Abstract
The polysaccharide capsule is the primary virulence factor in Streptococcus pneumoniae. There are at least 90 serotypes of S. pneumoniae, identified based on the immunogenicity of different capsular sugars. The aim of this study was to construct pneumococcal strains that are isogenic except for capsular type. Serotype 4 strain TIGR4 was rendered unencapsulated by recombinational replacement of the capsular polysaccharide synthesis (cps) locus with the bicistronic Janus cassette (C. K. Sung, J. P. Claverys, and D. A. Morrison, Appl. Environ. Microbiol. 67:5190-5196, 2001). In subsequent transformation with chromosomal DNA, the cassette was replaced by the cps locus derived from a strain of a different serotype, either 6B, 7F, 14, or 19F. To minimize the risk of uncontrolled recombinational replacements in loci other than cps, the TIGRcps::Janus strain was “backcross” transformed three times with chromosomal DNA of subsequently constructed capsular type transformants. Capsular serotypes were confirmed in all new capsule variants by the Quellung reaction. Restriction fragment length polymorphism (RFLP) analysis of the cps locus confirmed the integrity of the cps region transformed into the TIGR strain, and RFLP of the flanking regions confirmed their identities with the corresponding regions of the recipient. Transformants had in vitro growth rates greater than or equal to that of TIGR4. All four strains were able to colonize C57BL/6 mice (female, 6 weeks old) for at least 7 days when mice were intranasally inoculated with 6 × 106 to 8 × 106 CFU. The constructed capsular variants of TIGR4 are suitable for use in studies on the role of S. pneumoniae capsular polysaccharide in immunity, colonization, and pathogenesis.
A number of defined and putative virulence factors in Streptococcus pneumoniae have been described. Of these, the polysaccharide capsule, which protects pneumococcal cells against phagocytosis during infection, was the first to be identified and is the best studied. There are at least 90 different capsular types in S. pneumoniae, classified according to polysaccharide structure and immunogenicity (13), with considerable variation across serotypes in the tendency to colonize the human nasopharynx and to cause invasive disease (3, 12). Among the internationally distributed clones of S. pneumoniae, there are several for which variants with different capsular serotypes are known (21). It is believed that these variants arose through “capsular switching,” mediated by recombinational replacements within the capsular biosynthesis (cps) operon and its flanking regions (4-6), following natural transformation by DNA from other strains. The survival of such capsular variants is likely driven by the selective pressure within S. pneumoniae's natural environment, the human upper respiratory tract.
In order to perform studies on the role of capsule in immunity, colonization, and pathogenesis of S. pneumoniae, we constructed strains that were isogenic except for the capsular type. A laboratory procedure for capsule replacement has been known since Griffith's landmark 1928 paper on transformation (11). However, as Griffith's procedure involves selection of capsular transformants in a mouse, there is a potential for introducing additional, uncontrolled changes in the strains selected. More recent attempts to obtain capsular transformants in vitro have involved replacements of a part of the cps operon, and the type and number of possible constructs are limited to those of high homology between donor and recipient cps loci (22). The main limitations for controlled in vitro capsule transformation in S. pneumoniae are the large size of the DNA that had to be replaced and the lack of a suitable selective marker to identify successful transformants. The exception was serotype 3, distinguished by perhaps the smallest of all cps operons and by a colonial morphology distinctive enough to be used alone as a selective marker (1, 6, 15).
In an attempt to circumvent these limitations, we made use of the bicistronic, positively and negatively selectable Janus cassette recently constructed for S. pneumoniae by Sung et al. (27). We used Janus to replace the cps operon in encapsulated S. pneumoniae, leading to the unencapsulated, rough phenotype. In subsequent transformations, Janus was replaced by either the cps6B, cps7F, cps14, or cps19F capsule operon, leading to the construction of new encapsulated strains. The S. pneumoniae cps operon is a region of great variability, but it is flanked, in all pneumococcal isolates analyzed so far, by regions of high homology (9, 15). We selected these regions as targets for recombinational crossover in a controlled capsule transformation. The virulent TIGR4 S. pneumoniae strain was chosen as the host for the capsule replacements, since its complete genome sequence has been published (29) and susceptibility to a wide array of antimicrobial agents makes construction of strains with selectable resistant phenotypes relatively easy.
MATERIALS AND METHODS
Bacterial strains, DNA, and growth conditions.
S. pneumoniae strains used in this study are described in Table 1. Serotype 19F strain GA71 was kindly donated by Brian Spratt (6). Strains NY00216, GA03294, and GA03306 (all serotype 6B), GA02224 (serotype 7F), and GA02190 (serotype 14) were all clinical isolates collected by the Centers for Disease Control and Prevention's Active Bacterial Core Surveillance and kindly provided by Richard Facklam and Chris van Beneden (26). R6 (14) and TIGR4 (29) were obtained from the American Type Culture Collection. The genomic DNA of CP1296 was kindly donated by Don Morrison (27). All strains were maintained in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) or on blood agar base no. 2 medium (Becton Dickinson, Sparks, Md.) supplemented with 5% defibrinated sheep blood (Colorado Serum Company, Denver, Colo.) (SBA). Antibiotic concentrations in SBA selective media were 0.5 mg/liter for cefotaxime, 2.5 mg/liter for gentamicin, and 200 mg/liter for kanamycin and streptomycin, except for the SBA used to select streptomycin-resistant (Smr) mutants, which contained 300 mg of streptomycin per liter. DNA templates were prepared with a genomic DNA system (Qiagen, Valencia, Calif.). Growth media and culture methods for genetic transformations have been described by Pozzi et al. (23). The transformation of TIGR4 and its derivatives was induced by competence-stimulating peptide variant 2, while variant 1 was used for strains R6 and GA03306 and their derivatives. The concentrations of donor DNA used in transformation steps were 1 μg/ml for chromosomal DNA and 100 ng/ml for PCR products.
TABLE 1.
Bacterial strains and primers used in this study
| Strain or primer | Description | Source or reference |
|---|---|---|
| S. pneumoniae strains | ||
| R6 | 14 | |
| CP1296 | R6 derivative, cbp3::kan-rpsL+; Kmr Sms | 27 |
| R6S | R6 but Smr by selection of mutants | This study |
| R6J | R6S but cps::kan-rpsL+ | This study |
| TIGR4 | 29 | |
| TIGR4S | TIGR4 but Smr by selection of mutants | This study |
| TIGR4J | TIGR4S but cps::kan-rpsL+, by transformation with TTM01-04 PCR product from R6J; Kmr Sms | This study |
| NY00216 | Donor of type 6B capsule | 26 |
| GA02224 | Donor of type 7F capsule | 26 |
| GA02190 | Donor of type 14 capsule | 26 |
| GA71 | Donor of type 19F capsule | 6 |
| TIGR4:6B1 | TIGR4J derivative kan-rpsL+::cps6B, by transformation with NY00216 chromosomal DNA; Kms Smr | This study |
| TIGR4:6B2 | TIGR4J derivative kan-rpsL+::cps6B, by transformation with TIGR4:6B1 chromosomal DNA; Kms Smr; 1× backcross transformant | This study |
| TIGR4:6B3 | TIGR4J derivative kan-rpsL+::cps6B, by transformation with TIGR4:6B2 chromosomal DNA; Kms Smr, 2× backcross transformant | This study |
| TIGR4:6B4 | TIGR4J derivative kan-rpsL+::cps6B, by transformation with TIGR4:6B3 chromosomal DNA; Kms Smr; 3× backcross transformant | This study |
| TIGR4:7F4 | TIGR4J derivative kan-rpsL+::cps7F, serotype 7F 3× backcross capsule transformant of TIGR4 constructed as TIGR4:6B4; Kms Smr | This study |
| TIGR4:144 | TIGR4J derivative kan-rpsL+::cps14, serotype 14 3× backcross capsule transformant of TIGR4 constructed as TIGR4:6B4; Kms Smr | This study |
| TIGR4:19F4 | TIGR4J derivative kan-rpsL+::cps19F, serotype 19F 3× backcross capsule transformant of TIGR4 constructed as TIGR4:6B4; Kms Smr | This study |
| GA03294 | 6B serotype penicillin-resistant isolate, donor of pbp2x for 607S transformation | 26 |
| GA03306 | 6B serotype cefotaxime-susceptible isolate | 26 |
| 607S | GA03306 but Smr by selection of mutants | This study |
| 621 | 607S but cefotaxime resistant by transformation with pbp2xf-pbp2xr2 PCR product of GA03306 | This study |
| 622 | 621 but Sms Kmr by transformation with DAM313-DAM316 PCR product of CP1296 | This study |
| Primers | ||
| DAM406 | TCTATGCCTATTCCAGAGGAAATGGAT | D. A. Morrison |
| DAM351 | CTAGGGCCCCTTTCCTTATGCTTTTGGAC | 27 |
| DAM313 | AGCTTTCTCGTGGTGTAGAACAAC | 27 |
| DAM316 | CTCTCAAGGTCGCCCAGCTATG | 27 |
| TTM01 | ATCATGACCTCCCTCGTATTGT within dexB in R6; 932-953 | This study |
| TTM02 | CGCGGATCCTTAATAGTGGGAATTTG; positions 7-26 correspond to positions 131-150 downstream of the dexB 3′ end in R6 | This study |
| TTM03 | TTTGGGCCCGCCGGCGTCAGTCAGTTTT; positions 10-28 correspond to | This study |
| positions 618-600 upstream of aliA 5′ end in R6 | ||
| TTM04 | AGCTTTGACTGCCGCGTATTCTTC; within aliA in R6; 331-354 | This study |
| TTM05 | AAGGTGAGGAGATTGGGATGA; within dexB in R6; 1049-1069 | This study |
| TTM06 | TGTCGCAGCCTTAGCAGTTG; within aliA in R6; 155-174 | This study |
| 1430 | TGTCCAATGAAGAGCAAGACTTGACAGTAG | 15 |
| TTM07 | CTACTGTCAAGTCTTGCTCTTCATTGGACA; sequence reverse to 1430 | This study |
| 1402 | CAATAATGTCACGCCCGCAAGGGCAAGT | 15 |
| TTM08 | ACTTGCCCTTGCGGGCGTGACATTATTG; sequence reverse to 1402 | This study |
| TTM09 | CTAAAACAGGGGAAATTCTGGCAACAACGC; within pbp2x in TIGR4; 887-916 | This study |
| TTM10 | AATCGCGAAACGTCCCAGCCGTGGAAACTC; within pbp1a in TIGR4; 1280-1309 | This study |
| pbp2xf | CGTGGGACTATTTATGACCGAAATGGAG | 10 |
| pbp2xr2 | GGCGAATTCCAGCACTGATGGAAATAA | 10 |
Serotyping of isolates.
Polysaccharide capsule types were determined based on the Quellung test with factor-specific typing sera (Statens Serum Institute, Copenhagen, Denmark) (13, 20).
PCR amplification.
All products with sizes below 5 kb were amplified using 50 ng of genomic DNA in a final volume of 50 μl of 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 5 mM MgCl2, and 0.25 mM deoxynucleoside triphosphates with 0.3 pmol of each primer, 4.5 U of Taq DNA polymerase (Invitrogen, Chicago, Ill.), and 0.5 U of cloned Pfu DNA polymerase (Stratagene, La Jolla, Calif.). The PCR consisted of 30 cycles of 30 s at 94°C, 30 s at 60°C, and 1 min per kb of estimated product length at 72°C, followed by a final incubation at 72°C for 5 min. All products with sizes of 8.6 kb and above were amplified using a TripleMaster PCR system (Eppendorf, Hamburg, Germany) using cycling parameters recommended by the manufacturer.
Construction of the dexB-aphIII-rpsL+-aliA cassette.
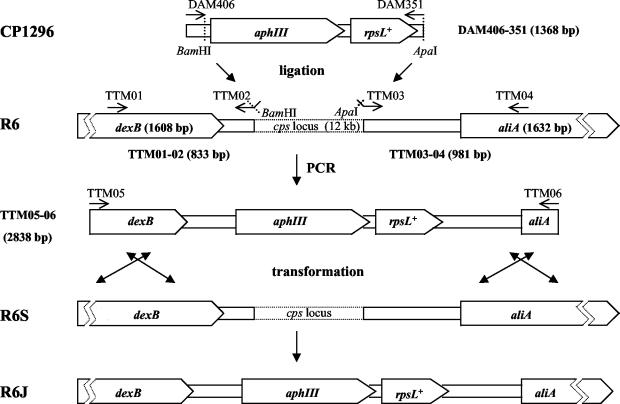
To construct a Janus-type cassette with kanamycin resistance and streptomycin sensitivity alleles embedded within DNA sequences corresponding to the genes that flank the cps locus, a 1,368-bp fragment containing aphIII-rpsL+ was first amplified from chromosomal DNA of CP1296 using the primer pair DAM 406 and DAM 351. Two DNA fragments, TTM01-02 (833 bp), which contains a BamHI 3′ terminus, and TTM03-04 (981 bp), which contains an ApaI 5′ terminus (both flanking the cps cassette), were prepared by PCR using chromosomal DNA of R6 as a template (Fig. 1). The aphIII-rpsL+ and TTM01-02 PCR products were digested with BamHI restriction nuclease, ligated by using T4 DNA ligase (New England BioLabs, Beverly, Mass.), and purified with a QIAquick PCR purification kit (Qiagen). The product of the ligation and the TTM03-04 fragment were digested with ApaI, ligated, and used as the template for a PCR with primers TTM05 and TTM06. Products of the PCR were run on a 1% agarose gel, and the fragment of 2,838 bp, corresponding to the expected size of kan-rpsL+ cassette with TTM01-02 and TTM03-04 flanking regions, was purified by using a gel extraction kit (Qiagen) and used to transform R6S with selection for resistance to kanamycin (Kmr) to create R6J. The structure of the insertion in R6J was verified by restriction fragment length polymorphism (RFLP) analysis of the TTM01-04 PCR product using BamHI and ApaI (25).
FIG. 1.
Construction of the Janus cassette in the R6 S. pneumoniae cps locus. Pentagons represent dexB and aliA genes of R6 and aphIII (Kmr) and rpsL+ (Sms) genes of Janus (size of the open reading frame is in parentheses). Oligonucleotides used to amplify cassette elements are in capital letters (see Table 1 for detailed description of the primers and annealing sites). Restriction sites of enzymes used to create sticky ends prior to the ligation step are shown by dotted lines. The two top lines show elements of CP1296 and R6 used to construct the dexB-Janus-aliA cassette by the two-step ligation procedure. The ligation product was used as a template to amplify the TTM05-06 fragment (third line) used to transform R6S (fourth line) to create R6J (fifth line) with selection for resistance to kanamycin. Sizes of the PCR products generated in this work are given in parentheses next to the product names.
Construction of cps transformants.
A 3,195-bp PCR product containing the kan-rpsL+ cassette amplified from chromosomal DNA of R6J with primer pair TTM01 and TTM04was used to transform TIGR4S (encapsulated, type 4) with selection for Kmr to make the unencapsulated (rough) TIGR4J strain, which was used as a recipient in all subsequent capsule transformation steps. DNA template from each of the following strains was used to transform TIGR4J with selection for Smr: type 6B strain NY00216, type 7F strain GA02224, type 14 strain GA02190 and type 19F strain GA71 (all listed in Table 1) to create encapsulated strains. For each transformation, up to five smooth colonies were picked from overnight growth on streptomycin-supplemented SBA and cultured separately. The effect of each capsule on the TIGR strain growth rate was evaluated by measuring optical density of growing broth cultures as described below. Once an isolate was identified with the appropriate capsule type and with no reduction in growth rate compared to that of TIGR4S, DNA was purified from that isolate and used to retransform TIGR4J into an encapsulated strain again (“backcross” transformation). The procedure was repeated three times to construct triple-backcross (3× backcross) transformants (16, 24). The structure of the cps locus and surrounding regions was confirmed in all four 3× backcross transformants by RFLP analysis of the following PCR products: 1430-1402 (dexB-cps-aliA locus; size in the TIGR4 strain, 22,332 bp) digested with RsaI, TTM07-09 (pbp2x-dexB flanking cps upstream region, 9,076 bp), and TTM08-10(aliA-pbp1a flanking cps downstream region, 8,618 bp), both digested with Tsp509I. Restriction patterns of the 3× backcross transformants were compared with those of TIGR4 and capsule donor strains after electrophoresis in 2% OmniPur PCR plus agarose (Merck, Darmstadt, Germany).
Effect of capsule transformation on the growth rate.
The cost of capsule replacement was evaluated by comparison of capsule transformants and TIGR4S strain growth curves. Cells harvested from overnight cultures on SBA were suspended in THY to an optical density (OD) at 600 nm of 0.1, diluted 50 times, and incubated at 37°C. The OD of broth cultures was measured every 30 min until early stationary phase was reached. For 3× backcross transformants, measurements were taken for three independent cultures and compared with those for TIGR4S. Based on OD values observed during the exponential phase of growth, the slopes were calculated as an increase in the natural logarithm per hour. The null hypothesis that the slopes of intertype transformants were identical to that of TIGR4S was tested by linear regression analysis, and t tests were performed for pairwise comparisons of each new capsule variant against TIGR4S using Prism 3.0 software (GraphPad Software, San Diego, Calif.). A P value of less than 0.05 was considered significant.
Assessment of the ratio of cotransformation.
To evaluate the probability that a single transformant takes up two unlinked marker loci, we transformed TIGR4S with chromosomal DNA from a laboratory-constructed strain, 622, carrying the original Janus cassette of Sung et al. (27) inserted into cbp3, along with a cefotaxime-resistant (Ctxr) allele of the gene encoding penicillin binding protein 2x, allowing selection for unlinked markers encoding Ctxr and Kmr. After transformation, the numbers of TIGR4 colonies resistant to cefotaxime, to kanamycin, and to both antibiotics simultaneously were counted and compared.
Mouse colonization.
To evaluate the ability of newly constructed strains to colonize mice, four groups of four C57BL/6 experimental mice (female, 6 weeks old; Jackson Laboratory) were inoculated intranasally (19, 30) with 6 × 106 to 8 × 106 CFU of the 3× backcross strains, TIGR4 and TIGR4J. After 7 days, nasal washes were carried out to determine the presence and the intensity (as defined by CFU per nasal wash) of pneumococcal colonization in the animals' upper respiratory tracts.
RESULTS
Isolation of encapsulated transformants.
In the course of the study, all successful capsule transformants were selected based on the smooth morphology of colonies that were selected for resistance to streptomycin but susceptibility to kanamycin. The frequency of Janus replacement by an intact (functional) cps locus was calculated only for serotype 19F transformants. Colonies of serotype 19F cells (true for both GA71 and TIGR4:19F4) have very distinctive smooth morphology and were relatively easy to identify among rough colonies of unencapsulated cells. The frequency of cps19F locus transformation was estimated to be 5.4 × 10−6 based on the number of smooth Smr Kms colonies growing out of TIGR4J culture transformed with chromosomal DNA of GA71. The genuine capsule transformants constituted 6.3% of all Smr colonies isolated following transformation. The remainder may have been some combination of recombinants that did not result in transformation at the capsule locus, resistant mutants, or spontaneous revertants (as observed previously with the use of Janus [27]).
The presence of capsule was confirmed in intertype transformants of the TIGR4 strain by the Quellung reaction, and the capsule type was congruent with that expected from the donor DNA. Only the original parent strain TIGR4S was positive for serotype 4. Results with backcrosses were similar to those obtained in primary transformations (Table 2).
TABLE 2.
Capsule type identification results for selected strains described in this study
| Strain | Result of Quellung test for serotypea:
|
||||
|---|---|---|---|---|---|
| 4 | 6B | 7F | 14 | 19F | |
| TIGR4 | Pos | Neg | Neg | Neg | Neg |
| TIGR4J | Neg | Neg | Neg | Neg | Neg |
| NY00216 | Neg | Pos | — | — | — |
| GA02224 | Neg | — | Pos | — | — |
| GA02190 | Neg | — | — | Pos | — |
| GA71 | Neg | — | — | — | Pos |
| TIGR4:6B4 | Neg | Pos | — | — | — |
| TIGR4:7F4 | Neg | — | Pos | — | — |
| TIGR4:144 | Neg | — | — | Pos | — |
| TIGR4:19F4 | Neg | — | — | — | Pos |
Pos, positive identification of particular serotype; Neg, negative result of the Quellung test; —, not determined.
Confirmation of intact capsule locus transformation.
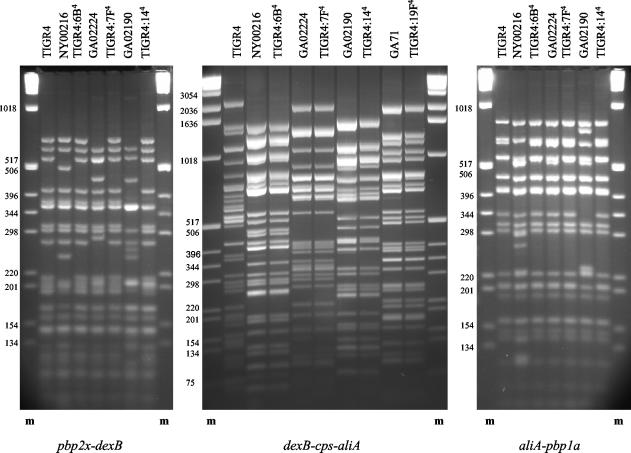
When RsaI restriction fragment patterns of the dexB-cps-aliA (1402-1430 PCR product) were compared among capsule donors, TIGR4S, and the 3× backcross transformants, the transformants in all cases matched the donor and not the recipient fingerprints (Fig. 2, center). The largest 1430-1402 PCR product was observed for TIGR4 (22.3 kb according to the published genome sequence [29]), followed by 21.5-kb products for TIGR4:7F4 and the cps7F donor and 18-, 16.8-, and 16.6-kb products for serotype 14, 19F, and 6B transformants and capsule donors, respectively. RsaI generated similar number of fragments for all five fingerprints (49 bands with sizes from 6 to 2,197 bp in TIGR4 [29] with no single band common to all five RFLP patterns [Fig. 2]).
FIG. 2.
RFLP patterns of the cps locus and its flanking regions in TIGR4, capsule transformants, and capsule donors. Lanes correspond to the Tsp509I fingerprints of pbp2x-dexB and pbp1a-aliA and the RsaI fingerprint of dexB-cps-aliA loci of indicated isolates. The order of fingerprints is repeated in all three modules of the picture with the exception of serotype 19F isolates GA71 and TIGR4:19F4, for which fingerprints of the dexB-cps-aliA locus are the only ones presented. RFLP patterns of the cps locus of isolates of the same serotype were identical for all four sets of strains (capsule donor and transformant), and the same was observed for flanking regions of the host strain (TIGR4) and all four capsule transformants. The exception was the TIGR4:144 fingerprint of the pbp2x-dexB fragment, for which a pattern different from that for TIGR4 was observed (see Results). Lanes m, 1-kb DNA ladder molecular size marker (sizes of fragments, in base pairs, are on the left of each gel).
Confirmation that flanking regions of transformants matched the recipient strains.
RFLP analysis of regions flanking the cps locus also revealed polymorphisms among analyzed strains; however, Tsp509I fingerprints of the pbp2x-dexB and aliA-pbp1a loci (TTM09-07 and TTM08-10 PCR products, respectively) showed homology between donors and recipients not observed for the dexB-cps-aliA locus. The size of the TTM09-07 PCR product in TIGR4 was ∼9 kb, as expected, and similar fragments were generated for NY00216 and all four 3× backcross capsule transformants. Products for GA02190, GA02224, and GA71 had sizes of 9.4, 9.7, and 2.8 kb, respectively. The Tsp509I patterns of TTM09-07 products generated for TIGR4 and all 3× backcross transformants except serotype 14 were identical. The RFLP fingerprints indicate that the capsule replacement crossover point in TIGR4:144 was located between enzyme restriction sites in TIGR4 at positions 700 bp upstream and 140 bp downstream of the dexB 5′ end. Analysis of the same region in four consecutive serotype 14 transformants of TIGR4 revealed an identical fingerprint for all of them. It indicated that the evidence of the crossover point of the first capsule replacement was retained through all three backcross transformation events. All consecutive crossover points were located at or upstream of the site of the first one. RFLP analysis of the smallest of the TTM09-07 PCR products, the 2.8-kb fragment amplified for GA71, revealed its homology with the 3′end of the TTM09-07 PCR product of TIGR4.
High homology was also observed among fingerprints generated for the second flanking region, the aliA-pbp1a locus. The size of the TTM08-10 PCR product in TIGR4 was ∼8.6 kb. Similarly sized fragments were generated for all other isolates except NY00216, for which an ∼10-kb product was amplified, and GA71, which yielded no product. The TTM08-10 PCR product fingerprints for all 3× backcross transformants were identical to that of TIGR4 and different from those of all capsule donor strains.
Calculation of backcross frequencies needed to ensure isogenic backgrounds.
Since chromosomal DNA was used to construct the original transformants, there was a risk that capsular transformants had acquired other, unlinked genetic material from the donor strains. To calculate the probability that a transformant with a change at a selected locus (the cps region) also picked up a gene at an unlinked region, we transformed our recipient, TIGR4S, with chromosomal DNA from a strain carrying two unlinked markers, Kmr and Ctxr. The pbp2x gene is located 345 kb downstream of the cbp3 gene in TIGR4 genome (approximately one-sixth of the genome away) (29). Under identical transformation conditions, with approximately 107 CFU of the recipient cells, we obtained 77 transformants with Ctxr and 303 transformants that were Kmr. With five times as many recipient cells, we obtained zero transformants that were simultaneously Kmr and Ctxr. This provided an approximate 95% confidence interval for the ratio of single to double transformants of 0 to 0.008. We reasoned that among our capsular transformants, the probability that any given locus was cotransformed was therefore less than 1/100, and that if each backcross independently reduced the probability of cotransformation by a factor of at least 100, then the 3× backcross transformants should have a probability of less than 10−8 of carrying any given gene from the donors apart from the capsule region.
Effect of capsule transformation on the growth rate.
When the growth rates for TIGR4S and 3× backcross capsule transformants were compared, no significant differences were observed between TIGR4S and the serotype 7F and 19F transformants. However, growth rates were significantly higher for serotype 6B and 14 transformants of the TIGR strain (P values of 0.002 and 0.04, respectively), indicating that capsular transformants actually grew faster than the recipient strain (Table 3).
TABLE 3.
Results of linear regression analysis of TIGR4S and its intertype transformants' growth rate slopes
| Strain | Slope (ΔN/Δt)a | 95% confidence interval | P value versus TIGR4S |
|---|---|---|---|
| TIGR4S | 0.56 | 0.51-0.61 | |
| TIGR4:6B4 | 0.69 | 0.65-0.72 | 0.002 |
| TIGR4:7F4 | 0.53 | 0.47-0.6 | 0.45 |
| TIGR4:144 | 0.63 | 0.59-0.67 | 0.04 |
| TIGR4:19F4 | 0.57 | 0.48-0.67 | 0.83 |
ΔN/Δt, exponential growth rate slope value calculated as change in lnOD at 600 nm per hour, where N is the OD and t is the time.
Ability of TIGR4 capsule transformants to colonize mice.
The presence of TIGR4 capsular transformants was demonstrated 7 days after mouse inoculation in nasal washes of all four animals colonized with serotype 7F or 14 (medians, 3,130 and 807 CFU/nasal wash, respectively) and in three of four mice colonized with serotype 6B or 19F strains (medians, 1,146 and 966 CFU/nasal wash, respectively). None of the four mice was colonized with TIGR4, and only one was colonized with TIGR4J.
DISCUSSION
Transformational recombination is a known mechanism by which S. pneumoniae obtains genetic variation that permits adaptation to changes in the environment. Natural transformation of the capsule locus leading to changes in polysaccharide structure and immunogenicity is a phenomenon that has been observed in pneumococci (5) and in other pathogens colonizing the human respiratory tract, i.e.,Neisseria meningitidis and Haemophilus influenzae (17, 28). Intertype transformation allows these microorganisms to avoid opsonization and neutralization by antibodies against serotypes to which the host was previously exposed. Uncovering the mechanisms that drive capsule switching is crucial for a better understanding of the interactions between the host and the pathogen and among different strains of pathogens competing for the host (18). Such interactions are difficult to trace in the naturally diverse population of S. pneumoniae due to the complexity of antigenic patterns observed. Isolates classified as closely related (members of the same epidemic clone) can be very different in their antigenic profiles (2, 4-6) and in their abilities to colonize (K. Trzcinski, C. M. Thompson, R. Malley, and M. Lipsitch, Abstr. 3rd Int. Symp. Pneumococci Pneumococcal Dis., p. 71, 2002) and probably to cause invasive disease.
For this reason, the availability of laboratory-constructed strains that are identical except for one feature (a single antigenic component) will be critical for testing these interactions in artificial or natural host models (16, 24). In the case of transformation into unrelated capsular types, such isolates can be constructed only by recombinational replacements of large fragments of chromosomal DNA, some over 20 kb in length, and crossover sites should be located within regions of high homology among pneumococcal strains. Suitable targets for recombinational crossovers seem to be cpsA, cpsB, cpsC, and cpsD, which are present at the 5′ end of cps operons, cpsN and cpsO, which are present at the 3′ end in most serotypes, regions of homology within intergenic regions surrounding the cps locus, and dexB and aliA genes, which flank the cps operon in all pneumococcal strains analyzed (15). To construct the dexB-Janus-aliA cassette suitable for capsule transformation, we chose the most external regions to eliminate any possible interactions between the cps operon of the recipient and newly introduced capsular genes. RFLP analysis of the fragments flanking the dexB-cps-aliA region in intertype transformants indicates that recombinational crossover points were located downstream of pbp2x and upstream of pbp1a but outside the cps operon in all of them.
Prior to this study, the construction of isogenic strains with variant polysaccharide capsule types was limited to intertype transformants of highly homologous cps regions (e.g., serogroup 19 variants) in which replacement of a smaller cps operon fragment was sufficient for serotype change (22). The exceptions were type 3 transformants, in which the presence of a relatively small operon of three genes determines the expression of that particular capsular type in any genetic background. However, such encapsulated-to-encapsulated transformations can result in isolates expressing both capsule donor and recipient serotypes (binary types) (7, 8). Our strategy for the replacement of the entire operon overcomes these limitations.
A major obstacle in capsule transformation was the lack of selective markers to allow the identification of extremely low-frequency transformants. We solved this problem by using the Janus cassette (27). Using this cassette, we constructed four different capsular variants of the TIGR4 strain. As has been documented for serotype 3 and 37 S. pneumoniae, genes located outside the cps locus can determine the expression of particular capsular types in some strains (9). Quellung test results confirmed the donor serotype in all constructed transformants, indicating that the generated replacements included all genes necessary for functional polysaccharide capsule biosynthesis in a TIGR4 background.
Natural capsule transformation through recombinational replacements of large fragments of chromosomal DNA in S. pneumoniae (covering not only the whole cps operon but also large fragments of its flanking regions) has been described by Coffey et al. in the studies on Spain23F-1 and Spain9V-3 pandemic clone capsular variants (4, 6). Our results show that similar transformation through recombinational replacement of DNA fragments of 20 kb and above (∼1% of the whole bacterial genome) can also be successfully conducted in vitro.
There were no detectable costs of capsule transformation in TIGR4S in terms of growth in vitro. Growth rates of serotype 7F and 19F transformants were not significantly different from that of TIGR4, whereas enhanced rates were observed for serotype 6B and 14 transformants. Since the cps6B and cps14 loci of the capsule donors used in this study were smaller than cps4 of TIGR4 and there was a significant increase in growth rate for these two transformants, one can speculate that the size of the cps operon might reflect lesser complexity and energetic cost of capsular polysaccharide biosynthesis in TIGR4 serotypes 6B and 14 than in serotype 4.
All four newly constructed capsule variants were able to colonize the upper respiratory tracts of laboratory mice, proving the strains to be suitable for use in a mouse colonization model. It is not surprising that the rough strain TIGR4J was not able to colonize, as there have been no reports to our knowledge of successful colonization of mice with rough strains and rough strain carriage is rare in humans. Previous experiments in two laboratories (19, 30; M. Lipsitch, unpublished data) have shown that different strains of S. pneumoniae differ widely in their ability to colonize mice at a standard dose, so the fact that the original TIGR4 strain did not colonize, while noteworthy, is consistent with prior experience.
The 3× backcross transformation steps ensured a very low likelihood of recombinational replacements involving exogenous (non-TIGR4) DNA taking place at any other locus than dexB-cps-aliA. Thus, the risk of changes in genotype and phenotype due to unwanted transformations was minimized. Overall, the unrelated and non-cross-reactive capsular variants of the virulent TIGR4 isolate described in this paper seem to be suitable for further studies on the role of S. pneumoniae capsule polysaccharide in immunity, colonization, and pathogenesis. The strategy described in this paper is in principle suitable for construction of any desired capsular variant of any S. pneumoniae strain.
Acknowledgments
We thank Brian G. Spratt, Richard Facklam, and Chris van Beneden for strains, Donald A. Morrison for the Janus cassette and technical advice, and Chris G. Dowson, Dorothy Fallows, and Noman Siddiqi for critical discussion of the results.
This work was supported by NIH grant 1R01AI48935 and by a New Investigator Award to M.L. from the Ellison Medical Foundation.
REFERENCES
- 1.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beall, B., G. Gherardi, R. R. Facklam, and S. K. Hollingshead. 2000. Pneumococcal pspA sequence types of prevalent multiresistant pneumococcal strains in the United States and of internationally disseminated clones. J. Clin. Microbiol. 38:3663-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 4.Coffey, T. J., M. Daniels, M. C. Enright, and B. G. Spratt. 1999. Serotype 14 variants of the Spanish penicillin-resistant serotype 9V clone of Streptococcus pneumoniae arose by large recombinational replacements of the cps-pbp1A region. Microbiology 145:2023-2031. [DOI] [PubMed] [Google Scholar]
- 5.Coffey, T. J., C. G. Dowson, M. Daniels, J. Zhou, C. Martin, B. G. Spratt, and J. M. Musser. 1991. Horizontal transfer of multiple penicillin-binding protein genes, and capsular biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol. Microbiol. 5:2255-2260. [DOI] [PubMed] [Google Scholar]
- 6.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype exchanges among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 7.Dillard, J. P., M. W. Wandersea, and J. Yother. 1995. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J. Exp. Med. 181:973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia, E., C. Arrecubieta, R. Munoz, M. Mollerach, and R. Lopez. 1997. A functional analysis of the Streptococcus pneumoniae genes involved in the synthesis of type 1 and type 3 capsular polysaccharides. Microb. Drug Resist. 3:73-88. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, E., D. Llull, R. Munoz, M. Mollerach, and R. Lopez. 2000. Current trends in capsular polysaccharide biosynthesis of Streptococcus pneumoniae. Res. Microbiol. 151:429-435. [DOI] [PubMed] [Google Scholar]
- 10.Gherardi, G., C. G. Whitney, R. R. Facklam, and B. Bell. 2000. Major related sets of antibiotic-resistant pneumococci in the United States as determined by pulsed-field gel electrophoresis and pbp1a-pbp2b-pbp2x-dhf restriction profiles. J. Infect. Dis. 181:216-229. [DOI] [PubMed] [Google Scholar]
- 11.Griffith, F. 1928. The significance of pneumococcal types. J. Hyg. 27:8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausdorff, W. P., J. Bryant, C. Kloek, P. R. Paradiso, and G. R. Siber. 2000. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin. Infect. Dis. 30:122-140. [DOI] [PubMed] [Google Scholar]
- 13.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, S.-M., L. Wang, and P. R. Reeves. 2001. Molecular characterization of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysaccharide gene cluster. Infect. Immun. 69:1244-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, T., J. P. Dillard, and J. Yother. 1994. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect. Immun. 62:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroll, J. S., and E. R. Moxon. 1990. Capsulation in distantly related strains of Haemophilus influenzae type b: genetic drift and gene transfer at the capsulation locus. J. Bacteriol. 172:1374-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsitch, M. 1999. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg. Infect. Dis. 5:336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipsitch, M., J. K. Dykes, S. E. Johnson, E. W. Ades, J. King, D. E. Briles, and G. M. Carlone. 2000. Competition among Streptococcus pneumoniae for intranasal colonization in a mouse model. Vaccine 15:2895-2901. [DOI] [PubMed] [Google Scholar]
- 20.Lund, E., and J. Henrichsen. 1978. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae. Methods Microbiol. 12:241-262. [Google Scholar]
- 21.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morona, J. K., R. Morona, and J. C. Paton. 1999. Comparative genetics of capsular polysaccharide biosynthesis in Streptococcus pneumoniae types belonging to serogroup 19. J. Bacteriol. 181:5355-5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozzi, G., L. Masala, F. Iannelli, R. Mangenelli, L. S. Håvarstein, L. Piccoli, D. Simon, and D. A. Morrison. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren, B., A. J. Szalai, O. Thomas, S. K. Hollingshead, and D. E. Briles. 2003. Both family 1 and 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 71:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russell. 2001. Molecular cloning, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Schuchat, A., T. Hilger, E. Zell, M. M. Farley, A. Reingold, L. Harrison, L. Lefkowitz, R. Danila, K. Stefonek, N. Barrett, D. Morse, and R. Pinner. 2001. Active bacterial core surveillance of the emerging infections program network. Emerg. Infect. Dis. 7:92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swartley, J. S., A. A. Marfin, S. Edupuganti, L. J. Liu, P. Cieslak, B. Perkins, J. D. Wenger, and D. S. Stephens. 1997. Capsule switching of Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 94:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 30.Wu, H. Y., A. Virolainen, B. Mathews, J. King, M. W. Russell, and D. E. Briles. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb. Pathog. 23:127-137. [DOI] [PubMed] [Google Scholar]