advance in our understanding of neural mechanisms of mating behaviour. Thus, the lordosis circuit within the VMH provides an exciting platform to define a feed-forward neuroendocrine mechanism to control a critical mammalian behaviour.
Acknowledgments
The authors thank Dr. Daniel K. Yee for his excellent graphical contributions. This work was supported by the National Institutes of Health Grants MH64371 and training grant MH017168.
References
- 1.Soma KK, Sinchak K, Lakhte rA, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3beta-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- 3.Mathews D, Edwards DA. The ventromedial nucleus of the hypothalamus and the hormonal arousal of sexual behaviors in the female rat. Horm Behav. 1977;8:40–51. doi: 10.1016/0018-506x(77)90019-8. [DOI] [PubMed] [Google Scholar]
- 4.Pfeifle JK, Shivers M, Edwards DA. Parasaggital hypothalamic knifecuts and sexual receptivity in the female rat. Physiol Behav. 1980;24:145–150. doi: 10.1016/0031-9384(80)90026-8. [DOI] [PubMed] [Google Scholar]
- 5.Davis PG, Krieger MS, Barfield RJ, McEwen BS, Pfaff DW. The site of action of intrahypothalamic estrogen implants in feminine sexual behavior: An autoradiographical analysis. Endocrinol. 1982;111:1581–1586. doi: 10.1210/endo-111-5-1581. [DOI] [PubMed] [Google Scholar]
- 6.Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–1812. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 7.Meisel RL, Dohanich GP, McEwen BS, Pfaff DW. Antagonism of sexual behavior in female rats by ventromedial hypothalamic implants of antiestrogen. Neuroendocrinology. 1987;45:201–207. doi: 10.1159/000124726. [DOI] [PubMed] [Google Scholar]
- 8.Etgen AM, Barfield RJ. Antagonism of female sexual behavior with intracerebral implants of antiprogestin RU38486: correlation with binding to neural progestin receptors. Endocrinology. 1986;119:1610–1617. doi: 10.1210/endo-119-4-1610. [DOI] [PubMed] [Google Scholar]
- 9.Millhouse OE. Certain ventromedial hypothalamic afferents. Brain Res. 1973;55:89–105. doi: 10.1016/0006-8993(73)90490-3. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan-Cato LM. Sex differences in the neural circuits mediating female sexual receptivity. Frontiers in Neuroendocrinology. 2011 doi: 10.1016/j.yfrne.2011.02.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 12.Millhouse OE. The organization of the ventromedial hyothalamic nucleus. Brain Res. 1973;55:71–87. [PubMed] [Google Scholar]
- 13.Calizo LH, Flanagan-Cato LM. Estrogen selectively induces dendritic spines within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin GD, Flanagan-Cato LM. Estradiol and progesterone differentially regulate the dendritic arbor of neurons in the hypothalamic ventromedial nucleus of the female rat (Rattus norvegicus) J Comp Neurol. 2008;510:631–640. doi: 10.1002/cne.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrer HF, Aoki A. Ultrastructural changes in the hypothalamic ventromedial nucleus of ovariectomized rats after estrogen treatment. Brain Research. 1982;240:221–233. doi: 10.1016/0006-8993(82)90218-9. [DOI] [PubMed] [Google Scholar]
- 16.Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: A golgi study in the adult rat. Neuroendocrinology. 1990;51:530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- 17.Madiera MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432:329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- 18.Frankfurt M, McEwen BS. Estrogen increases axodendritic synapses in the VMN of rats after ovariectomy. NeuroReport. 1991;2:380–382. doi: 10.1097/00001756-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Nishizuka M, Pfaff DW. Intrinsic synapses in the ventromedial nucleus of the hypothalamus: An ultrastructural study. J Comp Neurol. 1989;286:260–268. doi: 10.1002/cne.902860210. [DOI] [PubMed] [Google Scholar]
- 20.Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J Comp Neurol. 2002;447:234–248. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- 21.Griffin GD, Ferri-Kolwicz SL, Reyes BAS, VanBockstaele EJ, Flanagan-Cato LM. Ovarian hormone-induced reorganization of oxytocin-labeled dendrites and synapses lateral to the hypothalamic ventromedial nucleus in female rats. J Comp Neurol. 2010;518:4531–4545. doi: 10.1002/cne.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones KJ, McEwen BS, Pfaff DW. Quantitative assessment of the synergistic and independent effects of estradiol and progesterone on ventromedial hypothalamic and preoptic- area proteins in female rat brain. Metab Brain Dis. 1987;2:271–281. doi: 10.1007/BF00999697. [DOI] [PubMed] [Google Scholar]
- 23.Sinchak K, Micevych PE. Progesterone blockade of Estrogen activation of μ-opioid receptors regulates reproductive behavior. Journal of Neuroscience. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mani SK, Allen JMC, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely O, O’Malley BW. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Molec Endocrinol. 1996;10:1728–1737. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- 25.Boling JL, Blandau RJ. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinology. 1939;25:359–364. [Google Scholar]
- 26.Etgen AM. Estrogen regulation of noradrenergic signaling in the hypothalamus. Psychoneuroendocrinology. 1994;19:603–610. doi: 10.1016/0306-4530(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 27.Dudley CA, Moss RL. Facilitation of lordosis in female rats by CNS-site specific infusions of an LH-RH fragment, Ac-LH-RH (5–10) Brain Res. 1988;441:161–167. doi: 10.1016/0006-8993(88)91394-7. [DOI] [PubMed] [Google Scholar]
- 28.Willoughby WO, Blessing WW. Origin of serotonin innervation of the arcuate and ventromedial hypothalamic region. Brain Res. 1987;418:170–173. doi: 10.1016/0006-8993(87)90975-9. [DOI] [PubMed] [Google Scholar]
- 29.Daniels D, Flanagan-Cato LM. Functionally-defined compartments of the lordosis neural circuit in the ventromedial hypothalamus in female rats. J Neurobiol. 2000;45:1–13. [PubMed] [Google Scholar]
- 30.Schumacher M, Coirini H, Frankfurt M, McEwen BS. Localized actions of progesterone in hypothalamus involve oxytocin. Proc Natl Acad Sci USA. 1989;86:6798–6801. doi: 10.1073/pnas.86.17.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacher M, Coirini H, McEwen BS. Regulation of high affinity GABA-A receptors in specific brain regions by ovarian hormones. Neuroendocrinology. 1989;50:315–320. doi: 10.1159/000125239. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy MM, Malik KF, Feder HH. Increased GABAergic transmission in medial hypothalamus facilitates lordosis but has opposite effects in the preoptic area/anterior hypothalamus. Brain Res. 1990;507:40–44. doi: 10.1016/0006-8993(90)90519-h. [DOI] [PubMed] [Google Scholar]
- 33.Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothlamus in the regulation of female rat sexual behaviors. I. Behavioral effects of glutamate and its receptor agonists, AMPA, NMDA and kainate. Pharmacol Biochem Behav. 2006;83:322–332. doi: 10.1016/j.pbb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Booth C, Wayman CP, Jackson VM. An ex vivo multielectrode approach to evaluate endogenous hormones and receptor subtype pharmacology on evoked and spontaneous neural activity with the ventromedial hypothalamus; Translation from female receptivity. J Sexual Medicine. 2010;7:2411–2423. doi: 10.1111/j.1743-6109.2010.01843.x. [DOI] [PubMed] [Google Scholar]
- 35.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 36.Sofroniew MV, Glasmann W. Golgi-like immunoperoxidase staining of hypothalamic magnocellular neurons that contain vasopressin, oxytocin or neurophysin in the rat. Neuroscience. 1981;6:619–643. doi: 10.1016/0306-4522(81)90147-0. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell JD, Jirikowski GF, Greer ER, Stumpf WE, Pedersen CA. Ovarian steroids and sexual interaction alter oxytocinergic content and distribution in the basal forebrain. Brain Research. 1988;446:236–244. doi: 10.1016/0006-8993(88)90882-7. [DOI] [PubMed] [Google Scholar]
- 38.Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta. Progress in Brain Research. 2002;139:15–29. doi: 10.1016/s0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- 39.Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- 40.Sofroniew MV, Schrell U. Evidence for a direct projection from oxytocin and vasopressin neurons in the hypothalamic paraventricular nucleus to the medulla oblongata: Immunohistochemical visualization of both the horseradish peroxidase transported and the peptide produced by the same neurons. Neurosci Lett. 1981;22:211–217. [Google Scholar]
- 41.Swanson LW. Immunohistochemical evidence for a neurophysin-containing autonomic pathway arising in the paraventricular nucleus of the hypothalamus. Brain Res. 1977;128:346–353. doi: 10.1016/0006-8993(77)91000-9. [DOI] [PubMed] [Google Scholar]
- 42.Elands J, Barberis C, Jard S, Tribollet E, Dreifuss JJ, Bankowski K, Manning M, Sawyer WH. 125I-labelled d(CH2)5[Tyr(Me)2, Thr4, Tyr-NH29]OVT: a selective oxytocin receptor ligand. Eur j Pharm. 1987;147:197–207. doi: 10.1016/0014-2999(88)90778-9. [DOI] [PubMed] [Google Scholar]
- 43.Tribollet E, Barbaris C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- 44.De Kloet ER, Voorhuis DAM, Boschma Y, Elands J. Estradiol modulates density of putative ‘oxytocin receptors’ in discrete rat brain regions. Neuroendocrinology. 1986;44:415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- 45.Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats: an autoradiographical study. Brain Res. 1990;551:129–140. doi: 10.1016/0006-8993(90)90232-z. [DOI] [PubMed] [Google Scholar]
- 46.Coirini H, Schumacher M, Flanagan LM, McEwen BS. Transport of estrogen-induced oxytocin receptors in the ventromedial hypothalamus. J Neurosci. 1991;11:3317–3324. doi: 10.1523/JNEUROSCI.11-11-03317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schumacher M, Coirini H, Johnson AE, Flanagan LM, Frankfurt M, Pfaff DW, McEwen BS. The oxytocin receptor: a target for steroid hormones. Regulatory Peptides. 1993;45:115–119. doi: 10.1016/0167-0115(93)90192-b. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig MQJP. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 49.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 50.Hrabovszky E, Csapo AK, Kallo I, Wilheim T, Turi GF, Liposits Z. Localization and osmotic regulation of vesicular glutamate transporter-2 in magnocellular neurons of the rat hypothalamus. Neurochemistry International. 2006;48:753–761. doi: 10.1016/j.neuint.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Ponzio TA, Ni Y, Montana V, Parpura V, Hatton GI. Vesicular glutamate transporter expression in supraoptic neurones suggests a glutamatergic phenotype. Journal of Neuroendocrinology. 2006;18:253–265. doi: 10.1111/j.1365-2826.2006.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- 53.Felipo V, Grau E, Minana MD, Grisolia S. Activation of NMDA receptor mediates the toxicity of ammonia and the effects of ammonia on the microtubule-associated protein MAP-2. Advances in Experimental Medicine & Biology. 1993;341:83–93. doi: 10.1007/978-1-4615-2484-7_8. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Rubel EW. Rapid regulation of microtubule-associated protein 2 in dendrites of nucleus laminaris of the chick following deprivation of afferent activity. Neuroscience. 2008;154:381–389. doi: 10.1016/j.neuroscience.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu B, Goulding E, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor in the ventromedial hypothalamus is an anorexigenic factor regulated by melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarz JM, Liang S-L, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. Journal of Neuroscience. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocrine Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 59.Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum and amygdala: a morphological and biochemical study. Endocrinology. 1997;138:778–789. doi: 10.1210/endo.138.2.4937. [DOI] [PubMed] [Google Scholar]
- 60.Luine VN, Grattan DR, Selmanoff M. Gonadal hormones alter hypothalamic GABA and glutamate levels. Brain Res. 1997;747:165–168. doi: 10.1016/s0006-8993(96)01255-3. [DOI] [PubMed] [Google Scholar]