Abstract
The title compound, poly[[μ-aqua-tetraaqua{μ-5-[bis(carboxylatomethyl)amino]-3-carboxylatomethyl-4-cyanothiophene-2-carboxylato}distrontium(II)] tetrahydrate], [Sr2(C12H6N2O8S)(H2O)5]·3.79H2O, crystallizes with nine- and eight-coordinated Sr2+ cations. They are bound to seven of the eight ranelate O atoms and five of the water molecules. The SrO8 and SrO9 polyhedra are interconnected by edge-sharing, forming hollow layers parallel to (011). The layers are, in turn, interconnected by ranelate anions, forming a metal–organic framework (MOF) structure with channels along the a axis. The four water molecules not coordinated to strontium are located in these channels and hydrogen bonded to each other and to the ranelates. Part of the water H atoms are disordered. The compound dehydrates very easily and 0.210 (4) water molecules out of nine were lost during crystal mounting causing additional disorder in the water structure.
Related literature
For the effect of strontium on osteroporosis, see Schrooten et al. (2003) ▶. For a patent describing the synthesis and powder diffraction pattern of the title compound, see Horvath et al. (2008 ▶). For related strontium carboxylate structures, see, for example: Stahl et al. (2006 ▶). 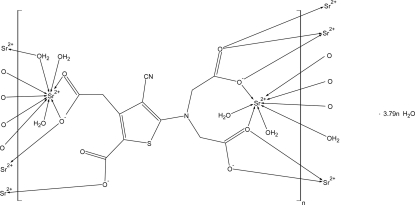
Experimental
Crystal data
[Sr2(C12H6N2O8S)(H2O)5]·3.79H2O
M r = 671.84
Triclinic,
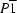
a = 8.3585 (3) Å
b = 12.3865 (5) Å
c = 12.6474 (5) Å
α = 109.880 (1)°
β = 97.148 (1)°
γ = 105.321 (1)°
V = 1154.00 (8) Å3
Z = 2
Mo Kα radiation
μ = 4.80 mm−1
T = 120 K
0.15 × 0.10 × 0.07 mm
Data collection
Bruker SMART APEX diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2002 ▶) T min = 0.574, T max = 0.710
17404 measured reflections
6617 independent reflections
5375 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.033
wR(F 2) = 0.075
S = 1.02
6617 reflections
375 parameters
21 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 1.48 e Å−3
Δρmin = −1.23 e Å−3
Data collection: SMART (Bruker, 1999 ▶); cell refinement: SAINT-Plus (Bruker, 1999 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and ATOMS (Dowty, 2000 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811010099/si2343sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811010099/si2343Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| Sr1—O8 | 2.4557 (18) |
| Sr1—O3i | 2.4782 (19) |
| Sr1—O5 | 2.5234 (16) |
| Sr1—O7ii | 2.6149 (19) |
| Sr1—O25 | 2.652 (2) |
| Sr1—O22 | 2.6560 (19) |
| Sr1—O27 | 2.657 (2) |
| Sr1—O8ii | 2.7834 (17) |
| Sr2—O6iii | 2.5452 (16) |
| Sr2—O23 | 2.5921 (18) |
| Sr2—O2i | 2.6222 (17) |
| Sr2—O21 | 2.6445 (17) |
| Sr2—O6 | 2.6628 (16) |
| Sr2—O2iv | 2.6848 (16) |
| Sr2—O1iv | 2.6944 (17) |
| Sr2—O22 | 2.7108 (18) |
| Sr2—O5 | 2.7228 (16) |
Symmetry codes: (i) 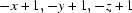 ; (ii)
; (ii) 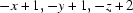 ; (iii)
; (iii) 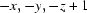 ; (iv)
; (iv) 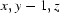 .
.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O21—H21A⋯O25 | 0.81 (2) | 1.98 (2) | 2.781 (3) | 169 (3) |
| O21—H21B⋯O24iii | 0.85 (2) | 1.93 (2) | 2.765 (3) | 169 (3) |
| O22—H22A⋯O2i | 0.81 (2) | 2.16 (3) | 2.761 (2) | 131 (3) |
| O22—H22B⋯O26iv | 0.82 (2) | 1.94 (2) | 2.755 (3) | 173 (3) |
| O23—H23A⋯O21iii | 0.81 (2) | 1.96 (2) | 2.766 (3) | 174 (4) |
| O23—H23B⋯O26i | 0.80 (2) | 2.11 (2) | 2.867 (3) | 159 (3) |
| O24—H24A⋯O1v | 0.82 (2) | 2.03 (2) | 2.760 (3) | 148 (3) |
| O24—H24B⋯O4 | 0.84 (2) | 1.93 (2) | 2.756 (3) | 172 (3) |
| O25—H25A⋯N1ii | 0.82 (2) | 2.15 (2) | 2.898 (3) | 152 (3) |
| O25—H25B⋯O27ii | 0.80 (2) | 1.93 (2) | 2.648 (3) | 150 (4) |
| O26—H26A⋯O28vi | 0.85 (2) | 1.90 (2) | 2.731 (3) | 164 (5) |
| O26—H26C⋯N1 | 0.85 (2) | 2.37 (4) | 3.108 (3) | 146 (5) |
| O27—H27A⋯O4i | 0.83 (2) | 1.79 (2) | 2.615 (3) | 172 (4) |
| O27—H27B⋯O29vii | 0.82 (2) | 1.94 (2) | 2.727 (4) | 160 (4) |
| O28—H28A⋯O24v | 0.80 (2) | 1.97 (2) | 2.756 (3) | 167 (4) |
| O28—H28B⋯O28viii | 0.82 (2) | 2.02 (2) | 2.835 (4) | 174 (8) |
| O28—H28C⋯O29 | 0.82 (2) | 2.04 (3) | 2.836 (4) | 164 (7) |
| O29—H29A⋯O7 | 0.83 (2) | 1.78 (2) | 2.595 (3) | 168 (7) |
| O29—H29B⋯O28 | 0.83 (2) | 2.03 (3) | 2.836 (4) | 164 (7) |
Symmetry codes: (i) 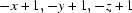 ; (ii)
; (ii) 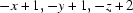 ; (iii)
; (iii) 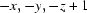 ; (iv)
; (iv) 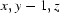 ; (v)
; (v) 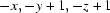 ; (vi)
; (vi) 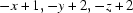 ; (vii)
; (vii) 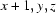 ; (viii)
; (viii) 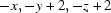 .
.
Acknowledgments
Ms L. Berring and Ms A. Schøneberg are gratefully acknowledged for the data collection and Dr Stephan Christgau for supplying the strontium ranelate.
supplementary crystallographic information
Comment
In recent years it has been found that Sr has a significant influence on the development and growth of bone and the effect of dose on bone structure has been investigated in great detail (Schrooten et al. 2003). Strontium ranelate (5-[bis(carboxymethyl) amino]-3-carboxymetyl- 4-cyano-2-thiophenecarboxylate) is one promising pharmaceutical compound for treating osteoporosis marketed as ProtelosR by Servier (Horvath et al., 2008). Strontium ranelate is known to form several hydrates with totally nine, eight, seven or four waters (Horvath et al., 2008). The initial dehydration observed here results from an expulsion of O27 or O29 and migration of the remaining water to site O30. As a consequence Sr1 is partially seven-coordinated (c.f. Table 1). The water hydrogen sites connected to O26, O28 and O29 are disordered. In essence, the alternating hydrogen bonding scheme between O28 and O29 is transmitted to a partial O26 - O28 hydrogen bond, and leaves H26B and H29C without hydrogen bond acceptors (c.f. Table 2).
Experimental
Strontium ranelate nona hydrate of 97% purity (Clauson-Kaas A/S) was recrystallized at different temperatures. Recrystallization at temperatures above 353 K appeared to produce the crystals of better quality. Upon cooling to room temperature large crystals of millimeter dimensions were obtained in the saturated solution. However, when the crystals were removed from the solution they rapidly degraded into smaller units of micron dimension. The smaller crystals showed out to contain less crystal water, as compared to the large crystals, presumably seven or five water molecules per formula unit. Thus, wet crystals were quickly transferred to the goniometer for X-ray data collection at 120 K. Several crystal were tried before an acceptable structure refinement was achieved. For all cases of lower quality data the SOF of O30 was about 0.3, confirming its role in the initial dehydration of strontium ranelate and the deterioration of the crystals.
Refinement
The H atoms of the CH2 groups were placed in calculated positions with C—H = 0.99, and refined as riding atoms. The H atoms of the water molecules were located in difference Fourier maps and refined with restrained O—H distances of 0.82 (2) Å. The H atoms of the partially occupied O30 (SOF=0.210 (4)) could not be located. The H displacement parameters were set to 1.2 (CH2) or 1.5 (H2O) times Ueq of the corresponding C or O atoms.
Figures
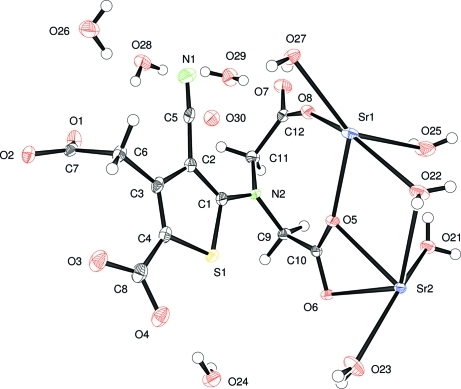
Fig. 1.
The asymmetric unit of (I) showing 50% probability displacement ellipsoids and the atomic numbering. Hydrogen atoms are represented by circles of arbitrary size and shows one consistent set of water H atoms.
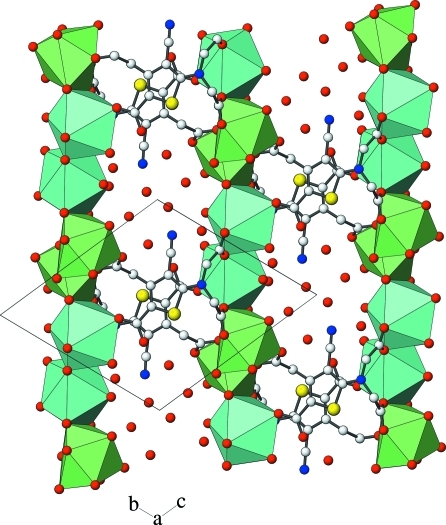
Fig. 2.
The crystal packing of (I) viewed down the a-axis. Hydrogen atoms are omitted for clarity.
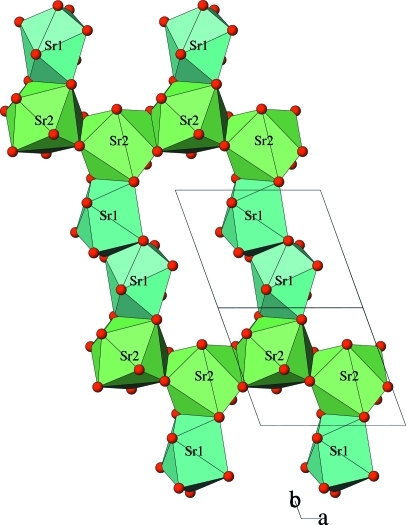
Fig. 3.
The polyhedral layer of (I) viewed down the (011) direction.
Crystal data
| [Sr2(C12H6N2O8S)(H2O)5]·3.79H2O | Z = 2 |
| Mr = 671.84 | F(000) = 671.8 |
| Triclinic, P1 | Dx = 1.933 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.3585 (3) Å | Cell parameters from 6752 reflections |
| b = 12.3865 (5) Å | θ = 2.6–30.6° |
| c = 12.6474 (5) Å | µ = 4.80 mm−1 |
| α = 109.880 (1)° | T = 120 K |
| β = 97.148 (1)° | Tabular, colorless |
| γ = 105.321 (1)° | 0.15 × 0.10 × 0.07 mm |
| V = 1154.00 (8) Å3 |
Data collection
| Bruker SMART APEX diffractometer | 6617 independent reflections |
| Radiation source: fine-focus sealed tube | 5375 reflections with I > 2σ(I) |
| graphite | Rint = 0.032 |
| ω scan, frame data integration | θmax = 30.9°, θmin = 2.6° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2002) | h = −12→12 |
| Tmin = 0.574, Tmax = 0.710 | k = −17→17 |
| 17404 measured reflections | l = −18→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.033 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.075 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0383P)2] where P = (Fo2 + 2Fc2)/3 |
| 6617 reflections | (Δ/σ)max = 0.002 |
| 375 parameters | Δρmax = 1.48 e Å−3 |
| 21 restraints | Δρmin = −1.23 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Sr1 | 0.52281 (3) | 0.38668 (2) | 0.836097 (18) | 0.01665 (6) | |
| Sr2 | 0.26745 (3) | 0.029136 (18) | 0.557006 (17) | 0.01072 (6) | |
| S1 | 0.13839 (8) | 0.40229 (5) | 0.50122 (5) | 0.01584 (12) | |
| C1 | 0.1878 (3) | 0.4919 (2) | 0.6477 (2) | 0.0160 (5) | |
| C2 | 0.3059 (3) | 0.6056 (2) | 0.6709 (2) | 0.0183 (5) | |
| C3 | 0.3561 (3) | 0.6182 (2) | 0.5698 (2) | 0.0168 (5) | |
| C4 | 0.2738 (3) | 0.5158 (2) | 0.4721 (2) | 0.0169 (5) | |
| C5 | 0.3781 (4) | 0.7012 (2) | 0.7831 (2) | 0.0237 (6) | |
| N1 | 0.4382 (4) | 0.7807 (2) | 0.8712 (2) | 0.0373 (7) | |
| C6 | 0.4862 (3) | 0.7305 (2) | 0.5736 (2) | 0.0187 (5) | |
| H6A | 0.5431 | 0.7077 | 0.5091 | 0.022* | |
| H6B | 0.5745 | 0.7663 | 0.6470 | 0.022* | |
| C7 | 0.4063 (3) | 0.8258 (2) | 0.5642 (2) | 0.0140 (4) | |
| O1 | 0.3026 (2) | 0.85078 (16) | 0.62454 (15) | 0.0218 (4) | |
| O2 | 0.4503 (2) | 0.87870 (15) | 0.49792 (15) | 0.0168 (3) | |
| C8 | 0.2835 (3) | 0.4871 (2) | 0.3498 (2) | 0.0208 (5) | |
| O3 | 0.3592 (3) | 0.57144 (17) | 0.32159 (17) | 0.0293 (4) | |
| O4 | 0.2095 (3) | 0.37659 (16) | 0.28052 (16) | 0.0253 (4) | |
| N2 | 0.1127 (3) | 0.44571 (17) | 0.71912 (16) | 0.0138 (4) | |
| C9 | 0.0097 (3) | 0.3176 (2) | 0.6705 (2) | 0.0147 (4) | |
| H9A | −0.0850 | 0.3036 | 0.6067 | 0.018* | |
| H9B | −0.0415 | 0.2978 | 0.7309 | 0.018* | |
| C10 | 0.1092 (3) | 0.2303 (2) | 0.62385 (19) | 0.0125 (4) | |
| O5 | 0.2644 (2) | 0.25859 (14) | 0.67064 (14) | 0.0150 (3) | |
| O6 | 0.0259 (2) | 0.13041 (14) | 0.54187 (13) | 0.0141 (3) | |
| C11 | 0.0937 (3) | 0.5241 (2) | 0.82968 (19) | 0.0167 (5) | |
| H11A | −0.0207 | 0.4870 | 0.8398 | 0.020* | |
| H11B | 0.0977 | 0.6033 | 0.8259 | 0.020* | |
| C12 | 0.2261 (3) | 0.5475 (2) | 0.9357 (2) | 0.0177 (5) | |
| O7 | 0.2069 (3) | 0.60995 (17) | 1.03249 (15) | 0.0266 (4) | |
| O8 | 0.3467 (2) | 0.50536 (16) | 0.92535 (15) | 0.0213 (4) | |
| O21 | 0.1336 (2) | 0.04188 (16) | 0.73788 (14) | 0.0177 (4) | |
| H21A | 0.195 (3) | 0.096 (2) | 0.7968 (19) | 0.027* | |
| H21B | 0.111 (4) | −0.021 (2) | 0.753 (3) | 0.027* | |
| O22 | 0.5486 (2) | 0.16837 (18) | 0.73157 (15) | 0.0218 (4) | |
| H22A | 0.608 (4) | 0.162 (3) | 0.686 (2) | 0.033* | |
| H22B | 0.572 (4) | 0.129 (3) | 0.768 (3) | 0.033* | |
| O23 | 0.2011 (2) | −0.02488 (19) | 0.33592 (16) | 0.0246 (4) | |
| H23A | 0.102 (3) | −0.035 (3) | 0.311 (3) | 0.037* | |
| H23B | 0.249 (4) | −0.006 (3) | 0.291 (2) | 0.037* | |
| O24 | −0.0724 (3) | 0.17943 (17) | 0.24069 (16) | 0.0233 (4) | |
| H24A | −0.138 (4) | 0.198 (3) | 0.282 (3) | 0.035* | |
| H24B | 0.015 (3) | 0.240 (2) | 0.260 (3) | 0.035* | |
| O25 | 0.3787 (3) | 0.23012 (19) | 0.92443 (16) | 0.0299 (5) | |
| H25A | 0.430 (4) | 0.204 (3) | 0.965 (3) | 0.045* | |
| H25B | 0.316 (4) | 0.259 (3) | 0.958 (3) | 0.045* | |
| O26 | 0.6112 (3) | 1.0182 (2) | 0.83803 (19) | 0.0321 (5) | |
| H26A | 0.686 (5) | 1.030 (5) | 0.897 (3) | 0.048* | 0.67 |
| H26B | 0.549 (6) | 1.037 (5) | 0.885 (4) | 0.048* | 0.67 |
| H26C | 0.532 (5) | 0.952 (3) | 0.823 (5) | 0.048* | 0.67 |
| O27 | 0.7186 (3) | 0.6180 (2) | 0.91336 (19) | 0.0194 (6) | 0.790 (4) |
| H27A | 0.740 (5) | 0.626 (4) | 0.854 (2) | 0.029* | 0.79 |
| H27B | 0.811 (4) | 0.640 (4) | 0.959 (3) | 0.029* | 0.79 |
| O28 | 0.1058 (3) | 0.92663 (19) | 0.99457 (17) | 0.0302 (5) | |
| H28A | 0.111 (5) | 0.896 (3) | 0.9290 (18) | 0.045* | |
| H28B | 0.041 (8) | 0.966 (6) | 1.000 (7) | 0.045* | 0.50 |
| H28C | 0.059 (8) | 0.873 (5) | 1.015 (6) | 0.045* | 0.50 |
| O29 | 0.0120 (4) | 0.7429 (3) | 1.0829 (2) | 0.0287 (7) | 0.790 (4) |
| H29A | 0.073 (7) | 0.701 (5) | 1.058 (6) | 0.043* | 0.53 |
| H29B | 0.045 (9) | 0.806 (4) | 1.071 (6) | 0.043* | 0.53 |
| H29C | −0.024 (10) | 0.732 (8) | 1.016 (3) | 0.043* | 0.53 |
| O30 | −0.0951 (12) | 0.6963 (8) | 0.9739 (8) | 0.023 (2)* | 0.210 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sr1 | 0.01489 (12) | 0.02075 (12) | 0.00915 (10) | 0.00204 (9) | 0.00133 (8) | 0.00307 (9) |
| Sr2 | 0.01086 (11) | 0.01068 (10) | 0.00940 (10) | 0.00340 (8) | 0.00144 (7) | 0.00290 (8) |
| S1 | 0.0195 (3) | 0.0115 (3) | 0.0149 (3) | 0.0032 (2) | 0.0029 (2) | 0.0051 (2) |
| C1 | 0.0158 (12) | 0.0144 (11) | 0.0177 (11) | 0.0064 (9) | −0.0008 (9) | 0.0067 (9) |
| C2 | 0.0197 (12) | 0.0133 (11) | 0.0215 (12) | 0.0061 (10) | −0.0004 (10) | 0.0078 (10) |
| C3 | 0.0133 (11) | 0.0122 (11) | 0.0255 (12) | 0.0049 (9) | 0.0006 (9) | 0.0087 (10) |
| C4 | 0.0164 (12) | 0.0138 (11) | 0.0241 (12) | 0.0060 (9) | 0.0057 (10) | 0.0105 (10) |
| C5 | 0.0287 (15) | 0.0139 (12) | 0.0256 (13) | 0.0010 (11) | −0.0027 (11) | 0.0120 (11) |
| N1 | 0.0485 (17) | 0.0204 (12) | 0.0261 (13) | −0.0078 (11) | −0.0058 (12) | 0.0083 (10) |
| C6 | 0.0129 (11) | 0.0152 (12) | 0.0301 (13) | 0.0046 (9) | 0.0032 (10) | 0.0122 (10) |
| C7 | 0.0120 (11) | 0.0104 (10) | 0.0167 (11) | 0.0015 (8) | 0.0008 (9) | 0.0042 (9) |
| O1 | 0.0269 (10) | 0.0231 (9) | 0.0262 (9) | 0.0140 (8) | 0.0147 (8) | 0.0152 (8) |
| O2 | 0.0174 (9) | 0.0160 (8) | 0.0201 (8) | 0.0055 (7) | 0.0065 (7) | 0.0101 (7) |
| C8 | 0.0220 (13) | 0.0200 (13) | 0.0260 (13) | 0.0100 (11) | 0.0119 (11) | 0.0111 (11) |
| O3 | 0.0386 (12) | 0.0209 (10) | 0.0317 (11) | 0.0058 (9) | 0.0167 (9) | 0.0141 (9) |
| O4 | 0.0348 (11) | 0.0179 (9) | 0.0252 (10) | 0.0072 (8) | 0.0152 (8) | 0.0092 (8) |
| N2 | 0.0185 (10) | 0.0084 (9) | 0.0109 (9) | 0.0030 (8) | 0.0002 (7) | 0.0016 (7) |
| C9 | 0.0142 (11) | 0.0121 (11) | 0.0150 (11) | 0.0029 (9) | 0.0014 (9) | 0.0038 (9) |
| C10 | 0.0157 (11) | 0.0109 (10) | 0.0106 (10) | 0.0022 (9) | 0.0027 (8) | 0.0056 (8) |
| O5 | 0.0125 (8) | 0.0144 (8) | 0.0140 (8) | 0.0033 (6) | −0.0023 (6) | 0.0032 (6) |
| O6 | 0.0140 (8) | 0.0104 (8) | 0.0127 (8) | 0.0016 (6) | −0.0014 (6) | 0.0016 (6) |
| C11 | 0.0195 (12) | 0.0164 (11) | 0.0125 (11) | 0.0096 (10) | 0.0008 (9) | 0.0017 (9) |
| C12 | 0.0235 (13) | 0.0113 (11) | 0.0151 (11) | 0.0023 (9) | 0.0003 (9) | 0.0055 (9) |
| O7 | 0.0378 (12) | 0.0255 (10) | 0.0124 (8) | 0.0109 (9) | 0.0040 (8) | 0.0028 (7) |
| O8 | 0.0213 (9) | 0.0194 (9) | 0.0199 (9) | 0.0077 (7) | −0.0036 (7) | 0.0056 (7) |
| O21 | 0.0200 (9) | 0.0184 (9) | 0.0120 (8) | 0.0043 (7) | 0.0025 (7) | 0.0046 (7) |
| O22 | 0.0186 (9) | 0.0305 (10) | 0.0139 (9) | 0.0109 (8) | 0.0024 (7) | 0.0043 (8) |
| O23 | 0.0172 (9) | 0.0421 (12) | 0.0195 (9) | 0.0106 (9) | 0.0070 (8) | 0.0164 (9) |
| O24 | 0.0270 (11) | 0.0201 (9) | 0.0198 (9) | 0.0028 (8) | 0.0098 (8) | 0.0066 (8) |
| O25 | 0.0413 (13) | 0.0248 (11) | 0.0151 (9) | 0.0009 (9) | 0.0046 (9) | 0.0060 (8) |
| O26 | 0.0299 (12) | 0.0322 (12) | 0.0283 (11) | 0.0011 (10) | 0.0029 (9) | 0.0131 (10) |
| O27 | 0.0243 (13) | 0.0161 (11) | 0.0129 (11) | 0.0003 (9) | 0.0054 (9) | 0.0042 (9) |
| O28 | 0.0422 (14) | 0.0295 (12) | 0.0163 (9) | 0.0133 (10) | 0.0022 (9) | 0.0060 (9) |
| O29 | 0.0313 (15) | 0.0330 (15) | 0.0249 (14) | 0.0161 (12) | 0.0070 (11) | 0.0107 (12) |
Geometric parameters (Å, °)
| Sr1—O8 | 2.4557 (18) | N2—C11 | 1.462 (3) |
| Sr1—O3i | 2.4782 (19) | C9—C10 | 1.540 (3) |
| Sr1—O5 | 2.5234 (16) | C9—H9A | 0.9900 |
| Sr1—O7ii | 2.6149 (19) | C9—H9B | 0.9900 |
| Sr1—O25 | 2.652 (2) | C10—O5 | 1.258 (3) |
| Sr1—O22 | 2.6560 (19) | C10—O6 | 1.258 (3) |
| Sr1—O27 | 2.657 (2) | C11—C12 | 1.517 (3) |
| Sr1—O8ii | 2.7834 (17) | C11—H11A | 0.9900 |
| Sr2—O6iii | 2.5452 (16) | C11—H11B | 0.9900 |
| Sr2—O23 | 2.5921 (18) | C12—O8 | 1.253 (3) |
| Sr2—O2i | 2.6222 (17) | C12—O7 | 1.262 (3) |
| Sr2—O21 | 2.6445 (17) | O21—H21A | 0.809 (18) |
| Sr2—O6 | 2.6628 (16) | O21—H21B | 0.845 (17) |
| Sr2—O2iv | 2.6848 (16) | O22—H22A | 0.808 (18) |
| Sr2—O1iv | 2.6944 (17) | O22—H22B | 0.819 (17) |
| Sr2—O22 | 2.7108 (18) | O23—H23A | 0.807 (18) |
| Sr2—O5 | 2.7228 (16) | O23—H23B | 0.798 (18) |
| S1—C1 | 1.733 (2) | O24—H24A | 0.823 (18) |
| S1—C4 | 1.735 (2) | O24—H24B | 0.837 (18) |
| C1—N2 | 1.358 (3) | O25—H25A | 0.819 (18) |
| C1—C2 | 1.398 (3) | O25—H25B | 0.796 (18) |
| C2—C5 | 1.431 (4) | O26—H26A | 0.850 (19) |
| C2—C3 | 1.439 (4) | O26—H26B | 0.849 (19) |
| C3—C4 | 1.368 (3) | O26—H26C | 0.85 (2) |
| C3—C6 | 1.504 (3) | O27—H27A | 0.827 (19) |
| C4—C8 | 1.482 (4) | O27—H27B | 0.823 (19) |
| C5—N1 | 1.148 (3) | O28—H28A | 0.799 (18) |
| C6—C7 | 1.532 (3) | O28—H28B | 0.82 (2) |
| C6—H6A | 0.9900 | O28—H28C | 0.82 (2) |
| C6—H6B | 0.9900 | O29—H29A | 0.83 (2) |
| C7—O1 | 1.256 (3) | O29—H29B | 0.83 (2) |
| C7—O2 | 1.261 (3) | O29—H29C | 0.82 (2) |
| C8—O3 | 1.257 (3) | O27—O30v | 1.534 (10) |
| C8—O4 | 1.275 (3) | O29—O30 | 1.383 (10) |
| N2—C9 | 1.456 (3) | ||
| O8—Sr1—O3i | 117.69 (6) | O22—Sr2—O5 | 67.10 (5) |
| O8—Sr1—O5 | 87.39 (5) | C1—S1—C4 | 92.44 (12) |
| O3i—Sr1—O5 | 81.32 (6) | N2—C1—C2 | 130.8 (2) |
| O8—Sr1—O7ii | 118.97 (6) | N2—C1—S1 | 119.08 (17) |
| O3i—Sr1—O7ii | 101.51 (7) | C2—C1—S1 | 110.09 (19) |
| O5—Sr1—O7ii | 146.34 (5) | C1—C2—C5 | 125.2 (2) |
| O8—Sr1—O25 | 85.98 (7) | C1—C2—C3 | 113.4 (2) |
| O3i—Sr1—O25 | 150.13 (6) | C5—C2—C3 | 121.3 (2) |
| O5—Sr1—O25 | 81.94 (6) | C4—C3—C2 | 111.8 (2) |
| O7ii—Sr1—O25 | 79.69 (7) | C4—C3—C6 | 124.9 (2) |
| O8—Sr1—O22 | 147.38 (6) | C2—C3—C6 | 123.3 (2) |
| O3i—Sr1—O22 | 83.58 (6) | C3—C4—C8 | 131.1 (2) |
| O5—Sr1—O22 | 70.82 (5) | C3—C4—S1 | 112.22 (19) |
| O7ii—Sr1—O22 | 76.14 (6) | C8—C4—S1 | 116.67 (18) |
| O25—Sr1—O22 | 67.59 (6) | N1—C5—C2 | 177.5 (3) |
| O8—Sr1—O27 | 74.49 (7) | C3—C6—C7 | 112.26 (19) |
| O3i—Sr1—O27 | 70.14 (7) | C3—C6—H6A | 109.2 |
| O5—Sr1—O27 | 132.72 (6) | C7—C6—H6A | 109.2 |
| O7ii—Sr1—O27 | 77.82 (7) | C3—C6—H6B | 109.2 |
| O25—Sr1—O27 | 137.67 (6) | C7—C6—H6B | 109.2 |
| O22—Sr1—O27 | 138.07 (7) | H6A—C6—H6B | 107.9 |
| O8—Sr1—O8ii | 70.87 (6) | O1—C7—O2 | 122.2 (2) |
| O3i—Sr1—O8ii | 130.40 (6) | O1—C7—C6 | 119.1 (2) |
| O5—Sr1—O8ii | 147.09 (6) | O2—C7—C6 | 118.7 (2) |
| O7ii—Sr1—O8ii | 48.19 (5) | O3—C8—O4 | 125.2 (2) |
| O25—Sr1—O8ii | 72.41 (6) | O3—C8—C4 | 118.9 (2) |
| O22—Sr1—O8ii | 115.58 (5) | O4—C8—C4 | 115.9 (2) |
| O27—Sr1—O8ii | 65.82 (6) | C1—N2—C9 | 117.63 (19) |
| O6iii—Sr2—O23 | 69.11 (6) | C1—N2—C11 | 121.82 (19) |
| O6iii—Sr2—O2i | 138.88 (5) | C9—N2—C11 | 118.5 (2) |
| O23—Sr2—O2i | 71.52 (6) | N2—C9—C10 | 114.24 (19) |
| O6iii—Sr2—O21 | 79.58 (5) | N2—C9—H9A | 108.7 |
| O23—Sr2—O21 | 145.03 (6) | C10—C9—H9A | 108.7 |
| O2i—Sr2—O21 | 141.51 (5) | N2—C9—H9B | 108.7 |
| O6iii—Sr2—O6 | 68.34 (6) | C10—C9—H9B | 108.7 |
| O23—Sr2—O6 | 81.03 (5) | H9A—C9—H9B | 107.6 |
| O2i—Sr2—O6 | 116.53 (5) | O5—C10—O6 | 123.3 (2) |
| O21—Sr2—O6 | 73.03 (5) | O5—C10—C9 | 119.92 (19) |
| O6iii—Sr2—O2iv | 96.94 (5) | O6—C10—C9 | 116.7 (2) |
| O23—Sr2—O2iv | 81.54 (6) | N2—C11—C12 | 115.7 (2) |
| O2i—Sr2—O2iv | 65.68 (6) | N2—C11—H11A | 108.3 |
| O21—Sr2—O2iv | 118.40 (5) | C12—C11—H11A | 108.3 |
| O6—Sr2—O2iv | 160.37 (5) | N2—C11—H11B | 108.3 |
| O6iii—Sr2—O1iv | 79.58 (5) | C12—C11—H11B | 108.3 |
| O23—Sr2—O1iv | 116.26 (6) | H11A—C11—H11B | 107.4 |
| O2i—Sr2—O1iv | 108.25 (5) | O8—C12—O7 | 122.9 (2) |
| O21—Sr2—O1iv | 71.00 (5) | O8—C12—C11 | 120.5 (2) |
| O6—Sr2—O1iv | 135.19 (5) | O7—C12—C11 | 116.7 (2) |
| O2iv—Sr2—O1iv | 48.34 (5) | H21A—O21—H21B | 106 (3) |
| O6iii—Sr2—O22 | 156.57 (6) | H22A—O22—H22B | 104 (3) |
| O23—Sr2—O22 | 133.65 (6) | H23A—O23—H23B | 104 (3) |
| O2i—Sr2—O22 | 62.33 (5) | H24A—O24—H24B | 108 (3) |
| O21—Sr2—O22 | 79.68 (6) | H25A—O25—H25B | 109 (4) |
| O6—Sr2—O22 | 115.27 (5) | H26A—O26—H26B | 87 (5) |
| O2iv—Sr2—O22 | 83.49 (5) | H26A—O26—H26C | 108 (5) |
| O1iv—Sr2—O22 | 83.47 (6) | H26B—O26—H26C | 75 (5) |
| O6iii—Sr2—O5 | 114.87 (5) | H27A—O27—H27B | 107 (4) |
| O23—Sr2—O5 | 109.66 (6) | H28A—O28—H28B | 111 (6) |
| O2i—Sr2—O5 | 89.11 (5) | H28A—O28—H28C | 109 (6) |
| O21—Sr2—O5 | 69.47 (5) | H28B—O28—H28C | 103 (7) |
| O6—Sr2—O5 | 48.56 (5) | H29A—O29—H29B | 107 (7) |
| O2iv—Sr2—O5 | 148.19 (5) | H29A—O29—H29C | 87 (6) |
| O1iv—Sr2—O5 | 133.93 (5) | H29B—O29—H29C | 68 (6) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x+1, −y+1, −z+2; (iii) −x, −y, −z+1; (iv) x, y−1, z; (v) x+1, y, z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O21—H21A···O25 | 0.81 (2) | 1.98 (2) | 2.781 (3) | 169 (3) |
| O21—H21B···O24iii | 0.85 (2) | 1.93 (2) | 2.765 (3) | 169 (3) |
| O22—H22A···O2i | 0.81 (2) | 2.16 (3) | 2.761 (2) | 131 (3) |
| O22—H22B···O26iv | 0.82 (2) | 1.94 (2) | 2.755 (3) | 173 (3) |
| O23—H23A···O21iii | 0.81 (2) | 1.96 (2) | 2.766 (3) | 174 (4) |
| O23—H23B···O26i | 0.80 (2) | 2.11 (2) | 2.867 (3) | 159 (3) |
| O24—H24A···O1vi | 0.82 (2) | 2.03 (2) | 2.760 (3) | 148 (3) |
| O24—H24B···O4 | 0.84 (2) | 1.93 (2) | 2.756 (3) | 172 (3) |
| O25—H25A···N1ii | 0.82 (2) | 2.15 (2) | 2.898 (3) | 152 (3) |
| O25—H25B···O27ii | 0.80 (2) | 1.93 (2) | 2.648 (3) | 150 (4) |
| O26—H26A···O28vii | 0.85 (2) | 1.90 (2) | 2.731 (3) | 164 (5) |
| O26—H26C···N1 | 0.85 (2) | 2.37 (4) | 3.108 (3) | 146 (5) |
| O27—H27A···O4i | 0.83 (2) | 1.79 (2) | 2.615 (3) | 172 (4) |
| O27—H27B···O29v | 0.82 (2) | 1.94 (2) | 2.727 (4) | 160 (4) |
| O28—H28A···O24vi | 0.80 (2) | 1.97 (2) | 2.756 (3) | 167 (4) |
| O28—H28B···O28viii | 0.82 (2) | 2.02 (2) | 2.835 (4) | 174 (8) |
| O28—H28C···O29 | 0.82 (2) | 2.04 (3) | 2.836 (4) | 164 (7) |
| O29—H29A···O7 | 0.83 (2) | 1.78 (2) | 2.595 (3) | 168 (7) |
| O29—H29B···O28 | 0.83 (2) | 2.03 (3) | 2.836 (4) | 164 (7) |
Symmetry codes: (iii) −x, −y, −z+1; (i) −x+1, −y+1, −z+1; (iv) x, y−1, z; (vi) −x, −y+1, −z+1; (ii) −x+1, −y+1, −z+2; (vii) −x+1, −y+2, −z+2; (v) x+1, y, z; (viii) −x, −y+2, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SI2343).
References
- Bruker (1999). SMART and SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
- Dowty, E. (2000). ATOMS Shape Software, Kingsport, Tennessee, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Horvath, S., Demuynck, I. & Damien, G. (2008). US Patent No. 7459568.
- Schrooten, I., Behets, G. J. S., Cabrera, W. E., Vercauteren, S. R., Lamberts, L. W., Verberckmoes, S. C., Bervoets, A. J., Dams, G., Goodman, W. G., De Broe, M. E. & D Haese, P. C. (2003). Kidney Intl, 63, 927–935. [DOI] [PubMed]
- Sheldrick, G. M. (2002). SADABS University of Göttingen, Germany
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stahl, K., Andersen, J. E. T. & Christgau, S. (2006). Acta Cryst. C62, m144–m149. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811010099/si2343sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811010099/si2343Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report