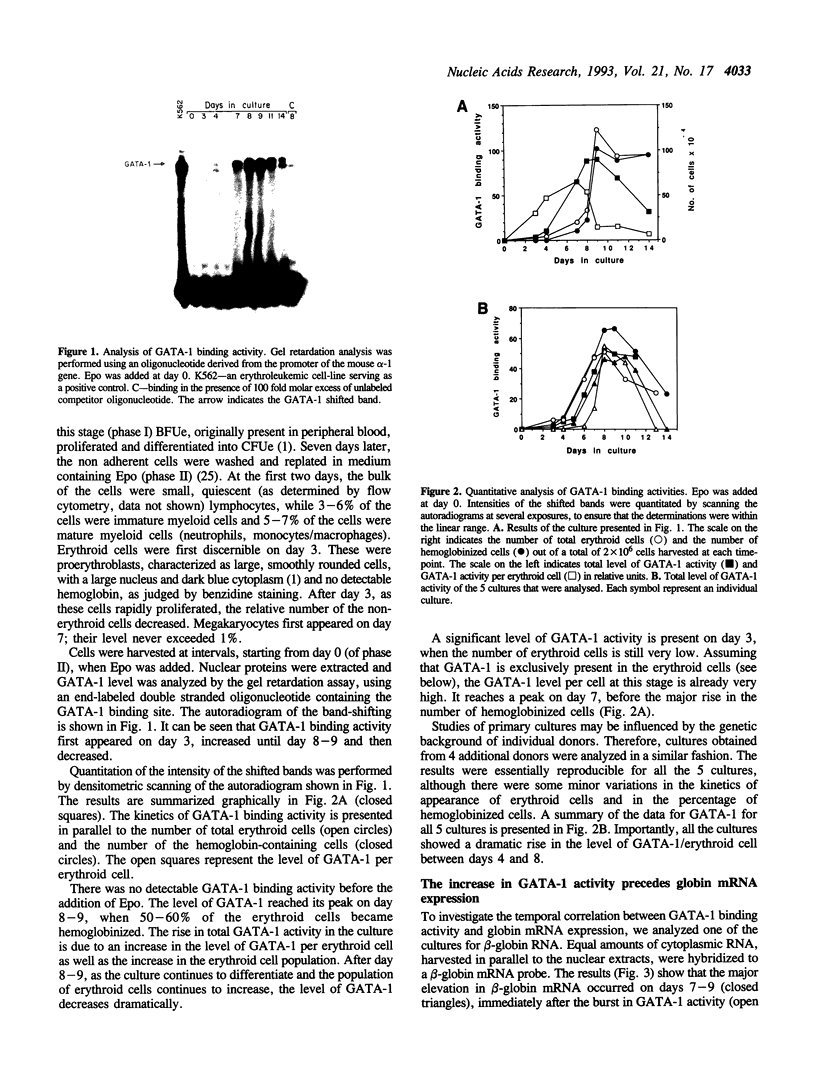
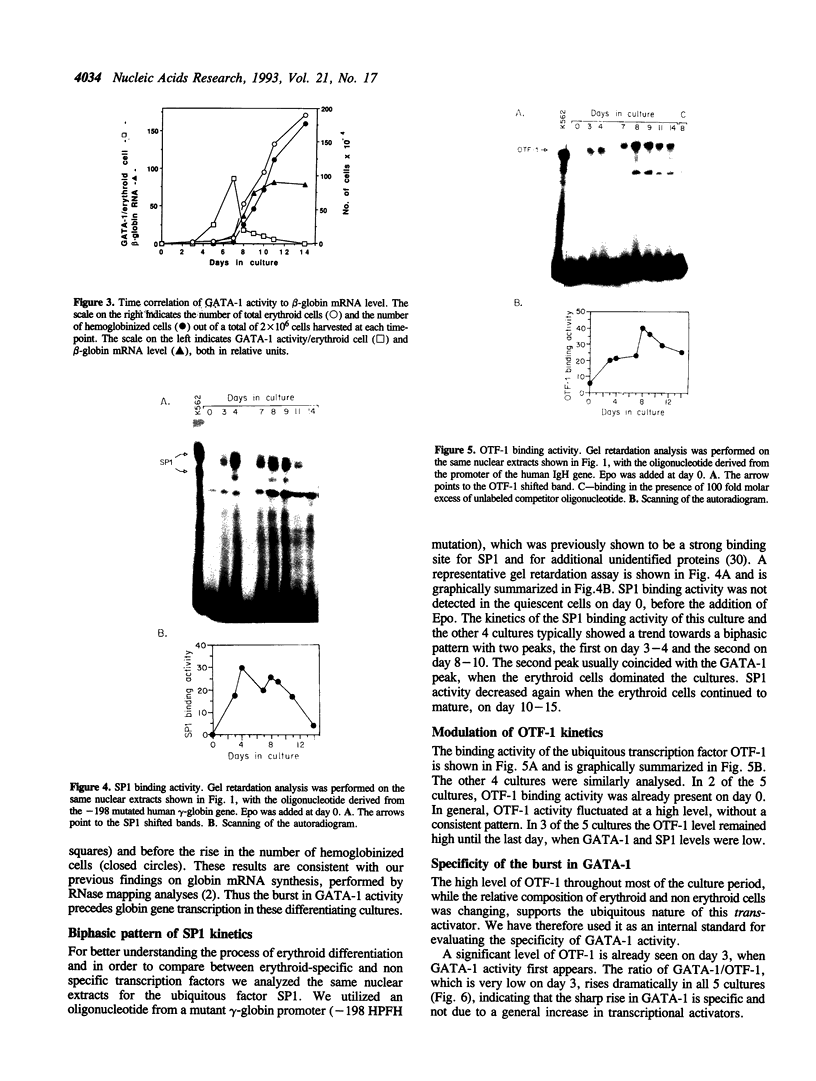
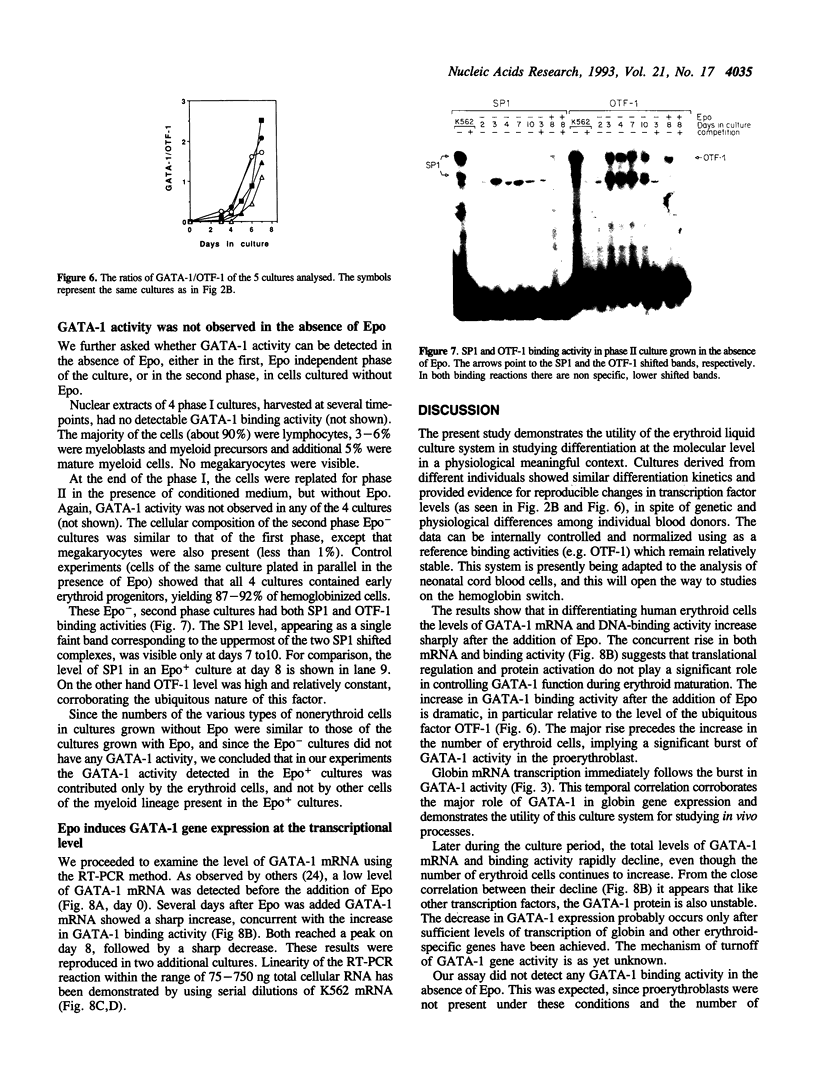
Abstract
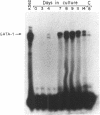
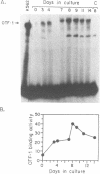
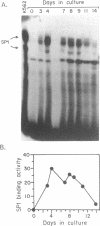
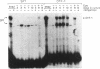
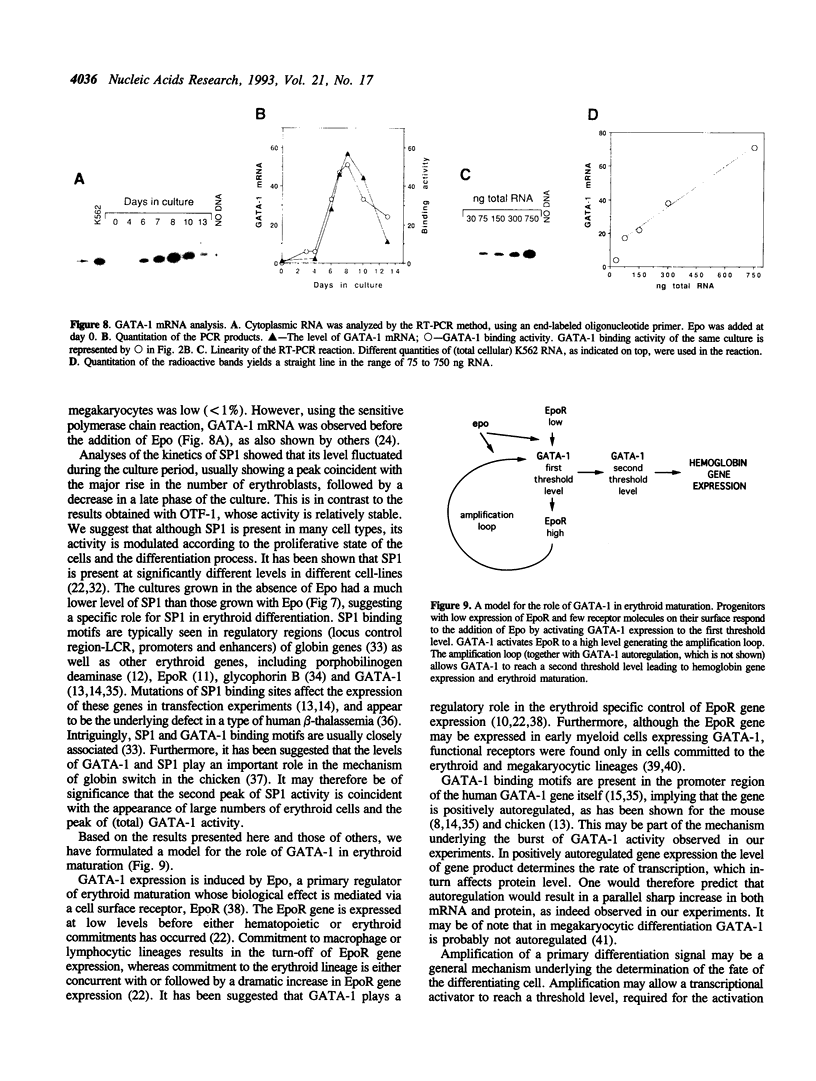
GATA-1 is a central transcription-activator of erythroid differentiation. In the present work we have studied the kinetics of its expression and activity during development of normal human erythroid progenitors, grown in primary cultures. In response to the addition of erythropoietin (Epo), the cells undergo proliferation and differentiation in a synchronized fashion. This recently developed experimental system allows biochemical dissection of erythroid differentiation in a physiological meaningful environment. No DNA-binding activity of GATA-1 could be detected before the addition of Epo, although a very low level of mRNA was observed. Following Epo addition there was a sharp parallel rise in both mRNA and DNA-binding activity, consistent with positive autoregulation of the GATA-1 gene. After reaching a peak on day 7-9, both mRNA and protein activity decreased. The binding activity of the ubiquitous factor SP1 showed a biphasic pattern; its second peak usually coincided with the GATA-1 peak, suggesting that SP1 also plays a specific role in erythroid maturation. The highest activity of GATA-1 per erythroid cell was found on day 6-8, immediately preceding the major rise in globin gene mRNA and in the number of hemoglobinized cells. The results imply that a high level of GATA-1 activity is necessary for globin gene expression and erythroid maturation, suggesting that a requirement for a threshold concentration of GATA-1 is part of the mechanism that determines the final steps of erythroid maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Erdjument-Bromage H., Davidson M. B., Tempst P., Orkin S. H. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993 Apr 22;362(6422):722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Chiba T., Ikawa Y., Todokoro K. GATA-1 transactivates erythropoietin receptor gene, and erythropoietin receptor-mediated signals enhance GATA-1 gene expression. Nucleic Acids Res. 1991 Jul 25;19(14):3843–3848. doi: 10.1093/nar/19.14.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crotta S., Nicolis S., Ronchi A., Ottolenghi S., Ruzzi L., Shimada Y., Migliaccio A. R., Migliaccio G. Progressive inactivation of the expression of an erythroid transcriptional factor in GM- and G-CSF-dependent myeloid cell lines. Nucleic Acids Res. 1990 Dec 11;18(23):6863–6869. doi: 10.1093/nar/18.23.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalyot N., Fibach E., Rachmilewitz E. A., Oppenheim A. Adult and neonatal patterns of human globin gene expression are recapitulated in liquid cultures. Exp Hematol. 1992 Oct;20(9):1141–1145. [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989 Jan 12;337(6203):138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- Engel J. D., George K. M., Ko L. J., Kornhauser J. M., Leonard M. W., Ting P., Yamamoto M. Transcription factor regulation of hematopoietic lineage cells. Semin Hematol. 1991 Apr;28(2):158–169. [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Fibach E., Manor D., Oppenheim A., Rachmilewitz E. A. Proliferation and maturation of human erythroid progenitors in liquid culture. Blood. 1989 Jan;73(1):100–103. [PubMed] [Google Scholar]
- Fibach E., Manor D., Treves A., Rachmilewitz E. A. Growth of human normal erythroid progenitors in liquid culture: a comparison with colony growth in semisolid culture. Int J Cell Cloning. 1991 Jan;9(1):57–64. doi: 10.1002/stem.5530090108. [DOI] [PubMed] [Google Scholar]
- Fraser J. K., Tan A. S., Lin F. K., Berridge M. V. Expression of specific high-affinity binding sites for erythropoietin on rat and mouse megakaryocytes. Exp Hematol. 1989 Jan;17(1):10–16. [PubMed] [Google Scholar]
- Hannon R., Evans T., Felsenfeld G., Gould H. Structure and promoter activity of the gene for the erythroid transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3004–3008. doi: 10.1073/pnas.88.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein C., Fischer K. D., Stoffel M., Nowock J., Ford A., Tessmer U., Stocking C. The gene for erythropoietin receptor is expressed in multipotential hematopoietic and embryonal stem cells: evidence for differentiation stage-specific regulation. Mol Cell Biol. 1992 Apr;12(4):1815–1826. doi: 10.1128/mcb.12.4.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E., Toki T., Ishihara H., Ohtani H., Gu L., Yokoyama M., Engel J. D., Yamamoto M. Erythroid transcription factor GATA-1 is abundantly transcribed in mouse testis. Nature. 1993 Apr 1;362(6419):466–468. doi: 10.1038/362466a0. [DOI] [PubMed] [Google Scholar]
- Laybourn P. J., Kadonaga J. T. Threshold phenomena and long-distance activation of transcription by RNA polymerase II. Science. 1992 Sep 18;257(5077):1682–1685. doi: 10.1126/science.1388287. [DOI] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Giglioni B., Comi P., Cappellini N., Nicolis S., Ottolenghi S. A protein factor binding to an octamer motif in the gamma-globin promoter disappears upon induction of differentiation and hemoglobin synthesis in K562 cells. Nucleic Acids Res. 1987 Nov 25;15(22):9349–9364. doi: 10.1093/nar/15.22.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Nicolis S., Giglioni B., Comi P., Cappellini N., Bertero M. T., Caligaris-Cappio F., Ottolenghi S. An erythroid specific nuclear factor binding to the proximal CACCC box of the beta-globin gene promoter. Nucleic Acids Res. 1988 May 25;16(10):4299–4313. doi: 10.1093/nar/16.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Nicolis S., Ronchi A., Giglioni B., Ottolenghi S. The effects of HPFH mutations in the human gamma-globin promoter on binding of ubiquitous and erythroid specific nuclear factors. Nucleic Acids Res. 1988 Aug 25;16(16):7783–7797. doi: 10.1093/nar/16.16.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. I., Orkin S. H. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990 Nov;4(11):1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Zon L. I., Mutter G., Orkin S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Mason-Garcia M., Clejan S., Tou J. S., Beckman B. S. Signal transduction by the erythropoietin receptor: evidence for the activation of phospholipases A2 and C. Am J Physiol. 1992 May;262(5 Pt 1):C1197–C1203. doi: 10.1152/ajpcell.1992.262.5.C1197. [DOI] [PubMed] [Google Scholar]
- Migliaccio A. R., Migliaccio G., D'Andrea A., Baiocchi M., Crotta S., Nicolis S., Ottolenghi S., Adamson J. W. Response to erythropoietin in erythroid subclones of the factor-dependent cell line 32D is determined by translocation of the erythropoietin receptor to the cell surface. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11086–11090. doi: 10.1073/pnas.88.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte V., Eleouet J. F., Raich N., Romeo P. H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6548–6552. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minie M. E., Kimura T., Felsenfeld G. The developmental switch in embryonic rho-globin expression is correlated with erythroid lineage-specific differences in transcription factor levels. Development. 1992 Aug;115(4):1149–1164. doi: 10.1242/dev.115.4.1149. [DOI] [PubMed] [Google Scholar]
- Nicolis S., Bertini C., Ronchi A., Crotta S., Lanfranco L., Moroni E., Giglioni B., Ottolenghi S. An erythroid specific enhancer upstream to the gene encoding the cell-type specific transcription factor GATA-1. Nucleic Acids Res. 1991 Oct 11;19(19):5285–5291. doi: 10.1093/nar/19.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H. GATA-binding transcription factors in hematopoietic cells. Blood. 1992 Aug 1;80(3):575–581. [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny L. A., Forget B. G. Genomic organization of the human erythropoietin receptor gene. Genomics. 1991 Dec;11(4):974–980. doi: 10.1016/0888-7543(91)90022-7. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Philipsen S., Talbot D., Fraser P., Grosveld F. The beta-globin dominant control region: hypersensitive site 2. EMBO J. 1990 Jul;9(7):2159–2167. doi: 10.1002/j.1460-2075.1990.tb07385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahuel C., Vinit M. A., Lemarchandel V., Cartron J. P., Roméo P. H. Erythroid-specific activity of the glycophorin B promoter requires GATA-1 mediated displacement of a repressor. EMBO J. 1992 Nov;11(11):4095–4102. doi: 10.1002/j.1460-2075.1992.tb05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Ronchi A., Nicolis S., Santoro C., Ottolenghi S. Increased Sp1 binding mediates erythroid-specific overexpression of a mutated (HPFH) gamma-globulin promoter. Nucleic Acids Res. 1989 Dec 25;17(24):10231–10241. doi: 10.1093/nar/17.24.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer J. D., Jackson S. P., Annarella M. B. Developmental expression of Sp1 in the mouse. Mol Cell Biol. 1991 Apr;11(4):2189–2199. doi: 10.1128/mcb.11.4.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Pevny L., Wiles M. V., Keller G., Costantini F., Orkin S. H. Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells. Nat Genet. 1992 May;1(2):92–98. doi: 10.1038/ng0592-92. [DOI] [PubMed] [Google Scholar]
- Sposi N. M., Zon L. I., Carè A., Valtieri M., Testa U., Gabbianelli M., Mariani G., Bottero L., Mather C., Orkin S. H. Cell cycle-dependent initiation and lineage-dependent abrogation of GATA-1 expression in pure differentiating hematopoietic progenitors. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6353–6357. doi: 10.1073/pnas.89.14.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Superti-Furga G., Barberis A., Schaffner G., Busslinger M. The -117 mutation in Greek HPFH affects the binding of three nuclear factors to the CCAAT region of the gamma-globin gene. EMBO J. 1988 Oct;7(10):3099–3107. doi: 10.1002/j.1460-2075.1988.tb03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Strauss E., Orkin S. H. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991 Jun;5(6):919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Elefanty A. G., Strasser A., Adams J. M. GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO J. 1992 Dec;11(12):4557–4564. doi: 10.1002/j.1460-2075.1992.tb05557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M., Martin D. I. Functional erythroid promoters created by interaction of the transcription factor GATA-1 with CACCC and AP-1/NFE-2 elements. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10444–10448. doi: 10.1073/pnas.89.21.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon L. I., Orkin S. H. Sequence of the human GATA-1 promoter. Nucleic Acids Res. 1992 Apr 11;20(7):1812–1812. doi: 10.1093/nar/20.7.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon L. I., Youssoufian H., Mather C., Lodish H. F., Orkin S. H. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]