Abstract
The title compound, 0.897C30H48O3.0.103C30H47O2F is a co-crystal of two triterpenes isolated from the resin of Canarium schweinfurthiiand Engl. Both triterpenes consists of four trans-fused rings having chair/half-chair/half-chair and envelope conformations. The molecular conformations are stabilized by intramolecular C—H⋯O hydrogen bonds, forming rings of S(7) graph-set motif. In the crystal, molecules are linked by intermolecular O—H⋯O and C—H⋯O interactions, forming sheets parallel to (001). All atoms. excepting the axially-oriented hydroxyl group in the major component and the equatorially-oriented fluorine atom in the minor component, are overlapping.
Related literature
For the crystal structure of 3α-hydroxytirucalla-7,24-diene-21-oic acid, see: Mora et al. (2001 ▶). For the crystal structure of 3α-hydroxytirucalla-8,24-diene-21-oic acid, see: Yousuf et al. (2011 ▶). For the biological activity of canarium schweinfurthiiand, see: Atawodi (2010) ▶; Dongmo et al. (2010 ▶). For the stability of the temperature controller used in the data collection, see: Cosier & Glazer (1986 ▶).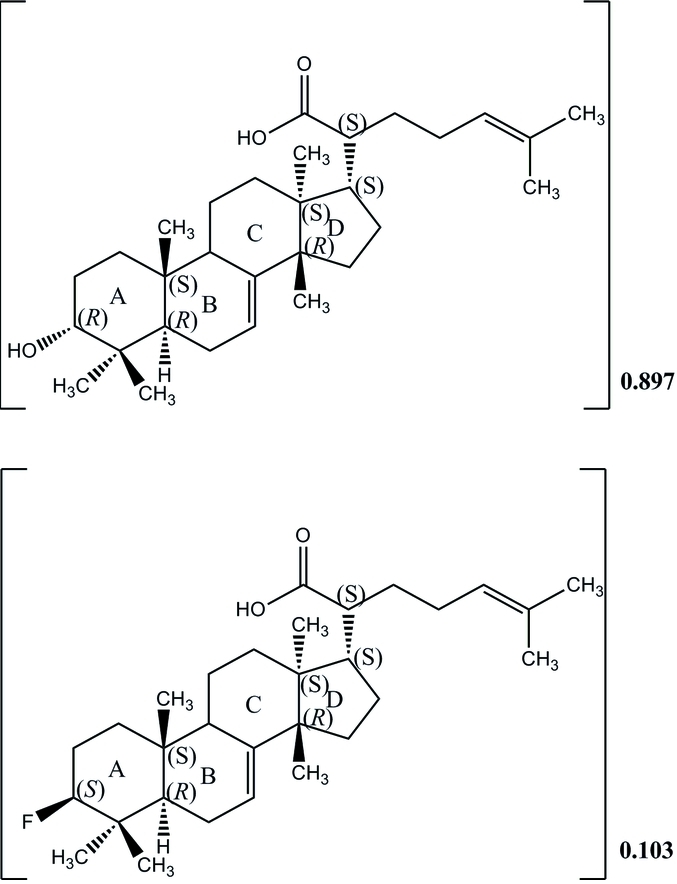
Experimental
Crystal data
0.897C30H48O3·0.103C30H47O2F
M r = 455.88
Trigonal,
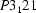
a = 11.2868 (9) Å
c = 36.446 (3) Å
V = 4020.9 (5) Å3
Z = 6
Mo Kα radiation
μ = 0.07 mm−1
T = 100 K
0.29 × 0.24 × 0.13 mm
Data collection
Bruker SMART APEXII DUO CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009) ▶ T min = 0.980, T max = 0.991
27808 measured reflections
4454 independent reflections
4347 reflections with I > 2σ(I)
R int = 0.105
Refinement
R[F 2 > 2σ(F 2)] = 0.058
wR(F 2) = 0.153
S = 1.18
4454 reflections
316 parameters
H-atom parameters constrained
Δρmax = 0.39 e Å−3
Δρmin = −0.33 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811011159/rz2572sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811011159/rz2572Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1O1⋯O2i | 0.87 | 1.81 | 2.654 (3) | 165 |
| O3—H3A⋯O2ii | 0.84 | 2.04 | 2.818 (4) | 154 |
| C12—H12B⋯O1 | 0.99 | 2.56 | 3.262 (4) | 128 |
| C22—H22A⋯O3iii | 0.99 | 2.40 | 3.300 (5) | 151 |
Symmetry codes: (i) 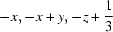 ; (ii)
; (ii) 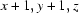 ; (iii)
; (iii) 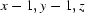 .
.
Acknowledgments
RSTK thanks the H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, for providing research facilities. SY thanks the School of Physics, Universiti Sains Malaysia, for providing X-ray diffraction research facilities. HKF thanks the Malaysian Government and Universiti Sains Malaysia for the Research University Grant No. 1001/PFIZIK/811160.
supplementary crystallographic information
Comment
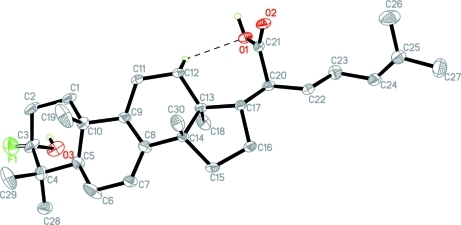
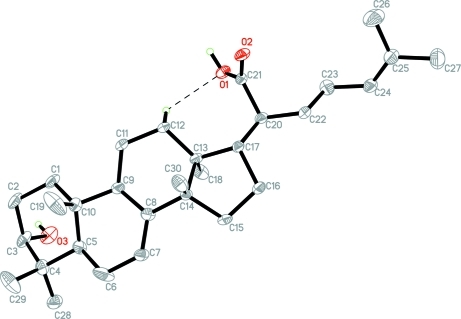
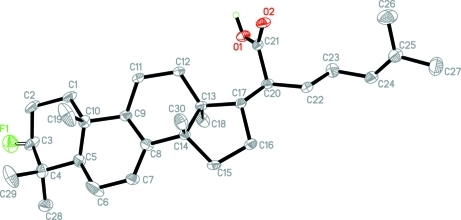
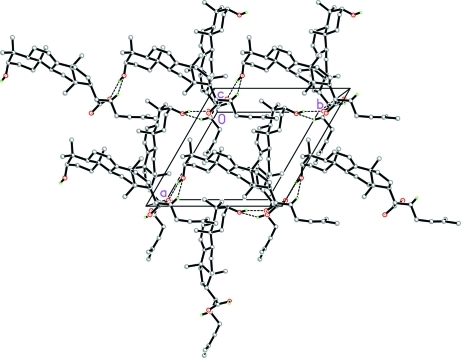
The title compound is a co-crystal of two triterpenes namely 3α-hydroxytirucalla-7,24-dien-21-oic acid (or epielemadienolic acid, I) as a major component (89.7%) and 3β-fluorotirucalla- 7,24-dien-21-oic acid (II) as minor component (10.3%). The co-crystal was isolated during the phytochemical investigation of the dichloromethane soluble part of the resins of the medicinally important plant Canarium schweinfurthii of Cameroon. The plant has been used for the treatment of a wide range of ailments including malaria, fever and diarrhea (Atawodi, 2010; Dongmo et al., 2010). The refinement of the crystal structure revealed I and II as major (89.7%) and minor (10.3%) component, respectively with the difference that in II the axially oriented hydroxyl group attached to C3 has been replaced by the equatorially oriented fluorine atom. The asymmetric unit of the co-crystal (Fig. 1) consists of the mixture of I (Fig. 2) and II (Fig. 3). The crystal structure of the major component I has already been reported and the space group (P3121) and cell parameters were found to be similar to those previously reported (Mora et al.. 2001, Yousuf et al., 2011). However the minor component II was found to be a new triterpene. In both components the molecular structure showed that the trans fused rings A/B/C and D adopt chair [Q= 0.550 (4) Å, θ = 7.1 (4)° and φ = 88 (3)°] / half-chair [Q= 0.530 (4) Å, θ = 49.5 (4)° and φ = 323.5 (6)°] / half-chair [Q= 0.652 (4) Å, θ = 100.4 (4)° and φ = 83.8 (3)°] and envelope [Q= 0.483 (2)Å and φ = 10.7 (4)°] conformations respectively. The chair and envelop conformations of rings C and D are stabilized by C12—H12B···O1 intramolecular hydrogen bond. In the crystal structure, the molecules are linked to form two-dimensional molecular sheets via O3—H3A···O2, O1—H1O1···O2 and C22—H22A···O3 intermolecular hydrogen bonds (symmetry codes as in Table 1) and arranged parallel to the (001) plane (Fig.2).The absolute configuration was assigned on the basis of our recently published triterpene crystal data (Yousuf et al., 2011).
Experimental
The resin (100 g) of Canarium schweinfurthii Engl. was collected in Yaounde, Cameroon, in May 2010 and identified by Professor Noumi, a botanist at the Department of Biology, University of Yaounde-1. A voucher specimen (HNC 25918.) was deposited at the National Herbarium of Cameroon in Yaounde. The resin (100 g) of C. schweinfurthii was allowed to dry under shade and extracted with dichloromethane. The extract (70 g) was subjected to column chromatography over silica gel (300 g, 60 × 5 cm) eluting with hexane followed by a mixture of n-hexane–EtOAc in order to increase polarity. The fractions eluted were monitored by thin layer chromatography and similar fractions were combined to give seven fraction FrA-FrG. Fraction FrA (200 mg), obtained on elution with a mixture of n-hexane-EtOAc (8:2 v/v), was subjected to further column chromatography over silica gel (70 g, 60 cm3 × 3, hexane-acetone equimolar solution) to yield crystals of the title compound. Recrystallization from n-hexane gave colourless crystals (60 mg).
Refinement
H atoms on the C of methyl, methylene, methine and oxygen were positioned geomatrically with C–H = 0.98–1.00 Å and O–H = 0.86 Å, respectively and constrained to ride on their parent atoms with Uiso(H) = 1.2Ueq(CH2, CH) and 1.5Ueq(CH3, OH). A rotating group model was applied to the methyl groups. The crystal is a twin with twin law -1 0 0 0 - 1 0 0 0 1 and BASF = 0.1815 (16). Friedel pairs were merged in the last refinement cycles.
Figures
Fig. 1.
The molecular structure of the title compound showing 50% probability displacement ellipsoids. The intramolecular hydrogen bond is shown as a dashed line. Hydrogen atoms not involved in hydrogen bonding are omitted for clarity.
Fig. 2.
The molecular structure of the major component I, showing 50% probability displacement ellipsoids. The intramolecular hydrogen bond is shown as a dashed line. Hydrogen atoms not involved in hydrogen bonding are omitted for clarity.
Fig. 3.
The molecular structure of the minor component II, showing 50% probability displacement ellipsoids. Hydrogen atoms are omitted for clarity.
Fig. 4.
Crystal packing of the major component of the title compound, showing a two-dimensional molecular sheet parallel to the (001) plane. Only hydrogen atoms involved in hydrogen bonding (dashed lines) are shown.
Crystal data
| 0.897C30H48O3·0.103C30H47O2F | Dx = 1.130 Mg m−3 |
| Mr = 455.88 | Mo Kα radiation, λ = 0.71073 Å |
| Trigonal, P3121 | Cell parameters from 10350 reflections |
| Hall symbol: p 31 2" | θ = 2.1–30.1° |
| a = 11.2868 (9) Å | µ = 0.07 mm−1 |
| c = 36.446 (3) Å | T = 100 K |
| V = 4020.9 (5) Å3 | Block, colourles |
| Z = 6 | 0.29 × 0.24 × 0.13 mm |
| F(000) = 1506 |
Data collection
| Bruker SMART APEXII DUO CCD area-detector diffractometer | 4454 independent reflections |
| Radiation source: fine-focus sealed tube | 4347 reflections with I > 2σ(I) |
| graphite | Rint = 0.105 |
| φ and ω scans | θmax = 30.1°, θmin = 2.1° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −15→15 |
| Tmin = 0.980, Tmax = 0.991 | k = −13→15 |
| 27808 measured reflections | l = −51→38 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.058 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.153 | H-atom parameters constrained |
| S = 1.18 | w = 1/[σ2(Fo2) + (0.0644P)2 + 1.6942P] where P = (Fo2 + 2Fc2)/3 |
| 4454 reflections | (Δ/σ)max < 0.001 |
| 316 parameters | Δρmax = 0.39 e Å−3 |
| 0 restraints | Δρmin = −0.33 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 0.1191 (2) | 0.0008 (2) | 0.12753 (5) | 0.0221 (4) | |
| H1O1 | 0.1119 | −0.0077 | 0.1511 | 0.033* | |
| O2 | −0.1057 (2) | −0.0844 (2) | 0.13357 (5) | 0.0230 (4) | |
| O3 | 0.6534 (3) | 0.8965 (3) | 0.10496 (7) | 0.0330 (8) | 0.898 (8) |
| H3A | 0.7204 | 0.9158 | 0.1187 | 0.049* | 0.898 (8) |
| F1 | 0.693 (3) | 1.048 (3) | 0.1287 (7) | 0.042 (7) | 0.102 (8) |
| C1 | 0.4098 (3) | 0.7220 (4) | 0.14860 (7) | 0.0268 (7) | |
| H1A | 0.3651 | 0.6772 | 0.1720 | 0.032* | |
| H1B | 0.4651 | 0.6811 | 0.1403 | 0.032* | |
| C2 | 0.5056 (4) | 0.8752 (4) | 0.15562 (8) | 0.0335 (8) | |
| H2A | 0.4524 | 0.9153 | 0.1660 | 0.040* | |
| H2B | 0.5761 | 0.8875 | 0.1738 | 0.040* | |
| C3 | 0.5750 (3) | 0.9496 (4) | 0.12011 (8) | 0.0278 (7) | |
| H3B | 0.6369 | 1.0487 | 0.1257 | 0.033* | 0.898 (8) |
| H3C | 0.6079 | 0.8925 | 0.1103 | 0.033* | 0.102 (8) |
| C4 | 0.4725 (3) | 0.9373 (3) | 0.09109 (9) | 0.0249 (6) | |
| C5 | 0.3685 (3) | 0.7821 (3) | 0.08498 (8) | 0.0241 (6) | |
| H5A | 0.4249 | 0.7444 | 0.0746 | 0.029* | |
| C6 | 0.2630 (5) | 0.7560 (4) | 0.05513 (13) | 0.0500 (13) | |
| H6A | 0.2107 | 0.8014 | 0.0622 | 0.060* | |
| H6B | 0.3116 | 0.7979 | 0.0319 | 0.060* | |
| C7 | 0.1654 (3) | 0.6078 (3) | 0.04875 (8) | 0.0257 (6) | |
| H7A | 0.1104 | 0.5813 | 0.0273 | 0.031* | |
| C8 | 0.1521 (3) | 0.5079 (3) | 0.07283 (8) | 0.0217 (5) | |
| C9 | 0.2374 (4) | 0.5422 (3) | 0.10692 (8) | 0.0299 (7) | |
| H9A | 0.3193 | 0.5367 | 0.0989 | 0.036* | |
| C10 | 0.2987 (3) | 0.6922 (3) | 0.11976 (8) | 0.0206 (5) | |
| C11 | 0.1746 (4) | 0.4342 (3) | 0.13671 (7) | 0.0252 (6) | |
| H11A | 0.1766 | 0.4589 | 0.1617 | 0.030* | |
| C12 | 0.1076 (4) | 0.2838 (3) | 0.12501 (7) | 0.0277 (7) | |
| H12A | 0.0164 | 0.2323 | 0.1367 | 0.033* | |
| H12B | 0.1639 | 0.2453 | 0.1342 | 0.033* | |
| C13 | 0.0908 (3) | 0.2624 (3) | 0.08335 (7) | 0.0180 (5) | |
| C14 | 0.0440 (3) | 0.3596 (3) | 0.06687 (7) | 0.0176 (5) | |
| C15 | 0.0125 (4) | 0.3087 (3) | 0.02691 (8) | 0.0273 (6) | |
| H15A | 0.0967 | 0.3517 | 0.0119 | 0.033* | |
| H15B | −0.0549 | 0.3301 | 0.0159 | 0.033* | |
| C16 | −0.0472 (3) | 0.1517 (3) | 0.02956 (7) | 0.0237 (6) | |
| H16A | 0.0009 | 0.1221 | 0.0124 | 0.028* | |
| H16B | −0.1458 | 0.1027 | 0.0234 | 0.028* | |
| C17 | −0.0255 (3) | 0.1210 (3) | 0.06995 (7) | 0.0180 (5) | |
| H17A | −0.1100 | 0.1000 | 0.0840 | 0.022* | |
| C18 | 0.2267 (3) | 0.2887 (4) | 0.06574 (10) | 0.0294 (7) | |
| H18A | 0.2646 | 0.2420 | 0.0801 | 0.044* | |
| H18B | 0.2920 | 0.3872 | 0.0654 | 0.044* | |
| H18C | 0.2094 | 0.2536 | 0.0406 | 0.044* | |
| C19 | 0.1860 (4) | 0.7155 (4) | 0.13545 (15) | 0.0473 (11) | |
| H19A | 0.1395 | 0.6511 | 0.1556 | 0.071* | |
| H19B | 0.1197 | 0.7011 | 0.1161 | 0.071* | |
| H19C | 0.2267 | 0.8094 | 0.1447 | 0.071* | |
| C20 | −0.0046 (3) | −0.0042 (3) | 0.07384 (7) | 0.0191 (5) | |
| H20A | 0.0854 | 0.0187 | 0.0627 | 0.023* | |
| C21 | −0.0021 (3) | −0.0331 (3) | 0.11447 (7) | 0.0184 (5) | |
| C22 | −0.1189 (3) | −0.1329 (3) | 0.05494 (7) | 0.0215 (5) | |
| H22A | −0.2073 | −0.1581 | 0.0668 | 0.026* | |
| H22B | −0.1249 | −0.1112 | 0.0289 | 0.026* | |
| C23 | −0.0965 (4) | −0.2561 (4) | 0.05649 (9) | 0.0274 (6) | |
| H23A | −0.0041 | −0.2285 | 0.0470 | 0.033* | |
| H23B | −0.1006 | −0.2846 | 0.0824 | 0.033* | |
| C24 | −0.2013 (4) | −0.3758 (4) | 0.03450 (8) | 0.0269 (6) | |
| H24A | −0.1861 | −0.3718 | 0.0088 | 0.032* | |
| C25 | −0.3113 (4) | −0.4852 (4) | 0.04663 (8) | 0.0288 (6) | |
| C26 | −0.3528 (5) | −0.5105 (6) | 0.08649 (10) | 0.0518 (12) | |
| H26A | −0.2775 | −0.4437 | 0.1018 | 0.078* | |
| H26B | −0.3741 | −0.6031 | 0.0933 | 0.078* | |
| H26C | −0.4337 | −0.5013 | 0.0903 | 0.078* | |
| C27 | −0.4081 (5) | −0.5985 (4) | 0.02111 (11) | 0.0418 (9) | |
| H27A | −0.3788 | −0.5722 | −0.0043 | 0.063* | |
| H27B | −0.5010 | −0.6138 | 0.0242 | 0.063* | |
| H27C | −0.4072 | −0.6828 | 0.0269 | 0.063* | |
| C28 | 0.5494 (4) | 0.9980 (4) | 0.05503 (9) | 0.0338 (8) | |
| H28A | 0.6234 | 1.0919 | 0.0593 | 0.051* | |
| H28B | 0.4860 | 0.9982 | 0.0367 | 0.051* | |
| H28C | 0.5880 | 0.9425 | 0.0461 | 0.051* | |
| C29 | 0.4101 (4) | 1.0252 (4) | 0.10225 (15) | 0.0462 (10) | |
| H29A | 0.4819 | 1.1217 | 0.1028 | 0.069* | |
| H29B | 0.3689 | 0.9975 | 0.1267 | 0.069* | |
| H29C | 0.3397 | 1.0127 | 0.0844 | 0.069* | |
| C30 | −0.0905 (3) | 0.3400 (3) | 0.08398 (10) | 0.0267 (6) | |
| H30A | −0.1171 | 0.4003 | 0.0716 | 0.040* | |
| H30B | −0.0763 | 0.3627 | 0.1102 | 0.040* | |
| H30C | −0.1631 | 0.2446 | 0.0810 | 0.040* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0217 (10) | 0.0320 (12) | 0.0167 (7) | 0.0165 (9) | 0.0016 (8) | 0.0012 (8) |
| O2 | 0.0198 (10) | 0.0313 (11) | 0.0140 (7) | 0.0099 (9) | 0.0026 (7) | 0.0030 (8) |
| O3 | 0.0163 (12) | 0.053 (2) | 0.0305 (13) | 0.0177 (13) | −0.0012 (10) | −0.0023 (13) |
| F1 | 0.040 (13) | 0.043 (14) | 0.053 (14) | 0.029 (11) | −0.008 (11) | −0.003 (10) |
| C1 | 0.0229 (14) | 0.0342 (17) | 0.0131 (10) | 0.0067 (13) | −0.0047 (10) | −0.0013 (11) |
| C2 | 0.0304 (16) | 0.0350 (18) | 0.0138 (11) | 0.0003 (15) | −0.0012 (12) | −0.0055 (12) |
| C3 | 0.0149 (12) | 0.0386 (18) | 0.0187 (11) | 0.0049 (13) | −0.0020 (11) | −0.0049 (12) |
| C4 | 0.0150 (12) | 0.0214 (14) | 0.0306 (14) | 0.0033 (11) | −0.0048 (11) | −0.0039 (12) |
| C5 | 0.0181 (13) | 0.0188 (13) | 0.0281 (13) | 0.0037 (11) | −0.0092 (11) | −0.0005 (11) |
| C6 | 0.042 (2) | 0.0203 (16) | 0.064 (3) | −0.0024 (15) | −0.035 (2) | 0.0129 (17) |
| C7 | 0.0215 (13) | 0.0218 (14) | 0.0230 (12) | 0.0027 (12) | −0.0109 (11) | 0.0036 (12) |
| C8 | 0.0145 (12) | 0.0195 (13) | 0.0246 (12) | 0.0036 (10) | −0.0066 (10) | 0.0032 (11) |
| C9 | 0.0392 (18) | 0.0205 (14) | 0.0191 (11) | 0.0068 (14) | −0.0115 (13) | 0.0016 (11) |
| C10 | 0.0146 (12) | 0.0222 (13) | 0.0229 (11) | 0.0078 (10) | −0.0029 (10) | −0.0015 (10) |
| C11 | 0.0350 (17) | 0.0234 (13) | 0.0114 (10) | 0.0103 (13) | 0.0001 (12) | 0.0007 (10) |
| C12 | 0.045 (2) | 0.0203 (13) | 0.0131 (10) | 0.0129 (14) | −0.0069 (13) | 0.0013 (10) |
| C13 | 0.0182 (12) | 0.0217 (13) | 0.0133 (9) | 0.0093 (11) | −0.0010 (9) | 0.0026 (9) |
| C14 | 0.0158 (12) | 0.0164 (12) | 0.0139 (10) | 0.0031 (10) | −0.0026 (9) | 0.0020 (10) |
| C15 | 0.0366 (17) | 0.0253 (14) | 0.0160 (11) | 0.0125 (14) | −0.0072 (12) | 0.0045 (11) |
| C16 | 0.0257 (14) | 0.0232 (14) | 0.0157 (10) | 0.0075 (12) | −0.0049 (11) | 0.0005 (11) |
| C17 | 0.0177 (12) | 0.0189 (12) | 0.0128 (9) | 0.0056 (10) | 0.0007 (9) | 0.0007 (9) |
| C18 | 0.0154 (13) | 0.0244 (16) | 0.0435 (17) | 0.0062 (12) | 0.0006 (13) | 0.0051 (14) |
| C19 | 0.0253 (17) | 0.0253 (17) | 0.090 (3) | 0.0118 (15) | 0.022 (2) | 0.010 (2) |
| C20 | 0.0190 (12) | 0.0225 (13) | 0.0137 (9) | 0.0088 (11) | 0.0023 (9) | 0.0024 (10) |
| C21 | 0.0219 (13) | 0.0179 (12) | 0.0159 (9) | 0.0104 (11) | 0.0010 (10) | −0.0002 (9) |
| C22 | 0.0256 (14) | 0.0227 (13) | 0.0142 (9) | 0.0107 (12) | −0.0007 (10) | −0.0005 (10) |
| C23 | 0.0264 (15) | 0.0269 (16) | 0.0289 (14) | 0.0134 (13) | 0.0018 (12) | −0.0012 (12) |
| C24 | 0.0357 (18) | 0.0276 (15) | 0.0185 (11) | 0.0166 (14) | 0.0020 (12) | −0.0010 (11) |
| C25 | 0.0286 (16) | 0.0325 (16) | 0.0245 (13) | 0.0147 (14) | −0.0019 (12) | −0.0009 (12) |
| C26 | 0.033 (2) | 0.068 (3) | 0.0296 (16) | 0.007 (2) | 0.0078 (16) | 0.0082 (19) |
| C27 | 0.040 (2) | 0.036 (2) | 0.0409 (18) | 0.0125 (18) | −0.0050 (17) | −0.0098 (17) |
| C28 | 0.0324 (18) | 0.0280 (16) | 0.0263 (14) | 0.0041 (14) | −0.0089 (13) | 0.0011 (13) |
| C29 | 0.0299 (19) | 0.0209 (16) | 0.084 (3) | 0.0097 (15) | 0.005 (2) | 0.0034 (19) |
| C30 | 0.0164 (13) | 0.0207 (14) | 0.0383 (16) | 0.0059 (12) | 0.0006 (12) | 0.0005 (13) |
Geometric parameters (Å, °)
| O1—C21 | 1.312 (4) | C14—C30 | 1.552 (4) |
| O1—H1O1 | 0.8651 | C15—C16 | 1.552 (5) |
| O2—C21 | 1.229 (3) | C15—H15A | 0.9900 |
| O3—C3 | 1.406 (5) | C15—H15B | 0.9900 |
| O3—H3A | 0.8400 | C16—C17 | 1.559 (4) |
| O3—H3C | 0.5288 | C16—H16A | 0.9900 |
| F1—C3 | 1.27 (3) | C16—H16B | 0.9900 |
| C1—C2 | 1.534 (5) | C17—C20 | 1.551 (4) |
| C1—C10 | 1.539 (4) | C17—H17A | 1.0000 |
| C1—H1A | 0.9900 | C18—H18A | 0.9800 |
| C1—H1B | 0.9900 | C18—H18B | 0.9800 |
| C2—C3 | 1.529 (4) | C18—H18C | 0.9800 |
| C2—H2A | 0.9900 | C19—H19A | 0.9800 |
| C2—H2B | 0.9900 | C19—H19B | 0.9800 |
| C3—C4 | 1.521 (4) | C19—H19C | 0.9800 |
| C3—H3B | 1.0000 | C20—C21 | 1.519 (3) |
| C3—H3C | 0.9601 | C20—C22 | 1.541 (4) |
| C4—C29 | 1.532 (5) | C20—H20A | 1.0000 |
| C4—C28 | 1.535 (5) | C22—C23 | 1.533 (5) |
| C4—C5 | 1.562 (4) | C22—H22A | 0.9900 |
| C5—C6 | 1.528 (4) | C22—H22B | 0.9900 |
| C5—C10 | 1.568 (4) | C23—C24 | 1.506 (5) |
| C5—H5A | 1.0000 | C23—H23A | 0.9900 |
| C6—C7 | 1.491 (5) | C23—H23B | 0.9900 |
| C6—H6A | 0.9900 | C24—C25 | 1.314 (5) |
| C6—H6B | 0.9900 | C24—H24A | 0.9500 |
| C7—C8 | 1.376 (4) | C25—C26 | 1.509 (5) |
| C7—H7A | 0.9500 | C25—C27 | 1.516 (5) |
| C8—C9 | 1.499 (4) | C26—H26A | 0.9800 |
| C8—C14 | 1.516 (4) | C26—H26B | 0.9800 |
| C9—C11 | 1.517 (4) | C26—H26C | 0.9800 |
| C9—C10 | 1.547 (4) | C27—H27A | 0.9800 |
| C9—H9A | 1.0000 | C27—H27B | 0.9800 |
| C10—C19 | 1.533 (5) | C27—H27C | 0.9800 |
| C11—C12 | 1.534 (4) | C28—H28A | 0.9800 |
| C11—H11A | 0.9500 | C28—H28B | 0.9800 |
| C12—C13 | 1.534 (4) | C28—H28C | 0.9800 |
| C12—H12A | 0.9900 | C29—H29A | 0.9800 |
| C12—H12B | 0.9900 | C29—H29B | 0.9800 |
| C13—C18 | 1.548 (4) | C29—H29C | 0.9800 |
| C13—C17 | 1.554 (4) | C30—H30A | 0.9800 |
| C13—C14 | 1.556 (4) | C30—H30B | 0.9800 |
| C14—C15 | 1.540 (4) | C30—H30C | 0.9800 |
| C21—O1—H1O1 | 107.6 | C14—C15—C16 | 104.7 (2) |
| C3—O3—H3A | 109.5 | C14—C15—H15A | 110.8 |
| C3—O3—H3C | 26.1 | C16—C15—H15A | 110.8 |
| H3A—O3—H3C | 121.0 | C14—C15—H15B | 110.8 |
| C2—C1—C10 | 113.4 (3) | C16—C15—H15B | 110.8 |
| C2—C1—H1A | 108.9 | H15A—C15—H15B | 108.9 |
| C10—C1—H1A | 108.9 | C15—C16—C17 | 106.6 (2) |
| C2—C1—H1B | 108.9 | C15—C16—H16A | 110.4 |
| C10—C1—H1B | 108.9 | C17—C16—H16A | 110.4 |
| H1A—C1—H1B | 107.7 | C15—C16—H16B | 110.4 |
| C3—C2—C1 | 110.9 (2) | C17—C16—H16B | 110.4 |
| C3—C2—H2A | 109.5 | H16A—C16—H16B | 108.6 |
| C1—C2—H2A | 109.5 | C20—C17—C13 | 118.1 (2) |
| C3—C2—H2B | 109.5 | C20—C17—C16 | 113.6 (2) |
| C1—C2—H2B | 109.5 | C13—C17—C16 | 102.4 (2) |
| H2A—C2—H2B | 108.0 | C20—C17—H17A | 107.4 |
| F1—C3—O3 | 82.0 (11) | C13—C17—H17A | 107.4 |
| F1—C3—C4 | 131.7 (11) | C16—C17—H17A | 107.4 |
| O3—C3—C4 | 107.4 (2) | C13—C18—H18A | 109.5 |
| F1—C3—C2 | 107.2 (11) | C13—C18—H18B | 109.5 |
| O3—C3—C2 | 110.9 (3) | H18A—C18—H18B | 109.5 |
| C4—C3—C2 | 112.4 (3) | C13—C18—H18C | 109.5 |
| F1—C3—H3B | 30.0 | H18A—C18—H18C | 109.5 |
| O3—C3—H3B | 108.7 | H18B—C18—H18C | 109.5 |
| C4—C3—H3B | 108.7 | C10—C19—H19A | 109.5 |
| C2—C3—H3B | 108.7 | C10—C19—H19B | 109.5 |
| F1—C3—H3C | 95.1 | H19A—C19—H19B | 109.5 |
| O3—C3—H3C | 14.0 | C10—C19—H19C | 109.5 |
| C4—C3—H3C | 102.1 | H19A—C19—H19C | 109.5 |
| C2—C3—H3C | 102.2 | H19B—C19—H19C | 109.5 |
| H3B—C3—H3C | 122.6 | C21—C20—C22 | 109.3 (2) |
| C3—C4—C29 | 109.3 (3) | C21—C20—C17 | 108.2 (2) |
| C3—C4—C28 | 108.7 (3) | C22—C20—C17 | 112.4 (2) |
| C29—C4—C28 | 106.2 (3) | C21—C20—H20A | 109.0 |
| C3—C4—C5 | 108.2 (3) | C22—C20—H20A | 109.0 |
| C29—C4—C5 | 115.7 (3) | C17—C20—H20A | 109.0 |
| C28—C4—C5 | 108.6 (3) | O2—C21—O1 | 122.7 (2) |
| C6—C5—C4 | 113.2 (3) | O2—C21—C20 | 122.5 (3) |
| C6—C5—C10 | 111.1 (3) | O1—C21—C20 | 114.8 (2) |
| C4—C5—C10 | 117.6 (2) | C23—C22—C20 | 113.5 (3) |
| C6—C5—H5A | 104.5 | C23—C22—H22A | 108.9 |
| C4—C5—H5A | 104.5 | C20—C22—H22A | 108.9 |
| C10—C5—H5A | 104.5 | C23—C22—H22B | 108.9 |
| C7—C6—C5 | 113.2 (3) | C20—C22—H22B | 108.9 |
| C7—C6—H6A | 108.9 | H22A—C22—H22B | 107.7 |
| C5—C6—H6A | 108.9 | C24—C23—C22 | 112.5 (3) |
| C7—C6—H6B | 108.9 | C24—C23—H23A | 109.1 |
| C5—C6—H6B | 108.9 | C22—C23—H23A | 109.1 |
| H6A—C6—H6B | 107.7 | C24—C23—H23B | 109.1 |
| C8—C7—C6 | 122.4 (3) | C22—C23—H23B | 109.1 |
| C8—C7—H7A | 118.8 | H23A—C23—H23B | 107.8 |
| C6—C7—H7A | 118.8 | C25—C24—C23 | 127.8 (3) |
| C7—C8—C9 | 121.6 (3) | C25—C24—H24A | 116.1 |
| C7—C8—C14 | 120.8 (2) | C23—C24—H24A | 116.1 |
| C9—C8—C14 | 117.5 (2) | C24—C25—C26 | 124.0 (3) |
| C8—C9—C11 | 113.8 (3) | C24—C25—C27 | 122.0 (3) |
| C8—C9—C10 | 114.3 (3) | C26—C25—C27 | 114.0 (3) |
| C11—C9—C10 | 115.9 (2) | C25—C26—H26A | 109.5 |
| C8—C9—H9A | 103.6 | C25—C26—H26B | 109.5 |
| C11—C9—H9A | 103.6 | H26A—C26—H26B | 109.5 |
| C10—C9—H9A | 103.6 | C25—C26—H26C | 109.5 |
| C19—C10—C1 | 111.3 (3) | H26A—C26—H26C | 109.5 |
| C19—C10—C9 | 110.2 (3) | H26B—C26—H26C | 109.5 |
| C1—C10—C9 | 108.4 (3) | C25—C27—H27A | 109.5 |
| C19—C10—C5 | 112.4 (3) | C25—C27—H27B | 109.5 |
| C1—C10—C5 | 108.7 (2) | H27A—C27—H27B | 109.5 |
| C9—C10—C5 | 105.6 (2) | C25—C27—H27C | 109.5 |
| C9—C11—C12 | 117.6 (2) | H27A—C27—H27C | 109.5 |
| C9—C11—H11A | 121.2 | H27B—C27—H27C | 109.5 |
| C12—C11—H11A | 121.2 | C4—C28—H28A | 109.5 |
| C11—C12—C13 | 113.8 (2) | C4—C28—H28B | 109.5 |
| C11—C12—H12A | 108.8 | H28A—C28—H28B | 109.5 |
| C13—C12—H12A | 108.8 | C4—C28—H28C | 109.5 |
| C11—C12—H12B | 108.8 | H28A—C28—H28C | 109.5 |
| C13—C12—H12B | 108.8 | H28B—C28—H28C | 109.5 |
| H12A—C12—H12B | 107.7 | C4—C29—H29A | 109.5 |
| C12—C13—C18 | 110.3 (3) | C4—C29—H29B | 109.5 |
| C12—C13—C17 | 116.6 (2) | H29A—C29—H29B | 109.5 |
| C18—C13—C17 | 108.3 (2) | C4—C29—H29C | 109.5 |
| C12—C13—C14 | 109.3 (2) | H29A—C29—H29C | 109.5 |
| C18—C13—C14 | 111.0 (2) | H29B—C29—H29C | 109.5 |
| C17—C13—C14 | 101.1 (2) | C14—C30—H30A | 109.5 |
| C8—C14—C15 | 117.1 (2) | C14—C30—H30B | 109.5 |
| C8—C14—C30 | 106.8 (3) | H30A—C30—H30B | 109.5 |
| C15—C14—C30 | 107.4 (3) | C14—C30—H30C | 109.5 |
| C8—C14—C13 | 110.7 (2) | H30A—C30—H30C | 109.5 |
| C15—C14—C13 | 101.5 (2) | H30B—C30—H30C | 109.5 |
| C30—C14—C13 | 113.4 (2) | ||
| C10—C1—C2—C3 | −57.4 (4) | C9—C11—C12—C13 | −10.5 (5) |
| C1—C2—C3—F1 | −148.0 (12) | C11—C12—C13—C18 | 81.3 (4) |
| C1—C2—C3—O3 | −60.1 (4) | C11—C12—C13—C17 | −154.6 (3) |
| C1—C2—C3—C4 | 60.2 (4) | C11—C12—C13—C14 | −40.9 (4) |
| F1—C3—C4—C29 | −70.9 (16) | C7—C8—C14—C15 | 35.8 (4) |
| O3—C3—C4—C29 | −165.7 (3) | C9—C8—C14—C15 | −147.8 (3) |
| C2—C3—C4—C29 | 72.0 (4) | C7—C8—C14—C30 | −84.6 (4) |
| F1—C3—C4—C28 | 44.6 (16) | C9—C8—C14—C30 | 91.8 (3) |
| O3—C3—C4—C28 | −50.3 (4) | C7—C8—C14—C13 | 151.4 (3) |
| C2—C3—C4—C28 | −172.6 (3) | C9—C8—C14—C13 | −32.2 (4) |
| F1—C3—C4—C5 | 162.4 (15) | C12—C13—C14—C8 | 63.1 (3) |
| O3—C3—C4—C5 | 67.5 (3) | C18—C13—C14—C8 | −58.8 (3) |
| C2—C3—C4—C5 | −54.8 (4) | C17—C13—C14—C8 | −173.5 (2) |
| C3—C4—C5—C6 | −177.7 (3) | C12—C13—C14—C15 | −171.9 (3) |
| C29—C4—C5—C6 | 59.3 (5) | C18—C13—C14—C15 | 66.2 (3) |
| C28—C4—C5—C6 | −59.9 (4) | C17—C13—C14—C15 | −48.5 (3) |
| C3—C4—C5—C10 | 50.5 (4) | C12—C13—C14—C30 | −57.0 (3) |
| C29—C4—C5—C10 | −72.5 (4) | C18—C13—C14—C30 | −178.9 (3) |
| C28—C4—C5—C10 | 168.3 (3) | C17—C13—C14—C30 | 66.4 (3) |
| C4—C5—C6—C7 | 178.8 (4) | C8—C14—C15—C16 | 156.0 (3) |
| C10—C5—C6—C7 | −46.3 (5) | C30—C14—C15—C16 | −83.9 (3) |
| C5—C6—C7—C8 | 14.2 (6) | C13—C14—C15—C16 | 35.4 (3) |
| C6—C7—C8—C9 | −1.1 (6) | C14—C15—C16—C17 | −9.2 (3) |
| C6—C7—C8—C14 | 175.2 (4) | C12—C13—C17—C20 | −73.7 (3) |
| C7—C8—C9—C11 | 157.2 (3) | C18—C13—C17—C20 | 51.4 (3) |
| C14—C8—C9—C11 | −19.2 (4) | C14—C13—C17—C20 | 168.0 (2) |
| C7—C8—C9—C10 | 21.0 (5) | C12—C13—C17—C16 | 160.6 (3) |
| C14—C8—C9—C10 | −155.4 (3) | C18—C13—C17—C16 | −74.3 (3) |
| C2—C1—C10—C19 | −74.9 (3) | C14—C13—C17—C16 | 42.4 (3) |
| C2—C1—C10—C9 | 163.8 (3) | C15—C16—C17—C20 | −149.2 (3) |
| C2—C1—C10—C5 | 49.5 (3) | C15—C16—C17—C13 | −20.7 (3) |
| C8—C9—C10—C19 | 71.6 (4) | C13—C17—C20—C21 | 66.8 (3) |
| C11—C9—C10—C19 | −63.7 (4) | C16—C17—C20—C21 | −173.3 (2) |
| C8—C9—C10—C1 | −166.4 (3) | C13—C17—C20—C22 | −172.4 (2) |
| C11—C9—C10—C1 | 58.4 (4) | C16—C17—C20—C22 | −52.5 (3) |
| C8—C9—C10—C5 | −50.0 (4) | C22—C20—C21—O2 | −49.3 (4) |
| C11—C9—C10—C5 | 174.7 (3) | C17—C20—C21—O2 | 73.4 (4) |
| C6—C5—C10—C19 | −56.8 (4) | C22—C20—C21—O1 | 130.6 (3) |
| C4—C5—C10—C19 | 75.9 (4) | C17—C20—C21—O1 | −106.7 (3) |
| C6—C5—C10—C1 | 179.5 (3) | C21—C20—C22—C23 | −63.1 (3) |
| C4—C5—C10—C1 | −47.7 (4) | C17—C20—C22—C23 | 176.8 (2) |
| C6—C5—C10—C9 | 63.4 (4) | C20—C22—C23—C24 | −174.0 (2) |
| C4—C5—C10—C9 | −163.9 (3) | C22—C23—C24—C25 | −99.7 (4) |
| C8—C9—C11—C12 | 42.2 (5) | C23—C24—C25—C26 | −0.2 (7) |
| C10—C9—C11—C12 | 177.7 (3) | C23—C24—C25—C27 | −179.9 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1O1···O2i | 0.87 | 1.81 | 2.654 (3) | 165 |
| O3—H3A···O2ii | 0.84 | 2.04 | 2.818 (4) | 154 |
| C12—H12B···O1 | 0.99 | 2.56 | 3.262 (4) | 128 |
| C22—H22A···O3iii | 0.99 | 2.40 | 3.300 (5) | 151 |
Symmetry codes: (i) −x, −x+y, −z+1/3; (ii) x+1, y+1, z; (iii) x−1, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RZ2572).
References
- Atawodi, S. E. (2010). Adv. Biol. Res. 4, 314–322.
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, 105–107.
- Dongmo, P. M., Tchoumbougnang, F., Ndongson, B., Agwannande, W., Sandjon, B., Zollo, P. H. A. & Menut, C. (2010). Agri. Biol. J. N. Am. 1 pp. 606–6011.
- Mora, A. J., Delgado, G., Díaz de Delgado, G., Usubillaga, A., Khouri, N. & Bahsas, A. (2001). Acta Cryst. C57, 638–640. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Yousuf, S., Kamdem, R. S. T., Ngadjui, B. T., Wafo, P. & Fun, H.-K. (2011). Acta Cryst. E67, o937–o938. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811011159/rz2572sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811011159/rz2572Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report