Abstract
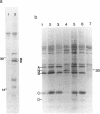
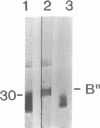
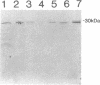
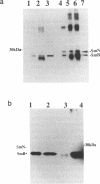
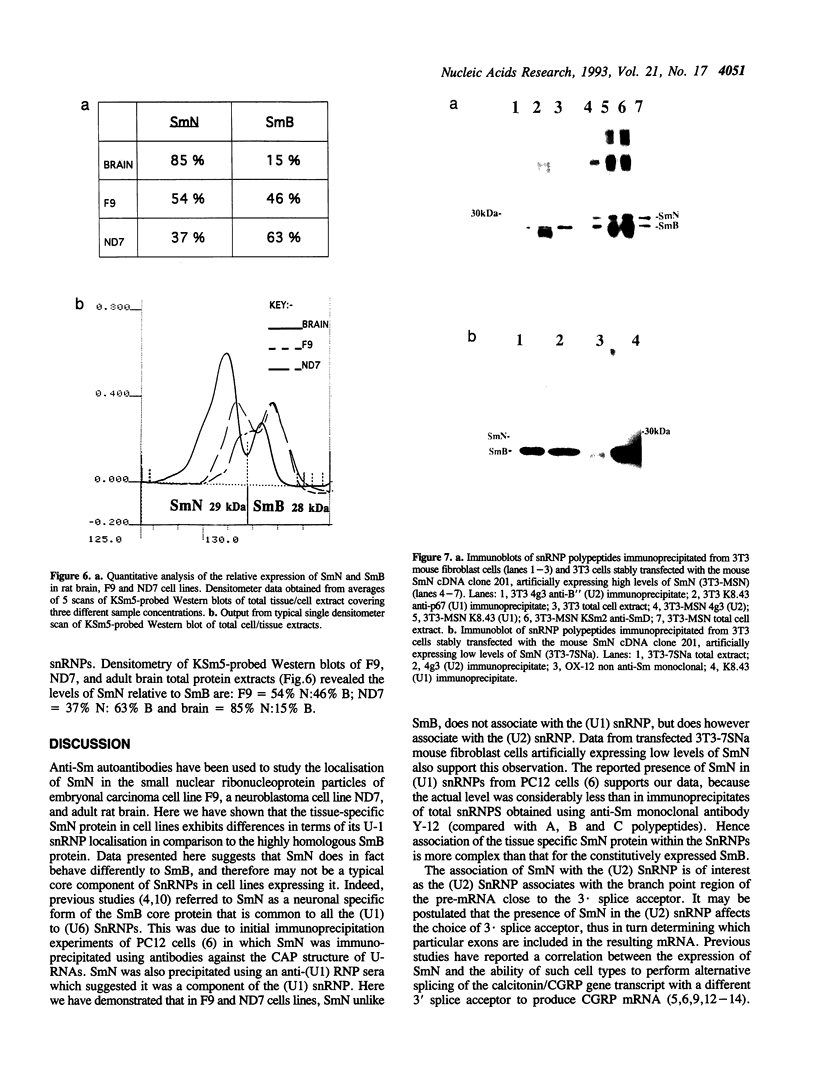
The SmN protein is a tissue specific component of the small nuclear ribonucleoprotein particle which is closely related to the ubiquitously expressed SmB protein but is expressed only in the brain and heart. To investigate the function of SmN, its localisation within different snRNP particles was investigated using a range of anti-snRNP monoclonal antibodies. SmN and SmB were found to exhibit different patterns of association with snRNP particles in two cell lines, ND7 and F9 which express SmN. In both cases, SmN was found to be present in the U-2 snRNP but was excluded from the U-1 snRNPs whereas SmB was present in both U-1 and U-2 snRNPs. Data from transfected 3T3 mouse fibroblasts cell lines artificially expressing a low level of SmN also confirm this observation. In contrast, SmN was found to be an integral component of both the U-1 and U-2 snRNPs in both 3T3 cells artificially expressing high levels of SmN and in adult rat brain which has a naturally high level of SmN expression. Taken together, the results suggest that the pre-U1 snRNP particle has a lower affinity for SmN than for SmB. Thus, SmN expressed at low levels incorporates into U2, but SmN expressed at high levels incorporates into both U1 and U2 snRNPs and replaces SmB. The significance of these effects is discussed in terms of the potential role played by SmN in constitutive and alternative splicing pathways in neuronal cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach B. M., Barr J. A., Evans E. P., Burtenshaw M., Beechey C. V., Leff S. E., Brannan C. I., Copeland N. G., Jenkins N. A., Jones J. A candidate mouse model for Prader-Willi syndrome which shows an absence of Snrpn expression. Nat Genet. 1992 Dec;2(4):270–274. doi: 10.1038/ng1292-270. [DOI] [PubMed] [Google Scholar]
- Crenshaw E. B., 3rd, Russo A. F., Swanson L. W., Rosenfeld M. G. Neuron-specific alternative RNA processing in transgenic mice expressing a metallothionein-calcitonin fusion gene. Cell. 1987 May 8;49(3):389–398. doi: 10.1016/0092-8674(87)90291-1. [DOI] [PubMed] [Google Scholar]
- Delsert C. D., Rosenfeld M. G. A tissue-specific small nuclear ribonucleoprotein and the regulated splicing of the calcitonin/calcitonin gene-related protein transcript. J Biol Chem. 1992 Jul 25;267(21):14573–14579. [PubMed] [Google Scholar]
- Eisenberg R. A., Tan E. M., Dixon F. J. Presence of anti-Sm reactivity in autoimmune mouse strains. J Exp Med. 1978 Feb 1;147(2):582–587. doi: 10.1084/jem.147.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evain-Brion D., Binet E., Donnadieu M., Laurent P., Anderson W. B. Production of immunoreactive calcitonin and parathyroid hormone by embryonal carcinoma cells: alteration with retinoic acid-induced differentiation. Dev Biol. 1984 Aug;104(2):406–412. doi: 10.1016/0012-1606(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Habets W. J., Hoet M. H., De Jong B. A., Van der Kemp A., Van Venrooij W. J. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J Immunol. 1989 Oct 15;143(8):2560–2566. [PubMed] [Google Scholar]
- Habets W., Hoet M., Bringmann P., Lührmann R., van Venrooij W. Autoantibodies to ribonucleoprotein particles containing U2 small nuclear RNA. EMBO J. 1985 Jun;4(6):1545–1550. doi: 10.1002/j.1460-2075.1985.tb03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D. A., Suburo A., Terenghi G., Hudson L. D., Polak J. M., Latchman D. S. Expression of the tissue specific splicing protein SmN in neuronal cell lines and in regions of the brain with different splicing capacities. Brain Res Mol Brain Res. 1992 Nov;16(1-2):13–19. doi: 10.1016/0169-328x(92)90188-h. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Latchman D. S. Cell-type-specific splicing factors and the regulation of alternative RNA splicing. New Biol. 1990 Apr;2(4):297–303. [PubMed] [Google Scholar]
- Leff S. E., Brannan C. I., Reed M. L., Ozçelik T., Francke U., Copeland N. G., Jenkins N. A. Maternal imprinting of the mouse Snrpn gene and conserved linkage homology with the human Prader-Willi syndrome region. Nat Genet. 1992 Dec;2(4):259–264. doi: 10.1038/ng1292-259. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Evans R. M., Rosenfeld M. G. Splice commitment dictates neuron-specific alternative RNA processing in calcitonin/CGRP gene expression. Cell. 1987 Feb 13;48(3):517–524. doi: 10.1016/0092-8674(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- McAllister G., Amara S. G., Lerner M. R. Tissue-specific expression and cDNA cloning of small nuclear ribonucleoprotein-associated polypeptide N. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5296–5300. doi: 10.1073/pnas.85.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R. D., Knoll J. H., Butler M. G., Karam S., Lalande M. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature. 1989 Nov 16;342(6247):281–285. doi: 10.1038/342281a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozçelik T., Leff S., Robinson W., Donlon T., Lalande M., Sanjines E., Schinzel A., Francke U. Small nuclear ribonucleoprotein polypeptide N (SNRPN), an expressed gene in the Prader-Willi syndrome critical region. Nat Genet. 1992 Dec;2(4):265–269. doi: 10.1038/ng1292-265. [DOI] [PubMed] [Google Scholar]
- Parker K. A., Steitz J. A. Determination of RNA-protein and RNA-ribonucleoprotein interactions by nuclease probing. Methods Enzymol. 1989;180:454–468. doi: 10.1016/0076-6879(89)80117-x. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Brines M. L., Lerner M. R. The gene encoding the small nuclear ribonucleoprotein-associated protein N is expressed at high levels in neurons. J Biol Chem. 1992 Apr 25;267(12):8521–8529. [PubMed] [Google Scholar]
- Schmauss C., Lerner M. R. The closely related small nuclear ribonucleoprotein polypeptides N and B/B' are distinguishable by antibodies as well as by differences in their mRNAs and gene structures. J Biol Chem. 1990 Jun 25;265(18):10733–10739. [PubMed] [Google Scholar]
- Schmauss C., McAllister G., Ohosone Y., Hardin J. A., Lerner M. R. A comparison of snRNP-associated Sm-autoantigens: human N, rat N and human B/B'. Nucleic Acids Res. 1989 Feb 25;17(4):1733–1743. doi: 10.1093/nar/17.4.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe N. G., Williams D. G., Latchman D. S. Regulated expression of the small nuclear ribonucleoprotein particle protein SmN in embryonic stem cell differentiation. Mol Cell Biol. 1990 Dec;10(12):6817–6820. doi: 10.1128/mcb.10.12.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe N. G., Williams D. G., Norton P., Latchman D. S. Expression of the SmB' splicing protein in rodent cells capable of following an alternative RNA splicing pathway. FEBS Lett. 1989 Jan 30;243(2):132–136. doi: 10.1016/0014-5793(89)80114-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. G., Sharpe N. G., Wallace G., Latchman D. S. A repeated proline-rich sequence in Sm B/B' and N is a dominant epitope recognized by human and murine autoantibodies. J Autoimmun. 1990 Dec;3(6):715–725. doi: 10.1016/s0896-8411(05)80038-1. [DOI] [PubMed] [Google Scholar]
- Williams D. G., Stocks M. R., Smith P. R., Maini R. N. Murine lupus monoclonal antibodies define five epitopes on two different Sm polypeptides. Immunology. 1986 Jul;58(3):495–500. [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Bevan S. J., Coote P. R., Dunn P. M., Harmar A., Hogan P., Latchman D. S., Morrison C., Rougon G., Theveniau M. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990 Sep 22;241(1302):187–194. doi: 10.1098/rspb.1990.0084. [DOI] [PubMed] [Google Scholar]
- Zieve G. W., Sauterer R. A. Cell biology of the snRNP particles. Crit Rev Biochem Mol Biol. 1990;25(1):1–46. doi: 10.3109/10409239009090604. [DOI] [PubMed] [Google Scholar]
- van Dam A., Winkel I., Zijlstra-Baalbergen J., Smeenk R., Cuypers H. T. Cloned human snRNP proteins B and B' differ only in their carboxy-terminal part. EMBO J. 1989 Dec 1;8(12):3853–3860. doi: 10.1002/j.1460-2075.1989.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]