Abstract
Newlyhatched juveniles of the Hawaiian squid Euprymna scolopes rapidly become colonized by the bioluminescent marine bacterium Vibrio fischeri. Motility is required to establish the symbiotic colonization, but the role of chemotaxis is unknown. In this study we analyzed chemotaxis of V. fischeri to a number of potential attractants. The bacterium migrated toward serine and most sugars tested. V. fischeri also exhibited the unusual ability to migrate to nucleosides and nucleotides as well as to N-acetylneuraminic acid, a component of squid mucus.
Upon hatching, the light organ of the juvenile Hawaiian squid, Euprymna scolopes, is specifically colonized by the luminous marine bacterium Vibrio fischeri (reviewed in references 7 and 13). V. fischeri cells present in the seawater aggregate in mucus secreted from the light organ and then appear to stream into the openings of the light organ (10), suggesting directed movement by the bacterium. The light-organ mucus, secreted upon bacterial exposure (10) and subsequently within the light organ in response to symbiotic colonization (9), contains two sugars, N-acetylgalactosamine (NAGal) and N-acetylneuraminic acid (NANA) (10). These sugars, as well as amino acids and peptides within the light organ (6), may serve as nutrient sources and/or chemoattractants to enhance entry by V. fischeri, which must be motile to colonize successfully (5). To begin investigating the potential role of chemotaxis in symbiotic initiation, we characterized the response of the bacterium to various nutrients.
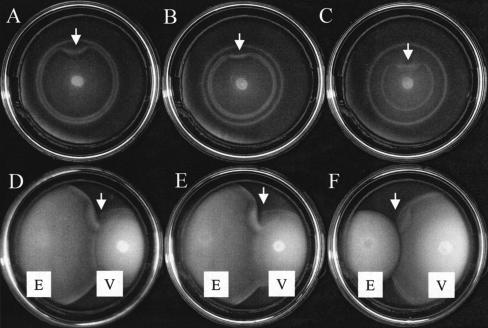
Motile cells inoculated onto a soft agar medium containing two attractants form an outermost ring in response to a spatial gradient that results from the consumption and subsequent diffusion of the preferred attractant (14). Similarly, an inner ring forms to the second attractant (11 and B. M. Pruss and A. J. Wolfe, unpublished data). When inoculated onto TB-SW soft agar plates (1% tryptone, 0.88% NaCl, 0.62% MgSO4, 0.072% CaCl2, 0.038% KCl, 0.25% agar), cells of V. fischeri strain ES114 (2) formed two concentric rings (Fig. 1A). Cells of Escherichia coli also form two rings on tryptone-based soft agar, with the outer and inner rings sensing serine and aspartate, respectively (1). We therefore tested whether V. fischeri also migrated to serine and aspartate. While the bacterium did not respond to aspartate (data not shown), the addition of increasing concentrations of serine to the soft agar slowed the migration of the inner ring of V. fischeri, indicating that the cells present in that ring consume, sense, and migrate to serine (Fig. 1B and C). We used an excess of serine to disrupt the gradient and found that serine perturbed migration of the inner ring (arrows in Fig. 1 depict the location of the spot of serine). Closer inspection of the rings revealed that a doublet we occasionally observed (e.g., Fig. 1B) consisted of faster-migrating cells on the surface of the plate and slower-migrating cells deeper in the agar, both of which responded to serine. Because the doublet responded as a unit to the addition of serine, we believe it should be considered a single, serine-responsive ring. Serine can be metabolized anaerobically by organisms such as E. coli (1); thus, the separation may result from a lag in migration due to the less-oxygenated environment deep in the plate. This would explain the apparent discrepancy between this report of two migrating rings and a recent report that mentions three (8).
FIG. 1.
(A-C) Migration of V. fischeri to serine. V. fischeri cells were inoculated onto TB-SW plates in the absence (A) or presence of increasing concentrations of serine (0.75 mM [B] or 2 mM [C]). Plates were incubated at 28°C for 5 h. Arrows indicate where an excess of serine was spotted directly onto the plate just beyond the migrating rings. (D-F) Cells of V. fischeri (V) and E. coli (E) were coinoculated on TBS soft agar plates. (D) E. coli strain RP437, wild-type for chemotaxis. (E) E. coli strain RP5854, tar. (F) E. coli strain RP5714, tsr. E. coli cells were inoculated at 28°C for 7 to 12 h prior to inoculation with V. fischeri, followed by incubation for an additional 5 h. Arrows indicate where an excess of serine was spotted directly onto the plate just beyond the migrating rings.
We sought further evidence that the cells in the inner ring sensed serine, taking advantage of the fact that rings of bacteria migrating toward the same attractant will fuse when they meet (1). Using TBS soft agar plates (1% tryptone, 2% NaCl, and 0.25% agar), we coinoculated V. fischeri cells with E. coli cells. The inner ring of V. fischeri fused with the outer (serine-sensing) ring formed either by wild-type E. coli (Fig. 1D) or a tar mutant that cannot sense aspartate (Fig. 1E); the fused ring was perturbed when an excess of serine was spotted just beyond the ring. In contrast, neither ring of V. fischeri fused with the single aspartate-sensing ring of an E. coli tsr mutant (Fig. 1F). These results confirmed that V. fischeri cells in the inner ring migrate toward serine and that those in the outer ring do not sense aspartate.
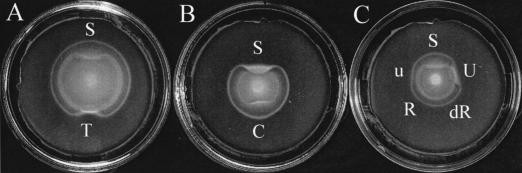
To identify the substrate sensed by the outer ring of V. fischeri, we spotted each of the other 18 amino acids onto Tris-buffered TB-SW soft agar plates just beyond the migrating rings. Although alanine, arginine, asparagine, histidine, and threonine slightly perturbed the inner ring, no amino acid perturbed the outer ring (data not shown), suggesting that the cells in the outer ring do not recognize an amino acid. We therefore investigated the ability of V. fischeri to migrate toward other components of tryptone and found that the outer ring of cells responded to the nucleoside thymidine (Fig. 2A). Increasing concentrations of thymidine added to Tris-buffered TB-SW plates caused cells in the outer ring to migrate more slowly, and an excess of thymidine perturbed that migration (data not shown). This suggests that in TB-SW this organism preferentially consumes and senses thymidine over serine. Indeed, V. fischeri cells grew with thymidine as a sole carbon source (data not shown). Supplementation with other ribonucleosides (adenosine, guanosine, uridine, and cytodine) similarly slowed the migration of the outer ring of V. fischeri cells, and spotting with an excess of these ribonucleosides just beyond the rings formed on TB-SW perturbed only the outer ring (Fig. 2B and C and data not shown), indicating that these cells could respond to any ribonucleoside. We also spotted with deoxynucleotide triphosphates (dATP, dCTP, dGTP, dTTP), which similarly perturbed only the outer ring (data not shown). Because deoxynucleoside triphosphates differ from ribonucleosides in two ways, the sugar moiety (deoxyribose versus ribose) and the phosphorylation state (triphosphate versus unphosphorylated), these data suggest that neither the sugar component nor the phosphorylation state constitutes a critical component of recognition.
FIG. 2.
Migration of V. fischeri to nucleosides and their components. V. fischeri cells were inoculated onto Tris-buffered TB-SW soft agar plates for 5 h at 28°C. (A) Aliquots (10 μl) of 0.121 M thymidine (T) and 2 M serine (S) were spotted just beyond the migrating rings of V. fischeri. (B) Serine (2 M) (S) and cytidine (0.171 M) (C) were spotted just beyond the migrating rings formed on Tris-buffered TB-SW plates containing 1 mM cytidine. Note that the ring perturbed by serine is now located on the outside of the ring perturbed by cytidine. (C) Aliquots (10 μl) of equimolar concentrations (0.066 M) of uridine (U), uracil (u), ribose (R), and deoxyribose (dR) were spotted onto plates just beyond the migrating rings. Serine (2 mM) was added to all plates to provide a better separation of the inner and outer rings for visualization of the response to nucleoside or nucleoside component addition. An excess of serine (S) was spotted at the top of each plate as a comparison.
We then tested whether V. fischeri cells in the outer ring responded to the whole nucleoside molecule or to its components (base or sugar) by spotting equimolar concentrations (0.066 M) of these substrates just beyond the outer ring. V. fischeri cells preferentially responded to the entire molecule (Fig. 2C and data not shown). Neither ribose nor deoxyribose perturbed the outer ring (Fig. 2C). Uracil and thymine occasionally caused only slight perturbations, while cytosine, guanine, or adenine did not (Fig. 2C and data not shown). When uracil and thymine were tested at higher concentrations, a slight perturbation was consistently observed (data not shown). Adenine, cytosine, and guanine were not tested at higher concentrations due to their insolubility. Thus, V. fischeri can consume, sense, and migrate to nucleosides and nucleotides. To our knowledge this is the first report of bacterial migration toward the building blocks of DNA and RNA. Whether this ability enhances colonization of E. scolopes by V. fischeri is unknown; however, it is not unreasonable to expect a higher concentration of nucleic acids in the vicinity of the squid, particularly because surface cells of the light organ undergo apoptosis during early stages of symbiotic colonization (4).
In addition to serine and nucleosides and nucleotides, V. fischeri may perform chemotaxis to sugars. Surface molecules of host cells frequently contain sugar moieties, and the mucus secreted by E. scolopes contains the sugars NANA and NAGal. We therefore tested the ability of V. fischeri to migrate in response to a variety of sugars and other substrates. Potential chemoattractants were either added to or spotted on one of two media: TB-SW and HEPES minimal medium (12) containing 25 mM mannitol (MM-M). Mannitol serves as a carbon source but not as an attractant for V. fischeri (Fig. 3, first row, column A). As shown in Table 1, V. fischeri migrated toward a variety of substrates (13 of 18 tested), including glucose, cellobiose, and to a lesser extent, ribose. Ribose caused a faint additional inner ring to form but did not slow migration of the outer ring, implying that the receptors for thymidine and ribose are distinct. The same was true for cyclic AMP (3).
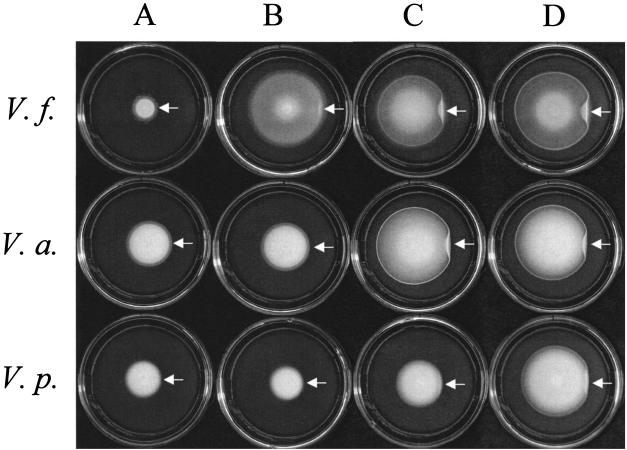
FIG. 3.
Migration of various Vibrio strains to NANA and other sugars. Cells of V. fischeri (V.f.), V. anguillarum strain PKJ (V.a.), and V. parahaemolyticus strain KNH1 (V.p.) were inoculated near the center of MM-M soft agar plates (column A) or MM-M soft agar plates containing either 1 mM NANA (column B), 1 mM glucose (column C), or 1 mM NAG (column D). Plates were incubated at 28°C for approximately 24 h. Arrows indicate where an excess of the respective carbon source was spotted onto the plate just beyond the migrating rings.
TABLE 1.
Response of V. fischeri to various substrates added to TB-SW or MM-M
| Substrate | Medium
|
|
|---|---|---|
| TB-SWa | MM-Mb | |
| Cyclic AMP | + | + |
| Cellobiose | ++ | ND |
| Choline | − | − |
| Deoxyribose | − | ND |
| Fructose | ++ | + |
| Galactose | + | ND |
| Glucose | ++ | ++ |
| Glucosamine | − | − |
| Glycerol | + | − |
| Maltose | + | ND |
| Mannitol | − | − |
| Mannose | + | ++ |
| NAGal | − | − |
| NAG | ++ | ++ |
| NANA | + | ++ |
| Raffinose | + | ND |
| Ribose | + | ND |
| Sucrose | ++ | ND |
For TB-SW, a positive response was noted if an additional concentric ring formed that was subsequently perturbed by spotting the appropriate substrate. ++, dense ring; +, faint ring; − no ring.
For MM-M, a positive response was noted if addition of a substrate, added at concentrations of 1 μM to 10 mM, allowed the cells to form a ring that migrated beyond the zone of growth formed in the presence of MM-M alone. ++, radius of >10 mm; +, radius of <10 mm; −, no difference. ND, migration to this substrate was not tested under these conditions.
When we tested the two sugars present in squid mucus, we found that whereas NANA served as a chemoattractant for V. fischeri, NAGal did not (Fig. 3, first row, column B, and Table 1). To test whether this attraction is a common trait among other vibrio species, we examined the ability of the marine isolates V. anguillarum and V. parahaemolyticus to migrate to the sugar. Unlike that of V. fischeri, migration of V. anguillarum and V. parahaemolyticus was not improved by the addition of NANA to MM-M, nor was migration perturbed by spotting with NANA (Fig. 3, columns A and B). In contrast, all three organisms formed rings in response to either 1 mM glucose or 1 mM N-acetylglucosamine (NAG), which were perturbed by an excess of either glucose or NAG, respectively (Fig. 3, columns C and D). This potentially unique chemotaxis to NANA, combined with the ability of V. fischeri to use NANA as a carbon source (data not shown), raises the intriguing possibility that migration toward this sugar could contribute to initiating symbiotic colonization.
Given the absolute requirement for motility in symbiotic colonization (5), the data reported here provide an important first step in assessing the contribution of chemotaxis to colonization. V. fischeri apparently encodes an unusually large number of chemoreceptors; over 40 genes contain putative chemotaxis signaling motifs (C. R. DeLoney-Marino, unpublished observations). We are presently working to identify receptors specific for the identified attractants by constructing and characterizing chemotaxis mutants. These mutants will permit more direct testing of a potential role for chemotaxis in establishing the Vibrio-squid symbiosis.
Acknowledgments
We thank Joy Campbell and Therese Bartley for expert technical assistance, E. G. Ruby for experimental suggestions, J. S. Parkinson and E. G. Ruby for strains, and members of our labs for critical reading of the manuscript. Genome information was provided by the Vibrio fischeri Genome Project, at http://ergo.integratedgenomics.com/Genomes/VFI, supported by the W. M. Keck Foundation.
This work was supported by NIH grant GM59690 awarded to K.L.V. and by the National Science Foundation under a Research Fellowship in Microbial Biology awarded in 2001 to C.R.D.
REFERENCES
- 1.Adler, J. 1966. Chemotaxis in bacteria. Science 153:708-716. [DOI] [PubMed] [Google Scholar]
- 2.Boettcher, K. J., and E. G. Ruby. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172:3701-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlap, P. V., U. Mueller, T. A. Lisa, and K. S. Lundberg. 1992. Growth of the marine luminous bacterium Vibrio fischeri on 3′:5′-cyclic AMP: correlation with a periplasmic 3′:5′-cyclic AMP phosphodiesterase. J. Gen. Microbiol. 138:115-123. [Google Scholar]
- 4.Foster, J. S., and M. J. McFall-Ngai. 1998. Induction of apoptosis by cooperative bacteria in the morphogenesis of host epithelial tissues. Dev. Genes Evol. 208:295-303. [DOI] [PubMed] [Google Scholar]
- 5.Graf, J., P. V. Dunlap, and E. G. Ruby. 1994. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 176:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graf, J., and E. G. Ruby. 1998. Host-derived amino acids support the proliferation of symbiotic bacteria. Proc. Natl. Acad. Sci. USA 95:1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFall-Ngai, M. J. 1999. Consequences of evolving with bacterial symbionts: insights from the squid-vibrio associations. Annu. Rev. Ecol. Syst. 30:235-256. [Google Scholar]
- 8.Millikan, D. S., and E. G. Ruby. 2002. Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects. Appl. Environ. Microbiol. 68:2519-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyholm, S. V., B. Deplancke, H. R. Gaskins, M. A. Apicella, and M. J. McFall-Ngai. 2002. Roles of Vibrio fischeri and nonsymbiotic bacteria in the dynamics of mucus secretion during symbiont colonization of the Euprymna scolopes light organ. Appl. Environ. Microbiol. 68:5113-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyholm, S. V., E. V. Stabb, E. G. Ruby, and M. J. McFall-Ngai. 2000. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. USA 97:10231-10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pruss, B. M., J. M. Nelms, C. Park, and A. J. Wolfe. 1994. Mutations in NADH:ubiquinone oxidoreductase of Escherichia coli affect growth on mixed amino acids. J. Bacteriol. 176:2143-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruby, E. G., and K. H. Nealson. 1977. Pyruvate production and excretion by the luminous marine bacteria. Appl. Environ. Microbiol. 34:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visick, K. L., and M. J. McFall-Ngai. 2000. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J. Bacteriol. 182:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe, A. J., and H. C. Berg. 1989. Migration of bacteria in semisolid agar. Proc. Natl. Acad. Sci. USA 86:6973-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]