Abstract
In the title compound, C30H52O5, the three six-membered rings are in chair conformations, the five-membered ring is in an envelope form and the tetrahydrofuran ring has a conformation intermediate between half-chair and sofa. Intramolecular O—H⋯O hydrogen bonds may influence the conformation of the molecule. In the crystal, molecules are linked by intermolecular O—H⋯O hydrogen bonds, forming a three-dimensional network.
Related literature
The title compound was prepared from 20(S)-protopanaxatriol which was degraded from Panax quinquefolium saponin. For background to and the medicinal properties of Panax ginseng and Panax quinquefolium, see: Shibata et al. (1985 ▶); Takano et al. (1999 ▶); Yu et al. (2007 ▶); Wang et al. (2010 ▶). For related structures, see: Shi et al. (1992 ▶); Meng et al. (2010 ▶).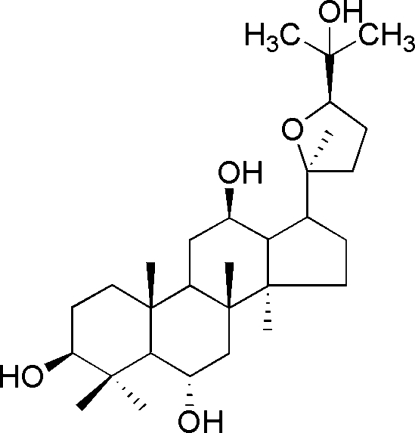
Experimental
Crystal data
C30H52O5
M r = 492.72
Orthorhombic,
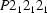
a = 12.7918 (6) Å
b = 13.7842 (7) Å
c = 16.0902 (8) Å
V = 2837.1 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 298 K
0.54 × 0.50 × 0.50 mm
Data collection
Bruker SMART CCD diffractometer
16320 measured reflections
3141 independent reflections
2911 reflections with I > 2σ(I)
R int = 0.020
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.113
S = 1.05
3141 reflections
325 parameters
6 restraints
H-atom parameters constrained
Δρmax = 0.26 e Å−3
Δρmin = −0.24 e Å−3
Data collection: SMART (Bruker, 1997 ▶); cell refinement: SAINT (Bruker, 1997 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811008609/lh5198sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811008609/lh5198Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1A⋯O2 | 0.82 | 2.41 | 2.882 (3) | 117 |
| O2—H2⋯O5 | 0.82 | 1.95 | 2.697 (2) | 152 |
| O3—H3⋯O4i | 0.82 | 2.12 | 2.929 (3) | 169 |
| O4—H4⋯O2ii | 0.82 | 2.13 | 2.890 (2) | 153 |
Symmetry codes: (i) 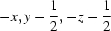 ; (ii)
; (ii) 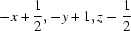 .
.
Acknowledgments
The authors thank Mr Lian-Dong Liu (College of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan 250014, People’s Republic of China) for his invaluable support of the X-ray data collection. The authors would like to thank Shandong Provincial Natural Science Foundation, China (Y2007C138,Y2008B29), the Scientific Reasearch Foundation of the Higher Education Institutions of Shandong Province, China (J07WE26), the National Natural Science Foundation of China (No.81001358) and the Promotive Research Fund for Excellent Young and Middle-aged sScientisits of Shandong Province (No. BS2010YY073) for research grants.
supplementary crystallographic information
Comment
Both Panax ginseng and Panax quinquefolium, belonging to Araliaceae, are well known traditional medicinal herbs. They are used as tonics and for the treatment for diseases, such as tumor and myocardial ischemia. Panax ginseng contains numbers of saponins, namely ginsenoside and an oleanolic acid-type saponin in addition to the major protopanaxadiol and protopanaxatriol-type saponins (Shibata et al.,1985). Panax quinquefolium contains an ocotillol-type (20S, 24R-epoxyside) saponin with high anti-tumor activity (Takano et al.,1999), as well as oleanolic acid-type, protopanaxadiol and protopanaxatriol-type saponins. (3R,6R,12R,20S,24S)-20,24-Epoxy-dammarane-3,6,12,25-tetraol and (3R,12R,20S,24R)-20,24-epoxy-dammarane-3,12,25-triol are found to possess cardioprotective effect on myocardial injury induced by isoproterenol in rats (Yu et al.,2007; Wang et al., 2010). As part of our ongoing investigation of ocotillol-type compounds and their cardioprotective effect on myocardial injury, we report herein the crystal structure of the title compound, (I).
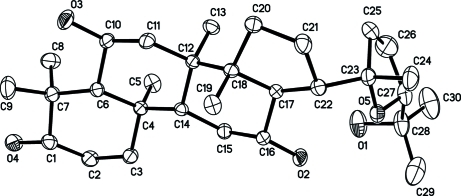
The molecular structure of (I) is shown in Fig. 1. In the molecule, all bond lengths and angles are within normal ranges (Shi et al.,1992; Meng et al., 2010). The six-membered rings A(C11,C13,C16,C17,C18,C19), B(C13,C15,C16,C22,C32,C33), and C(C22,C24,C25,C26,C29,C32) are in chair conformations. Ring D(C8,C9,C10,C11,C19) has an envelope form with atom C11 forming the flap. The tetrahydrofuran ring has a conformation intermediate between half-chair and sofa forms. In the crystal, molecules are linked by O—H···O hydrogen bonds (Table 1) to form a three-dimensional network.
Experimental
20(S)-protopanaxatriol was degraded from Panax quinquefolium saponin with sodium in glycerine at about 473 - 503K and seperated by silica flash chromatography. (3R,6R,12R,20S,24R)-20,24-Epoxy-dammarane-3,6,12,25-tetraol was synthesized from 20(S)-protopanaxatriol in the presence of N,N-dimethylaminopyridine, pyridine and acetic anhydride. The esters were oxidized by m-CPBA and the title compound was obtained by saponification with sodium hydroxide in DMSO and seperated by silica flash chromatography. Finally, the crystals were dried at room temperature the title compound was crystallized from ethyl acetate. Single crystals suitable for X-ray measurements were obtained by recrystallization of an acetone solution of the title compound at room temperature.
Refinement
In the absence of significant anomalous dispersion effects the Friedel pairs were merged. All H atoms were fixed geometrically and allowed to ride on their attached atoms, with C—H distances in the range 0.93–0.97 Å; O—H = 0.86Å and with Uiso(H) = 1.2Ueq(C) or 1.5Ueq(Cmethyl,O). The absolute configuration is based on unchanging stereochemical centers in the synthesis
Figures
Fig. 1.
The molecular structure of the title compound showing ellipsoids drawn at the 30% probability level. H atoms are not shown.
Crystal data
| C30H52O5 | F(000) = 1088 |
| Mr = 492.72 | Dx = 1.154 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 8789 reflections |
| a = 12.7918 (6) Å | θ = 2.2–28.0° |
| b = 13.7842 (7) Å | µ = 0.08 mm−1 |
| c = 16.0902 (8) Å | T = 298 K |
| V = 2837.1 (2) Å3 | Block, colourless |
| Z = 4 | 0.54 × 0.50 × 0.50 mm |
Data collection
| Bruker SMART CCD diffractometer | 2911 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.020 |
| graphite | θmax = 26.0°, θmin = 2.0° |
| φ and ω scans | h = −14→15 |
| 16320 measured reflections | k = −16→17 |
| 3141 independent reflections | l = −17→19 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.042 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.113 | H-atom parameters constrained |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0809P)2 + 0.2644P] where P = (Fo2 + 2Fc2)/3 |
| 3141 reflections | (Δ/σ)max < 0.001 |
| 325 parameters | Δρmax = 0.26 e Å−3 |
| 6 restraints | Δρmin = −0.24 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.17647 (17) | 0.49883 (16) | −0.21123 (14) | 0.0410 (5) | |
| H1 | 0.2435 | 0.4696 | −0.2267 | 0.049* | |
| C2 | 0.18569 (19) | 0.53311 (15) | −0.12319 (15) | 0.0449 (5) | |
| H2A | 0.1238 | 0.5700 | −0.1085 | 0.054* | |
| H2B | 0.2457 | 0.5757 | −0.1182 | 0.054* | |
| C3 | 0.19786 (18) | 0.44819 (14) | −0.06334 (13) | 0.0403 (5) | |
| H3A | 0.2030 | 0.4730 | −0.0071 | 0.048* | |
| H3B | 0.2625 | 0.4144 | −0.0758 | 0.048* | |
| C4 | 0.10599 (15) | 0.37543 (13) | −0.06804 (12) | 0.0301 (4) | |
| C5 | 0.00765 (18) | 0.42793 (16) | −0.03552 (15) | 0.0450 (5) | |
| H5A | 0.0050 | 0.4229 | 0.0240 | 0.067* | |
| H5B | −0.0536 | 0.3985 | −0.0590 | 0.067* | |
| H5C | 0.0104 | 0.4951 | −0.0512 | 0.067* | |
| C6 | 0.09851 (15) | 0.34065 (13) | −0.16103 (11) | 0.0308 (4) | |
| H6 | 0.1650 | 0.3078 | −0.1723 | 0.037* | |
| C7 | 0.09008 (17) | 0.42290 (15) | −0.22863 (13) | 0.0400 (5) | |
| C8 | −0.0189 (2) | 0.4691 (2) | −0.23163 (19) | 0.0588 (7) | |
| H8A | −0.0280 | 0.5106 | −0.1843 | 0.088* | |
| H8B | −0.0711 | 0.4191 | −0.2309 | 0.088* | |
| H8C | −0.0258 | 0.5066 | −0.2816 | 0.088* | |
| C9 | 0.1152 (3) | 0.3832 (2) | −0.31603 (15) | 0.0661 (8) | |
| H9A | 0.1302 | 0.4362 | −0.3528 | 0.099* | |
| H9B | 0.0561 | 0.3476 | −0.3367 | 0.099* | |
| H9C | 0.1748 | 0.3410 | −0.3130 | 0.099* | |
| C10 | 0.01445 (17) | 0.26102 (15) | −0.16760 (12) | 0.0360 (4) | |
| H10 | −0.0525 | 0.2870 | −0.1481 | 0.043* | |
| C11 | 0.04459 (17) | 0.17408 (14) | −0.11425 (12) | 0.0362 (4) | |
| H11A | 0.1093 | 0.1471 | −0.1355 | 0.043* | |
| H11B | −0.0091 | 0.1248 | −0.1200 | 0.043* | |
| C12 | 0.05889 (14) | 0.19643 (13) | −0.02208 (11) | 0.0282 (4) | |
| C13 | −0.05053 (15) | 0.21586 (16) | 0.01561 (14) | 0.0406 (5) | |
| H13A | −0.0895 | 0.2574 | −0.0209 | 0.061* | |
| H13B | −0.0429 | 0.2468 | 0.0687 | 0.061* | |
| H13C | −0.0870 | 0.1555 | 0.0224 | 0.061* | |
| C14 | 0.13479 (14) | 0.28469 (13) | −0.01349 (11) | 0.0279 (4) | |
| H14 | 0.2012 | 0.2615 | −0.0364 | 0.033* | |
| C15 | 0.15847 (17) | 0.30546 (14) | 0.07854 (12) | 0.0363 (4) | |
| H15A | 0.2095 | 0.3574 | 0.0819 | 0.044* | |
| H15B | 0.0950 | 0.3278 | 0.1054 | 0.044* | |
| C16 | 0.20003 (16) | 0.21773 (15) | 0.12529 (11) | 0.0344 (4) | |
| H16 | 0.2676 | 0.1994 | 0.1013 | 0.041* | |
| C17 | 0.12547 (14) | 0.13222 (13) | 0.11688 (11) | 0.0298 (4) | |
| H17 | 0.0574 | 0.1534 | 0.1381 | 0.036* | |
| C18 | 0.11087 (14) | 0.10824 (13) | 0.02336 (11) | 0.0300 (4) | |
| C19 | 0.21614 (17) | 0.07853 (14) | −0.01680 (13) | 0.0385 (4) | |
| H19A | 0.2556 | 0.0401 | 0.0218 | 0.058* | |
| H19B | 0.2551 | 0.1357 | −0.0310 | 0.058* | |
| H19C | 0.2029 | 0.0413 | −0.0661 | 0.058* | |
| C20 | 0.04667 (18) | 0.01370 (14) | 0.02911 (13) | 0.0395 (5) | |
| H20A | −0.0254 | 0.0270 | 0.0439 | 0.047* | |
| H20B | 0.0482 | −0.0216 | −0.0230 | 0.047* | |
| C21 | 0.1027 (2) | −0.04328 (15) | 0.09838 (14) | 0.0446 (5) | |
| H21A | 0.1572 | −0.0839 | 0.0751 | 0.054* | |
| H21B | 0.0534 | −0.0843 | 0.1278 | 0.054* | |
| C22 | 0.15043 (17) | 0.03336 (14) | 0.15818 (12) | 0.0363 (4) | |
| H22 | 0.2265 | 0.0248 | 0.1588 | 0.044* | |
| C23 | 0.10904 (18) | 0.01715 (16) | 0.24673 (13) | 0.0407 (5) | |
| C24 | 0.1500 (3) | −0.07978 (18) | 0.28034 (17) | 0.0605 (7) | |
| H24A | 0.2250 | −0.0802 | 0.2781 | 0.091* | |
| H24B | 0.1231 | −0.1319 | 0.2471 | 0.091* | |
| H24C | 0.1277 | −0.0879 | 0.3369 | 0.091* | |
| C25 | −0.0109 (2) | 0.0249 (2) | 0.25591 (16) | 0.0551 (6) | |
| H25A | −0.0426 | 0.0461 | 0.2042 | 0.066* | |
| H25B | −0.0407 | −0.0372 | 0.2714 | 0.066* | |
| C26 | −0.0286 (3) | 0.0990 (3) | 0.3237 (2) | 0.0756 (9) | |
| H26A | −0.0801 | 0.0759 | 0.3633 | 0.091* | |
| H26B | −0.0525 | 0.1600 | 0.3004 | 0.091* | |
| C27 | 0.0770 (2) | 0.11130 (18) | 0.36501 (14) | 0.0534 (6) | |
| H27 | 0.0855 | 0.0604 | 0.4070 | 0.064* | |
| C28 | 0.0987 (3) | 0.20971 (19) | 0.40561 (17) | 0.0663 (8) | |
| C29 | 0.2120 (4) | 0.2150 (2) | 0.4350 (2) | 0.0942 (13) | |
| H29A | 0.2579 | 0.2094 | 0.3880 | 0.141* | |
| H29B | 0.2255 | 0.1629 | 0.4731 | 0.141* | |
| H29C | 0.2238 | 0.2759 | 0.4623 | 0.141* | |
| C30 | 0.0201 (4) | 0.2255 (3) | 0.4772 (2) | 0.1030 (14) | |
| H30A | 0.0299 | 0.2890 | 0.5004 | 0.155* | |
| H30B | 0.0314 | 0.1775 | 0.5195 | 0.155* | |
| H30C | −0.0499 | 0.2198 | 0.4562 | 0.155* | |
| O2 | 0.21633 (15) | 0.24775 (12) | 0.21002 (9) | 0.0491 (4) | |
| H2 | 0.2176 | 0.1999 | 0.2403 | 0.074* | |
| O3 | 0.00324 (17) | 0.22871 (13) | −0.25149 (10) | 0.0572 (5) | |
| H3 | −0.0404 | 0.1852 | −0.2535 | 0.086* | |
| O4 | 0.15864 (14) | 0.57985 (12) | −0.26633 (12) | 0.0549 (4) | |
| H4 | 0.2056 | 0.6198 | −0.2605 | 0.082* | |
| O5 | 0.14937 (13) | 0.09411 (11) | 0.29913 (9) | 0.0426 (4) | |
| O1 | 0.0805 (2) | 0.28725 (13) | 0.34886 (13) | 0.0759 (7) | |
| H1A | 0.0692 | 0.2651 | 0.3024 | 0.114* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0381 (10) | 0.0370 (10) | 0.0478 (11) | −0.0032 (9) | −0.0025 (9) | 0.0145 (10) |
| C2 | 0.0528 (13) | 0.0297 (9) | 0.0523 (12) | −0.0109 (9) | −0.0068 (10) | 0.0062 (9) |
| C3 | 0.0486 (11) | 0.0319 (9) | 0.0404 (10) | −0.0103 (9) | −0.0084 (9) | 0.0051 (9) |
| C4 | 0.0328 (9) | 0.0250 (8) | 0.0325 (9) | −0.0007 (7) | −0.0008 (8) | 0.0003 (7) |
| C5 | 0.0497 (12) | 0.0345 (10) | 0.0506 (12) | 0.0115 (9) | 0.0102 (10) | −0.0001 (9) |
| C6 | 0.0314 (9) | 0.0294 (8) | 0.0316 (9) | −0.0007 (8) | −0.0010 (8) | 0.0027 (7) |
| C7 | 0.0443 (11) | 0.0367 (10) | 0.0392 (10) | −0.0067 (9) | −0.0064 (9) | 0.0094 (8) |
| C8 | 0.0474 (13) | 0.0524 (14) | 0.0767 (18) | −0.0077 (11) | −0.0191 (13) | 0.0309 (13) |
| C9 | 0.101 (2) | 0.0597 (15) | 0.0372 (12) | −0.0213 (16) | 0.0000 (14) | 0.0098 (11) |
| C10 | 0.0418 (10) | 0.0342 (10) | 0.0320 (9) | −0.0079 (8) | −0.0071 (8) | 0.0030 (8) |
| C11 | 0.0455 (11) | 0.0275 (9) | 0.0356 (10) | −0.0072 (8) | −0.0075 (9) | −0.0016 (8) |
| C12 | 0.0299 (9) | 0.0253 (8) | 0.0294 (8) | −0.0028 (7) | −0.0015 (7) | 0.0011 (7) |
| C13 | 0.0282 (9) | 0.0448 (11) | 0.0488 (11) | −0.0021 (8) | 0.0024 (8) | 0.0086 (10) |
| C14 | 0.0277 (8) | 0.0264 (8) | 0.0295 (8) | −0.0035 (7) | 0.0004 (7) | −0.0007 (7) |
| C15 | 0.0470 (11) | 0.0305 (9) | 0.0315 (9) | −0.0093 (9) | −0.0028 (8) | −0.0016 (8) |
| C16 | 0.0359 (9) | 0.0395 (10) | 0.0279 (9) | −0.0100 (8) | −0.0030 (8) | 0.0016 (8) |
| C17 | 0.0286 (9) | 0.0328 (9) | 0.0279 (8) | −0.0036 (7) | 0.0001 (7) | 0.0026 (7) |
| C18 | 0.0311 (9) | 0.0272 (9) | 0.0318 (9) | −0.0030 (7) | −0.0011 (8) | −0.0006 (7) |
| C19 | 0.0437 (11) | 0.0328 (9) | 0.0390 (10) | 0.0067 (8) | 0.0070 (9) | 0.0011 (8) |
| C20 | 0.0494 (11) | 0.0307 (9) | 0.0386 (10) | −0.0109 (9) | −0.0044 (9) | −0.0005 (8) |
| C21 | 0.0600 (13) | 0.0328 (10) | 0.0411 (11) | −0.0061 (10) | −0.0015 (10) | 0.0038 (9) |
| C22 | 0.0369 (10) | 0.0344 (9) | 0.0377 (10) | −0.0020 (8) | −0.0017 (8) | 0.0059 (8) |
| C23 | 0.0485 (11) | 0.0377 (11) | 0.0359 (10) | −0.0063 (9) | −0.0050 (9) | 0.0084 (9) |
| C24 | 0.091 (2) | 0.0439 (13) | 0.0467 (13) | −0.0027 (13) | −0.0142 (14) | 0.0139 (11) |
| C25 | 0.0490 (13) | 0.0685 (16) | 0.0480 (12) | −0.0168 (12) | 0.0038 (10) | 0.0152 (12) |
| C26 | 0.0608 (17) | 0.091 (2) | 0.0746 (19) | 0.0033 (17) | 0.0135 (15) | 0.0000 (19) |
| C27 | 0.0756 (17) | 0.0478 (12) | 0.0369 (11) | 0.0024 (12) | 0.0091 (11) | 0.0098 (10) |
| C28 | 0.112 (2) | 0.0402 (12) | 0.0463 (13) | 0.0050 (15) | 0.0070 (15) | 0.0074 (11) |
| C29 | 0.161 (4) | 0.0472 (15) | 0.074 (2) | −0.010 (2) | −0.046 (2) | 0.0026 (15) |
| C30 | 0.171 (4) | 0.067 (2) | 0.071 (2) | 0.009 (2) | 0.040 (2) | −0.0019 (16) |
| O2 | 0.0687 (10) | 0.0474 (9) | 0.0311 (7) | −0.0241 (8) | −0.0123 (7) | 0.0037 (7) |
| O3 | 0.0882 (13) | 0.0489 (10) | 0.0346 (7) | −0.0240 (9) | −0.0183 (8) | 0.0033 (7) |
| O4 | 0.0598 (10) | 0.0438 (9) | 0.0610 (10) | −0.0155 (8) | −0.0113 (9) | 0.0241 (8) |
| O5 | 0.0519 (9) | 0.0414 (8) | 0.0346 (7) | −0.0059 (7) | −0.0038 (6) | 0.0064 (6) |
| O1 | 0.1141 (18) | 0.0473 (10) | 0.0663 (12) | 0.0204 (12) | 0.0115 (12) | 0.0160 (9) |
Geometric parameters (Å, °)
| C1—O4 | 1.444 (2) | C16—H16 | 0.9800 |
| C1—C2 | 1.498 (3) | C17—C22 | 1.549 (3) |
| C1—C7 | 1.548 (3) | C17—C18 | 1.552 (2) |
| C1—H1 | 0.9800 | C17—H17 | 0.9800 |
| C2—C3 | 1.524 (3) | C18—C20 | 1.543 (2) |
| C2—H2A | 0.9700 | C18—C19 | 1.549 (3) |
| C2—H2B | 0.9700 | C19—H19A | 0.9600 |
| C3—C4 | 1.547 (3) | C19—H19B | 0.9600 |
| C3—H3A | 0.9700 | C19—H19C | 0.9600 |
| C3—H3B | 0.9700 | C20—C21 | 1.540 (3) |
| C4—C5 | 1.543 (3) | C20—H20A | 0.9700 |
| C4—C14 | 1.572 (2) | C20—H20B | 0.9700 |
| C4—C6 | 1.574 (3) | C21—C22 | 1.554 (3) |
| C5—H5A | 0.9600 | C21—H21A | 0.9700 |
| C5—H5B | 0.9600 | C21—H21B | 0.9700 |
| C5—H5C | 0.9600 | C22—C23 | 1.536 (3) |
| C6—C10 | 1.540 (3) | C22—H22 | 0.9800 |
| C6—C7 | 1.575 (3) | C23—O5 | 1.450 (3) |
| C6—H6 | 0.9800 | C23—C24 | 1.534 (3) |
| C7—C8 | 1.534 (3) | C23—C25 | 1.546 (3) |
| C7—C9 | 1.543 (3) | C24—H24A | 0.9600 |
| C8—H8A | 0.9600 | C24—H24B | 0.9600 |
| C8—H8B | 0.9600 | C24—H24C | 0.9600 |
| C8—H8C | 0.9600 | C25—C26 | 1.510 (4) |
| C9—H9A | 0.9600 | C25—H25A | 0.9700 |
| C9—H9B | 0.9600 | C25—H25B | 0.9700 |
| C9—H9C | 0.9600 | C26—C27 | 1.515 (4) |
| C10—O3 | 1.428 (2) | C26—H26A | 0.9700 |
| C10—C11 | 1.524 (3) | C26—H26B | 0.9700 |
| C10—H10 | 0.9800 | C27—O5 | 1.427 (3) |
| C11—C12 | 1.526 (3) | C27—C28 | 1.531 (4) |
| C11—H11A | 0.9700 | C27—H27 | 0.9800 |
| C11—H11B | 0.9700 | C28—O1 | 1.425 (3) |
| C12—C13 | 1.549 (3) | C28—C29 | 1.525 (5) |
| C12—C14 | 1.563 (2) | C28—C30 | 1.544 (5) |
| C12—C18 | 1.567 (2) | C29—H29A | 0.9600 |
| C13—H13A | 0.9600 | C29—H29B | 0.9600 |
| C13—H13B | 0.9600 | C29—H29C | 0.9600 |
| C13—H13C | 0.9600 | C30—H30A | 0.9600 |
| C14—C15 | 1.538 (3) | C30—H30B | 0.9600 |
| C14—H14 | 0.9800 | C30—H30C | 0.9600 |
| C15—C16 | 1.520 (3) | O2—H2 | 0.8200 |
| C15—H15A | 0.9700 | O3—H3 | 0.8200 |
| C15—H15B | 0.9700 | O4—H4 | 0.8200 |
| C16—O2 | 1.440 (2) | O1—H1A | 0.8200 |
| C16—C17 | 1.522 (2) | ||
| O4—C1—C2 | 110.43 (18) | O2—C16—H16 | 108.6 |
| O4—C1—C7 | 107.41 (16) | C15—C16—H16 | 108.6 |
| C2—C1—C7 | 116.14 (18) | C17—C16—H16 | 108.6 |
| O4—C1—H1 | 107.5 | C16—C17—C22 | 120.91 (15) |
| C2—C1—H1 | 107.5 | C16—C17—C18 | 109.06 (15) |
| C7—C1—H1 | 107.5 | C22—C17—C18 | 104.68 (15) |
| C1—C2—C3 | 111.31 (17) | C16—C17—H17 | 107.2 |
| C1—C2—H2A | 109.4 | C22—C17—H17 | 107.2 |
| C3—C2—H2A | 109.4 | C18—C17—H17 | 107.2 |
| C1—C2—H2B | 109.4 | C20—C18—C19 | 105.34 (16) |
| C3—C2—H2B | 109.4 | C20—C18—C17 | 100.71 (15) |
| H2A—C2—H2B | 108.0 | C19—C18—C17 | 110.87 (15) |
| C2—C3—C4 | 112.93 (17) | C20—C18—C12 | 117.21 (15) |
| C2—C3—H3A | 109.0 | C19—C18—C12 | 112.30 (15) |
| C4—C3—H3A | 109.0 | C17—C18—C12 | 109.77 (15) |
| C2—C3—H3B | 109.0 | C18—C19—H19A | 109.5 |
| C4—C3—H3B | 109.0 | C18—C19—H19B | 109.5 |
| H3A—C3—H3B | 107.8 | H19A—C19—H19B | 109.5 |
| C5—C4—C3 | 107.38 (16) | C18—C19—H19C | 109.5 |
| C5—C4—C14 | 112.02 (16) | H19A—C19—H19C | 109.5 |
| C3—C4—C14 | 108.10 (15) | H19B—C19—H19C | 109.5 |
| C5—C4—C6 | 114.56 (17) | C21—C20—C18 | 103.09 (16) |
| C3—C4—C6 | 106.87 (15) | C21—C20—H20A | 111.1 |
| C14—C4—C6 | 107.61 (14) | C18—C20—H20A | 111.1 |
| C4—C5—H5A | 109.5 | C21—C20—H20B | 111.1 |
| C4—C5—H5B | 109.5 | C18—C20—H20B | 111.1 |
| H5A—C5—H5B | 109.5 | H20A—C20—H20B | 109.1 |
| C4—C5—H5C | 109.5 | C20—C21—C22 | 106.51 (16) |
| H5A—C5—H5C | 109.5 | C20—C21—H21A | 110.4 |
| H5B—C5—H5C | 109.5 | C22—C21—H21A | 110.4 |
| C10—C6—C4 | 108.96 (15) | C20—C21—H21B | 110.4 |
| C10—C6—C7 | 114.70 (15) | C22—C21—H21B | 110.4 |
| C4—C6—C7 | 116.19 (16) | H21A—C21—H21B | 108.6 |
| C10—C6—H6 | 105.3 | C23—C22—C17 | 117.05 (17) |
| C4—C6—H6 | 105.3 | C23—C22—C21 | 109.87 (17) |
| C7—C6—H6 | 105.3 | C17—C22—C21 | 104.55 (15) |
| C8—C7—C9 | 107.9 (2) | C23—C22—H22 | 108.4 |
| C8—C7—C1 | 111.95 (19) | C17—C22—H22 | 108.4 |
| C9—C7—C1 | 104.9 (2) | C21—C22—H22 | 108.4 |
| C8—C7—C6 | 112.53 (18) | O5—C23—C24 | 108.09 (17) |
| C9—C7—C6 | 111.07 (19) | O5—C23—C22 | 108.07 (16) |
| C1—C7—C6 | 108.25 (16) | C24—C23—C22 | 109.6 (2) |
| C7—C8—H8A | 109.5 | O5—C23—C25 | 104.3 (2) |
| C7—C8—H8B | 109.5 | C24—C23—C25 | 111.5 (2) |
| H8A—C8—H8B | 109.5 | C22—C23—C25 | 114.89 (19) |
| C7—C8—H8C | 109.5 | C23—C24—H24A | 109.5 |
| H8A—C8—H8C | 109.5 | C23—C24—H24B | 109.5 |
| H8B—C8—H8C | 109.5 | H24A—C24—H24B | 109.5 |
| C7—C9—H9A | 109.5 | C23—C24—H24C | 109.5 |
| C7—C9—H9B | 109.5 | H24A—C24—H24C | 109.5 |
| H9A—C9—H9B | 109.5 | H24B—C24—H24C | 109.5 |
| C7—C9—H9C | 109.5 | C26—C25—C23 | 105.3 (2) |
| H9A—C9—H9C | 109.5 | C26—C25—H25A | 110.7 |
| H9B—C9—H9C | 109.5 | C23—C25—H25A | 110.7 |
| O3—C10—C11 | 108.21 (17) | C26—C25—H25B | 110.7 |
| O3—C10—C6 | 110.93 (17) | C23—C25—H25B | 110.7 |
| C11—C10—C6 | 110.19 (16) | H25A—C25—H25B | 108.8 |
| O3—C10—H10 | 109.2 | C25—C26—C27 | 105.0 (2) |
| C11—C10—H10 | 109.2 | C25—C26—H26A | 110.7 |
| C6—C10—H10 | 109.2 | C27—C26—H26A | 110.7 |
| C10—C11—C12 | 114.79 (16) | C25—C26—H26B | 110.7 |
| C10—C11—H11A | 108.6 | C27—C26—H26B | 110.7 |
| C12—C11—H11A | 108.6 | H26A—C26—H26B | 108.8 |
| C10—C11—H11B | 108.6 | O5—C27—C26 | 103.5 (2) |
| C12—C11—H11B | 108.6 | O5—C27—C28 | 110.3 (2) |
| H11A—C11—H11B | 107.5 | C26—C27—C28 | 116.6 (3) |
| C11—C12—C13 | 107.89 (16) | O5—C27—H27 | 108.7 |
| C11—C12—C14 | 108.52 (15) | C26—C27—H27 | 108.7 |
| C13—C12—C14 | 113.09 (16) | C28—C27—H27 | 108.7 |
| C11—C12—C18 | 110.36 (15) | O1—C28—C29 | 108.6 (3) |
| C13—C12—C18 | 109.58 (15) | O1—C28—C27 | 111.2 (2) |
| C14—C12—C18 | 107.41 (14) | C29—C28—C27 | 110.3 (3) |
| C12—C13—H13A | 109.5 | O1—C28—C30 | 105.4 (3) |
| C12—C13—H13B | 109.5 | C29—C28—C30 | 112.4 (3) |
| H13A—C13—H13B | 109.5 | C27—C28—C30 | 108.9 (3) |
| C12—C13—H13C | 109.5 | C28—C29—H29A | 109.5 |
| H13A—C13—H13C | 109.5 | C28—C29—H29B | 109.5 |
| H13B—C13—H13C | 109.5 | H29A—C29—H29B | 109.5 |
| C15—C14—C12 | 110.63 (14) | C28—C29—H29C | 109.5 |
| C15—C14—C4 | 115.82 (15) | H29A—C29—H29C | 109.5 |
| C12—C14—C4 | 115.13 (14) | H29B—C29—H29C | 109.5 |
| C15—C14—H14 | 104.6 | C28—C30—H30A | 109.5 |
| C12—C14—H14 | 104.6 | C28—C30—H30B | 109.5 |
| C4—C14—H14 | 104.6 | H30A—C30—H30B | 109.5 |
| C16—C15—C14 | 113.40 (16) | C28—C30—H30C | 109.5 |
| C16—C15—H15A | 108.9 | H30A—C30—H30C | 109.5 |
| C14—C15—H15A | 108.9 | H30B—C30—H30C | 109.5 |
| C16—C15—H15B | 108.9 | C16—O2—H2 | 109.5 |
| C14—C15—H15B | 108.9 | C10—O3—H3 | 109.5 |
| H15A—C15—H15B | 107.7 | C1—O4—H4 | 109.5 |
| O2—C16—C15 | 106.89 (16) | C27—O5—C23 | 108.82 (18) |
| O2—C16—C17 | 113.42 (15) | C28—O1—H1A | 109.5 |
| C15—C16—C17 | 110.66 (15) | ||
| O4—C1—C2—C3 | 176.51 (18) | O2—C16—C17—C18 | 178.19 (16) |
| C7—C1—C2—C3 | 53.9 (3) | C15—C16—C17—C18 | 58.1 (2) |
| C1—C2—C3—C4 | −58.2 (3) | C16—C17—C18—C20 | 172.77 (15) |
| C2—C3—C4—C5 | −66.8 (2) | C22—C17—C18—C20 | 42.05 (18) |
| C2—C3—C4—C14 | 172.11 (17) | C16—C17—C18—C19 | 61.7 (2) |
| C2—C3—C4—C6 | 56.5 (2) | C22—C17—C18—C19 | −69.06 (18) |
| C5—C4—C6—C10 | −66.2 (2) | C16—C17—C18—C12 | −63.00 (19) |
| C3—C4—C6—C10 | 174.97 (15) | C22—C17—C18—C12 | 166.28 (14) |
| C14—C4—C6—C10 | 59.06 (19) | C11—C12—C18—C20 | −66.3 (2) |
| C5—C4—C6—C7 | 65.2 (2) | C13—C12—C18—C20 | 52.4 (2) |
| C3—C4—C6—C7 | −53.6 (2) | C14—C12—C18—C20 | 175.57 (15) |
| C14—C4—C6—C7 | −169.54 (15) | C11—C12—C18—C19 | 55.9 (2) |
| O4—C1—C7—C8 | −47.7 (3) | C13—C12—C18—C19 | 174.53 (16) |
| C2—C1—C7—C8 | 76.5 (2) | C14—C12—C18—C19 | −62.25 (19) |
| O4—C1—C7—C9 | 69.1 (2) | C11—C12—C18—C17 | 179.69 (15) |
| C2—C1—C7—C9 | −166.7 (2) | C13—C12—C18—C17 | −61.64 (19) |
| O4—C1—C7—C6 | −172.26 (17) | C14—C12—C18—C17 | 61.57 (18) |
| C2—C1—C7—C6 | −48.1 (2) | C19—C18—C20—C21 | 71.6 (2) |
| C10—C6—C7—C8 | 53.4 (3) | C17—C18—C20—C21 | −43.78 (19) |
| C4—C6—C7—C8 | −75.3 (2) | C12—C18—C20—C21 | −162.75 (17) |
| C10—C6—C7—C9 | −67.8 (2) | C18—C20—C21—C22 | 29.8 (2) |
| C4—C6—C7—C9 | 163.6 (2) | C16—C17—C22—C23 | 90.9 (2) |
| C10—C6—C7—C1 | 177.61 (18) | C18—C17—C22—C23 | −145.68 (17) |
| C4—C6—C7—C1 | 49.0 (2) | C16—C17—C22—C21 | −147.28 (18) |
| C4—C6—C10—O3 | 178.68 (17) | C18—C17—C22—C21 | −23.9 (2) |
| C7—C6—C10—O3 | 46.5 (2) | C20—C21—C22—C23 | 122.8 (2) |
| C4—C6—C10—C11 | −61.5 (2) | C20—C21—C22—C17 | −3.6 (2) |
| C7—C6—C10—C11 | 166.30 (17) | C17—C22—C23—O5 | −56.9 (2) |
| O3—C10—C11—C12 | −179.57 (18) | C21—C22—C23—O5 | −175.92 (17) |
| C6—C10—C11—C12 | 59.0 (2) | C17—C22—C23—C24 | −174.54 (19) |
| C10—C11—C12—C13 | 71.6 (2) | C21—C22—C23—C24 | 66.5 (2) |
| C10—C11—C12—C14 | −51.3 (2) | C17—C22—C23—C25 | 59.0 (3) |
| C10—C11—C12—C18 | −168.72 (16) | C21—C22—C23—C25 | −60.0 (3) |
| C11—C12—C14—C15 | −175.74 (16) | O5—C23—C25—C26 | −7.7 (3) |
| C13—C12—C14—C15 | 64.6 (2) | C24—C23—C25—C26 | 108.7 (2) |
| C18—C12—C14—C15 | −56.43 (19) | C22—C23—C25—C26 | −125.8 (2) |
| C11—C12—C14—C4 | 50.6 (2) | C23—C25—C26—C27 | −13.9 (3) |
| C13—C12—C14—C4 | −69.1 (2) | C25—C26—C27—O5 | 30.9 (3) |
| C18—C12—C14—C4 | 169.89 (14) | C25—C26—C27—C28 | 152.2 (2) |
| C5—C4—C14—C15 | −60.1 (2) | O5—C27—C28—O1 | 64.9 (3) |
| C3—C4—C14—C15 | 58.1 (2) | C26—C27—C28—O1 | −52.7 (3) |
| C6—C4—C14—C15 | 173.16 (15) | O5—C27—C28—C29 | −55.5 (3) |
| C5—C4—C14—C12 | 71.2 (2) | C26—C27—C28—C29 | −173.2 (3) |
| C3—C4—C14—C12 | −170.70 (16) | O5—C27—C28—C30 | −179.3 (3) |
| C6—C4—C14—C12 | −55.6 (2) | C26—C27—C28—C30 | 63.0 (3) |
| C12—C14—C15—C16 | 54.5 (2) | C26—C27—O5—C23 | −37.5 (2) |
| C4—C14—C15—C16 | −172.13 (16) | C28—C27—O5—C23 | −163.0 (2) |
| C14—C15—C16—O2 | −178.89 (16) | C24—C23—O5—C27 | −90.3 (2) |
| C14—C15—C16—C17 | −54.9 (2) | C22—C23—O5—C27 | 151.15 (18) |
| O2—C16—C17—C22 | −60.5 (2) | C25—C23—O5—C27 | 28.5 (2) |
| C15—C16—C17—C22 | 179.36 (16) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···O2 | 0.82 | 2.41 | 2.882 (3) | 117 |
| O2—H2···O5 | 0.82 | 1.95 | 2.697 (2) | 152 |
| O3—H3···O4i | 0.82 | 2.12 | 2.929 (3) | 169 |
| O4—H4···O2ii | 0.82 | 2.13 | 2.890 (2) | 153 |
Symmetry codes: (i) −x, y−1/2, −z−1/2; (ii) −x+1/2, −y+1, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5198).
References
- Bruker (1997). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Meng, Q.-G., Liu, L.-D., Guo, H.-M., Bi, Y. & Wang, L. (2010). Acta Cryst. E66, o3210. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shi, Q., Hen, K., Jioka, T. & Mhiwada, Y. (1992). J. Nat. Prod. pp. 1488–1497.
- Shibata, S., Tanaka, L., Shoji, L. & Saito, H. (1985). Econ. Med. Res. 1, 217–284.
- Takano, K., Midori, T., Eiichiro, I. & Teruo, M. (1999). Cancer Lett. 147, 11–16.
- Wang, T., Meng, Q. G., Zhang, J. F., Bi, Y. & Jiang, N. C. (2010). Fitoterapia, 81, 783–787. [DOI] [PubMed]
- Yu, C., Fu, F. H., Yu, X., Han, B. & Zhu, M. (2007). Arzneimittelforschung, 57, 568–572.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811008609/lh5198sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811008609/lh5198Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report