Abstract
Although most evident in the skin, the process of scarring, or fibrosis, occurs in all major organs because of impaired epithelial self-renewal. No current therapy exists for Idiopathic pulmonary fibrosis. The major profibrotic factor is TGF-β1 and developing inhibitors is an area of active research. Recently, IGFBP-5 has also been identified as a profibrotic factor, and studies suggest that, while both TGF-β1 and IGFBP-5 activate mesenchymal cells to increase collagen and fibronectin production, their effects on epithelial cells are distinct. TGF-β1 induces cell death and/or EMT in the epithelial cells, exacerbating the disruption of tissue architecture. In contrast, IGFBP-5 induces epithelial cell spreading over collagen or fibronectin matrices, increases secretion of laminin, the epithelial basement membrane, and enhances the survival of epithelial cells in nutrient-poor conditions, as exists in scar tissue. Thus, IGFBP-5 may enhance repair and may be an important target for antifibrotic therapies.
1. Introduction
Idiopathic Pulmonary fibrosis (IPF) is a nonneoplastic chronic lung syndrome that is characterized by aberrant accumulation of fibroblasts/myofibroblasts and progressive abnormal remodelling of lung parenchyma, with subsequent scarring and disruption of its structure and function. It belongs to a broad category of 200 disorders called diffuse pulmonary lung diseases (DPLDs) or simply interstitial lung diseases (ILDs). This is further classified into a subgroup known as idiopathic interstitial pneumonia (IIP), where IPF is one of the seven diseases, being the most prevalent and pernicious disorder. It is subclassified with a pathological condition known as usual interstitial pneumonia (UIP). Major symptoms include chronic dyspnea (shortness of breath) induced by 6-minute walk test, persistent dry cough, reduced lung volume, and impaired gas exchange. Constitutional symptoms include weight loss, fatigue, finger clubbing, and general malaise [1]. IPF has a poor prognosis and is five times more prevalent than cystic fibrosis and amyotrophic lateral sclerosis, with no FDA approved treatments. Despite considerable recent progress, current treatment regimens are largely unpromising with a median survival rate of less than three years from the date of diagnosis [2]. The precise mechanisms involved in the pathogenesis of IPF have not been completely understood. Here, in this paper, we attempt to highlight the pathology of pulmonary fibrogenesis from its history, possible cellular and molecular mechanisms, and draw comparisons between the major profibrotic factor TGF-β1 and a more recently described player, IGFBP-5.
2. IPF Incidence and Pathophysiology
IPF is usually a disease of the elderly and occurs after five decades of life, with the probability of incidence markedly increasing thereafter. The onset of disease is slow but with age and time, the symptoms increase. There are five million sufferers, with increasing world-wide incidence, that are affected by IPF [3]. The number of affected patients has doubled in the past decade [4] and inevitably will increase in developed countries due to increasing age of the population. The disease is slightly more prevalent in men [5] and strongly associated with cigarette smoking [6]. Recent statistics of large United States and UK population-based studies indicated a significant increase in mortality [7, 8]. Annually, 40,000 and 4,000 new cases were diagnosed each year in US and UK, respectively.
Pulmonary fibrosis can also occur due to a variety of stimuli ranging from life style habits such as cigarette smoking [6], occupational exposure to polluted environments, notably asbestos or silica [9], induced by pharmacological agents [10], radiation exposure [11], and genetic predisposition [5], or associated with other autoimmune abnormalities such as collagen vascular diseases [12, 13].
3. Epithelial Cell Injury and IPF
The complex signalling mechanisms of pulmonary epithelial cell injury in response to fibrosis are of considerable research interest. As intact alveolar epithelium is critical for maintaining lung homeostasis, initiation of repair mechanisms following the insult and injury, without compromising its structure and function, is of great importance. Our understanding of the relationship between injury of epithelia and development of IPF has increased dramatically in recent years. Current hypotheses suggest that microrepetitive injury to pulmonary epithelial cells results in ineffective repair with subsequent fibrogenesis. As previously mentioned, the sources of recurrent injury could be wide ranging and include toxic or drug-induced insults, autoimmune disease, traumatic or hypoxic injuries, and bacterial or viral infections. Recently, a high occurrence of Epstein Barr Virus (EBV) expressing latent membrane protein (LMB-1) has been reported in IPF patients with synergizing effects with TGF-β-induced EMT in lung epithelial cells [14]. Observations suggest that the release of soluble growth factors after injury including chemokines, eicosanoids, and interleukins has also shown to be involved in the resolution of epithelial repair processes. However, there may be compromise with respect to structure and functional organisation with varying degree of injuries.
4. The Role of Fibroblasts/Myofibroblasts in IPF
Effective therapies to prevent tissue fibrosis require a complete understanding of the mechanisms involved in the development of the disease, and one mechanism of particular interest is fibroblast development. Resident fibroblasts and resident activated fibroblasts (myofibroblasts) are critical cell types for the process of wound healing and also for the formation of fibrotic lesions in the pathogenesis of lung fibrosis. Fibroblasts express receptors for a number of cytokines including PDGF [15], TGF-β1 [16], and TNF-α [16]. These cytokines and others may mediate their recruitment and activation during injury. In addition to their involvement in skin wound healing [17–19], myofibroblasts are most commonly identified and well characterized in idiopathic pulmonary fibrosis [20]. Myofibroblasts are mesenchymal cells with characteristics of both fibroblasts and smooth muscle cells [21]. The origin of myofibroblasts is still under debate. However, persistence of fibroblasts/myofibroblasts at the site of injury and extracellular matrix (ECM) are critical for fibrotic lesion formation in IPF [22]. Thus, understanding the mechanism of the gradual decrease of myofibroblasts, as occurs in normal wound healing, should be important for the resolution of pulmonary fibrosis.
5. Epithelial to Mesenchymal Transition
EMT is a prominent manifestation of cell plasticity observed in three distinct cellular processes: embryonic development, metastasis, and fibrosis. Here, as per the context, determination of the cellular source of myofibroblasts is crucial in understanding the pathogenesis of tissue fibrosis. Myofibroblasts appear to have at least three possible origins. Alveolar epithelial damage with consequent EMT, activation of fibroblasts, and circulating fibrocytes occur through detection of injurious signals including TGF-β, platelet-derived growth factor (PDGF), IL-13, and connective tissue growth factor (CTGF). This results in the transdifferentiation of myofibroblasts, with smooth muscle actin, MMP-2 and MMP-9 expression, and excessive collagen production [20]. Resident fibroblasts can respond to a variety of profibrotic mediators and differentiate into myofibroblasts [23]. TGF-β1 induces transdifferentiation of fibroblasts, through a Smad3-dependent mechanism, to myofibroblasts [24]. In vivo overexpression of IGFBP-5 induces increased expression of α-SMA and vimentin in dermal and lung fibroblasts suggesting fibroblast transdifferentiation [25, 26].
Epithelial-myofibroblast transdifferentiation from epithelial cells is a specialized version of epithelial-mesenchymal transition (EMT), a physiological process in which epithelial cells can acquire the invasive and motile properties of mesenchymal cells [27]. Alveolar epithelial cells have been shown to undergo EMT in vivo during the development of pulmonary fibrosis [28]. There is also compelling evidence suggesting EMT in alveolar epithelial cells following exposure to TGF-β both in vitro and in vivo [29–31]. In addition, circulating bone marrow-derived fibrocytes behave like mesenchymal stem cells and migrate into sites of lung injury and become myofibroblasts. These fibrocytes express type 1 collagen and α-SMA and contribute to lung fibrosis [32]. It is interesting to note that TGF-β1-induced EMT is reversed by fibroblast growth factor-1, through ERK phosphorylation and Smad2 dephosphorylation [33]. This implies that the therapeutic role of FGF-1 should be defined in IPF, as inhibiting TGF-β globally may have adverse effects on its role in tissue homeostasis.
6. Extracellular Matrix and IPF
There is now mounting evidence suggesting that extracellular matrix (ECM) is involved in both normal physiology as well as in wide variety of pathophysiological processes. One possible mechanism of pulmonary fibrosis is the disruption of ECM which enables cell-to-cell contact of epithelial cells with fibroblasts, leading to the epithelial cell induction of fibroblast- and myofibroblast-derived signalling molecules. The end process of EMT is the degradation of underlying extracellular matrix with the formation of additional mesenchymal cells. Epithelial cell injury causes the release of a wide variety of growth factors, chemokines and MMPs, notably MMP-2, MMP-3, and MMP-9, interleukins, and prostaglandins. Under the influence of these signalling molecules, epithelial cells, acting together with inflammatory cells, induce basement membrane disruption and focal degradation of type IV collagen and laminin [34].
7. Role of TGF-β in IPF
Transforming growth factor beta (TGF-β) is a pleiotropic regulatory cytokine that is ubiquitously expressed by all cells and tissues within the body. TGF-β1, of the three highly homologous mammalian isoforms (TGF-β1-3), is thought to play a central mediator role in wound healing and fibrosis [35–37]. There is compelling evidence that TGF-β1 plays a pivotal role in pathophysiology of IPF. There is increasing evidence pointing to the profibrogenic effects of TGF-β both in animal models of IPF and in IPF patients. Induction of EMT in alveolar epithelial cells following TGF-β1 overexpression in triple transgenic mice and via adenoviral-mediated gene transfer induces severe pulmonary fibrosis. In contrast, ex vivo alveolar epithelial cells cultured on laminin/collagen mixtures undergo programmed cell death when exposed to active TGF-β [28].
8. Integrin-Mediated Activation of TGF-β
The integrins are a large family of transmembrane heterodimeric cell adhesion receptors which mediate cell-to-surface interactions either to the extracellular cellular matrix or to specific cell-surface receptors during cell-cell interactions [38, 39]. They are composed of α and β dimers in a noncovalent complex, comprising 24 combinations of 18 α and 8 β subunits. These receptors function as important regulators that control whole sets of cellular processes including cell spreading, retraction, migration, and proliferation. Integrins contain the specialized binding regions for physical attachment of cells to the ECM, and through intracellular domains they form connections to various components of the actin cytoskeleton and a wide variety of interconnecting signalling adaptors [40].
The cross talk between integrins and TGF-β signalling is of considerable interest in a wide variety of physiological and pathophysiological processes including fibrosis, cancer, and wound healing. The molecular interactions between TGF-β and integrins αVβ6 and αVβ8 are of particular interest in the pathogenetic mechanisms of pulmonary fibrogenesis. The αVβ6 integrin-dependent activation of TGF-β requires the binding of the β6 cytoplasmic tail to the actin cytoskeleton [41]. This activation of TGF-β is highly dependent on the association of inactive (latent) forms of TGF-β and TGF-β binding protein-1 (LTBP-1) of the large latent complex with αVβ6 integrin [42]. Furthermore, lysophosphatidic acid and stimulation of protease-activated receptor 1 have shown to activate αVβ6-mediated activation of TGF-β1 via RhoA and are implicated in the pathogenensis of acute lung injury and pulmonary fibrosis [43, 44]. Partial inhibition of αVβ6 integrin-mediated activation of TGF-β1 using small molecule inhibitors has been shown to possess antifibrotic activity, without pulmonary inflammation and emphysema [45].
9. Role of IGFBP-5 in IPF
IGFBP-5 is one of the members of insulin-like growth-factor-binding protein (IGFBP) family which bind to and modulate the biological actions of insulin-like growth factors by interference with receptor binding although these IGFBPs also have IGF-independent actions [46]. Increased expression of IGFBP-5 has been described in fibrosis [47, 48]. Increased expression of IGFBP-5 at mRNA and protein levels was also evident in vitro in primary fibroblasts cultured from disease-affected skin of patients with systemic sclerosis/scleroderma, compared with the skin from their healthy twins. It has been demonstrated that IGFBP-5 induces collagen and fibronectin production from fibroblasts and induces fibroblast/myofibroblast transdifferentiation in vitro and in vivo [25, 26]. Finally, in vivo overexpression of IGFBP5, using replication-deficient adenovirus, induced skin fibrosis in mice which included increased thickness of the dermis and increased collagen bundle thickness [25] and induced pulmonary fibrosis with myofibroblastic changes [26]. Increased expression of α-SMA and vimentin in dermal fibroblasts was also evident with overexpression of IGFBP-5. Collectively, these findings suggest that upregulated expression of IGFBP5 could be an initiating event in ECM production and indicate its involvement in the development of fibrosis.
10. Integrin-Dependent Interaction of IGFBP-5
Integrin αVβ6 expression is constitutively expressed at low levels in epithelial tissues and was significantly increased during injury but was unable to potentiate fibrosis [49]. Recently, we demonstrated that αVβ6 integrin is also essential in addition to α2 and β1 integrins for the IGFBP-5-induced cell to extracellular matrix adhesion in MCF-7 cells. Using biosensor technology, we showed a novel, direct, high affinity interaction of IGFBP-5 with α2 and β1 integrins leading to the activation of ILK and Akt and improved epithelial cell adhesion and survival (our unpublished observations). Apoptosis of epithelial cells induces IGFBP-5 which activates tissue plasminogen activator (tPA), generating plasmin, and also interacts with several matricellular proteins [50]. Thus, IGFBP-5 may act as a central mediator in the initial response of epithelial cell injury.
This provides an intriguing possibility in which IGFBP-5 acts indirectly, extracellularly, in a similar fashion to connective tissue growth factor (CTGF), which also serves as a mediator of the actions of TGF-β1 [51]. CTGF is structurally related to IGFBP-5 and interacts with integrins to elicit its actions, rather than using classic cell-surface receptors. In fact, there are similarities between IGFBP-5 and other members of the secreted cysteine-rich (CCN) protein family which includes CTGF, CYR61, and Nov. The CCN molecules were temporarily renamed IGFBP8-10 because of their structural relationship with the IGFBP family. However, we believe that IGFBP-5 may be a member of the CCN family since it binds and activates integrins, interacts with the ECM via a heparin-binding domain, and influences growth factor actions (IGFs) indirectly by binding to them. In analogous fashion, CTGF as well as binding to integrins and the ECM binds to VEGF [52] to inhibit its actions and bind to, and enhances the actions of, TGF-β1 [53].
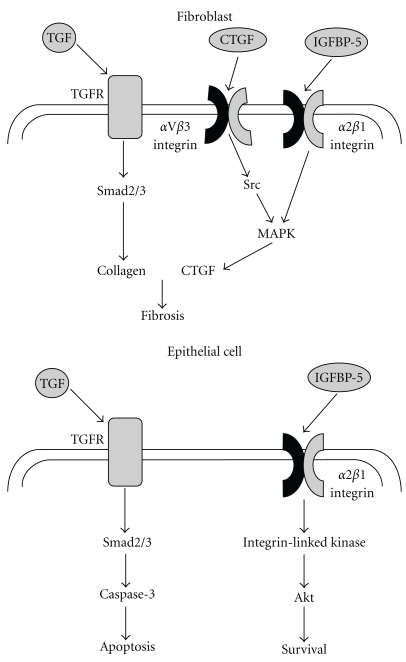
The adhesive responses to IGFBP-5 could be anticipated to enhance re-epithelialisation during injury by increasing the surface area of epithelial cells when epithelia are exposed to a mesenchymal environment, whilst concurrently inhibiting their migration into the mesenchymal compartment. Thus, IGFBP-5 would be anticipated to display antagonistic actions to TGF-β1 in the epithelial compartment whilst enhancing its actions in the mesenchymal compartment (Figure 1).
Figure 1.
Differential effects of TGF-β1 and IGFBP-5 on mesenchymal and epithelial cells. In fibroblasts, TGF-β1 activates the Smad pathway resulting in activation of genes associated with fibrosis such as collagen, fibronectin, and CTGF. CTGF in turn further activates this process via an aVb3 integrin-dependent mechanism. IGFBP-5 binds to and activates α2β1 integrins and is capable of activating MAPK pathways in similar fashion to CTGF. In contrast, TGF-β1 activation of the Smad pathway results in an apoptotic pathway in epithelial cells, whereas IGFBP-5, again acting through α2β1 integrin, stimulates an adhesive, prosurvival pathway. Thus, IGFBP-5 enhances TGF-β1 actions in the mesenchymal compartment but inhibits them in the epithelium.
11. TGF-β and IGFBP-5: Redundancy or Complementarity of Actions?
The recent studies implicating IGFBP-5 in the development of fibrosis begs the question “Do TGF-β1 and IGFBP-5 serve similar roles and, if so, why? We believe that, although their roles in the mesenchymal compartment are similar and certainly complementary, their actions in the epithelial compartment are different. Whereas TGF-β1 is clearly apoptotic and induces EMT in a proportion of the epithelial cells, thus disrupting the epithelial compartment and its functions both as a physical barrier and in, for example, gas exchange in the lung, the actions of IGFBP-5 are quite distinct. We have shown that IGFBP-5 induces an adhesive action [50] in epithelial cells exposed to a mesenchymal environment, leading to increased cell spreading and decreased migration and propose that this serves as a mechanism to prevent epithelial egress from, and mesenchymal ingress into, the epithelial compartment. IGFBP-5 also increases epithelial production of the basement membrane protein, laminin [54]. These actions would be anticipated to limit the boundary of the fibrotic response to the underlying stroma. Thus, IGFBP-5 may be a more relevant target in therapies of wound repair than TGF-β1, since it enhances fibrosis but limits scar formation to the mesenchymal compartment, thereby preventing excessive disruption of the epithelium. This contrasts with the uncontrolled fibrotic response characteristically produced by TGF-β1.
12. Clinical Implications
Fibrotic diseases in general, and idiopathic pulmonary fibrosis in particular, represent disease states for which no therapies currently exist. Targeting the major fibrotic agents may provide the prospect of developing such therapies, and TGF-β1 is, indeed, the target of research programmes seeking pharmacological inhibitors. The complementary roles of TGF-β1 and IGFBP-5 in alveolar epithelial cell injury provide additional options for drug targets although a better understanding of their precise mechanisms of action will be essential to enhance the prospects of success. Nevertheless, the potential protective role of IGFBP-5 in the epithelium is particularly amenable to aerosol-mediated drug delivery systems, since this would provide direct access to the site of action required for effective re-epithelialisation to occur. Possible approaches could include smaller domains of the IGFBP-5 molecule, which can exert these effects, drugs which mimic its adhesive, survival effects, or drugs, such as retinoic acid and vitamin D analogues, that are known to stimulate its production in epithelial cells [55–57].
Acknowledgment
The authors thank the BBSRC for funding parts of the research referred to in this paper.
References
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) American Journal of Respiratory and Critical Care Medicine. 2000;161(2 part 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Tzilas V, Koti A, Papandrinopoulou D, Tsoukalas G. Prognostic factors in idiopathic pulmonary fibrosis. American Journal of the Medical Sciences. 2009;338(6):481–485. doi: 10.1097/MAJ.0b013e3181ad5984. [DOI] [PubMed] [Google Scholar]
- 3.Sharma OP, Chan K. Idiopathic interstitial pneumonitis/fibrosis: a historical note. Current Opinion in Pulmonary Medicine. 1999;5(5):275–277. doi: 10.1097/00063198-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Slutsky AS. Idiopathic pulmonary fibrosis—new insights. New England Journal of Medicine. 2007;356(13):1370–1372. doi: 10.1056/NEJMcibr070490. [DOI] [PubMed] [Google Scholar]
- 5.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. New England Journal of Medicine. 2001;345(7):517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 1997;155(1):242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 7.Gribbin J, Hubbard RB, Le Jeune I, Smith CJP, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61(11):980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. American Journal of Respiratory and Critical Care Medicine. 2007;176(3):277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 9.Governa M, Amati M, Fontana S, et al. Role of iron in asbestos-body-induced oxidant radical generation. Journal of Toxicology and Environmental Health A. 1999;58(5):279–287. doi: 10.1080/009841099157241. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira KC, Soares FS, Rocha LGC, et al. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulmonary Pharmacology and Therapeutics. 2008;21(2):309–316. doi: 10.1016/j.pupt.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Newman LS, Mroz MM, Ruttenber AJ. Lung fibrosis in plutonium workers. Radiation Research. 2005;164(2):123–131. doi: 10.1667/rr3407. [DOI] [PubMed] [Google Scholar]
- 12.Lynch DA. Lung disease related to collagen vascular disease. Journal of Thoracic Imaging. 2009;24(4):299–309. doi: 10.1097/RTI.0b013e3181c1acec. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. American Journal of Respiratory and Critical Care Medicine. 2008;178(12):1257–1261. doi: 10.1164/rccm.200805-725OC. [DOI] [PubMed] [Google Scholar]
- 14.Sides MD, Klingsberg RC, Shan B, et al. The Epstein-Barr virus LMP 1 and TGF-β1 synergistically induce EMT in lung epithelial cells. doi: 10.1165/rcmb.2009-0232OC. American Journal of Respiratory Cell and Molecular Biology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alpers CE, Hudkins KL, Floege J, Johnson RJ. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. Journal of the American Society of Nephrology. 1994;5(2):201–210. doi: 10.1681/ASN.V52201. [DOI] [PubMed] [Google Scholar]
- 16.Noronha IL, Niemir Z, Stein H, Waldherr R. Cytokines and growth factors in renal disease. Nephrology Dialysis Transplantation. 1995;10(6):775–786. [PubMed] [Google Scholar]
- 17.Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971;173(3996):548–550. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- 18.Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 19.Hebda PA, Collins MA, Tharp MD. Mast cell and myofibroblast in wound healing. Dermatologic Clinics. 1993;11(4):685–696. [PubMed] [Google Scholar]
- 20.Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent advances in molecular targets and treatment of Idiopathic Pulmonary Fibrosis: focus on TGFβ signaling and the myofibroblast. Current Medicinal Chemistry. 2009;16(11):1400–1417. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 21.Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Laboratory Investigation. 1990;63(2):144–161. [PubMed] [Google Scholar]
- 22.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis: a combined immunohistochemical and in situ hybridization study. American Journal of Pathology. 1994;145(1):114–125. [PMC free article] [PubMed] [Google Scholar]
- 23.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 24.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-β-induced α-smooth muscle actin expression. American Journal of Respiratory Cell and Molecular Biology. 2003;29(3, part 1):397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 25.Yasuoka H, Jukic DM, Zhou Z, Choi AMK, Feghali-Bostwick CA. Insulin-like growth factor binding protein 5 induces skin fibrosis: a novel murine model for dermal fibrosis. Arthritis and Rheumatism. 2006;54(9):3001–3010. doi: 10.1002/art.22084. [DOI] [PubMed] [Google Scholar]
- 26.Yasuoka H, Zhou Z, Pilewski JM, Oury TD, Choi AMK, Feghali-Bostwick CA. Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltration. American Journal of Pathology. 2006;169(5):1633–1642. doi: 10.2353/ajpath.2006.060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radisky DC. Epithellial-mesenchymal transition. Journal of Cell Science. 2005;118(19):4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 28.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respiratory Research. 2005;6, article 56 doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z, Yang L, Cai L, et al. Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse. Respiratory Research. 2007;8, article 1 doi: 10.1186/1465-9921-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willis BC, DuBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proceedings of the American Thoracic Society. 2006;3(4):377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. Journal of Clinical Investigation. 2004;113(2):243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos C, Becerril C, Montaño M, et al. FGF-1 reverts epithelial-mesenchymal transition induced by TGF-β1 through MAPK/ERK kinase pathway. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2010;299(2):L222–L231. doi: 10.1152/ajplung.00070.2010. [DOI] [PubMed] [Google Scholar]
- 34.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. Journal of Clinical Investigation. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CG, Kang HR, Homer RJ, Chupp G, Elias JA. Transgenic modeling of transforming growth factor-β1: role of apoptosis in fibrosis and alveolar remodeling. Proceedings of the American Thoracic Society. 2006;3(5):418–423. doi: 10.1513/pats.200602-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts AB, Sporn MB. Transforming growth factor-b. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. New York, NY, USA: Plenum Press; 1996. pp. 275–308. [Google Scholar]
- 37.Flanders KC. Smad3 as a mediator of the fibrotic response. International Journal of Experimental Pathology. 2004;85(2):47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nature Cell Biology. 2002;4(4):E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 39.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 40.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nature Cell Biology. 2007;9(8):858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munger JS, Huang X, Kawakatsu H, et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 42.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin αβ-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. Journal of Cell Biology. 2004;165(5):723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howell DC, Laurent GJ, Chambers RC. Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis. Biochemical Society Transactions. 2002;30(2):211–216. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 44.Xu MY, Porte J, Knox AJ, et al. Lysophosphatidic acid induces αvβ36 integrin-mediated TGF-β activation via the LPA2 receptor and the small G protein Gα(q) American Journal of Pathology. 2009;174(4):1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura SL. Integrin-mediated transforming growth factor-β activation, a potential therapeutic target in fibrogenic disorders. American Journal of Pathology. 2009;175(4):1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochemical Journal. 2006;395(1):1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. American Journal of Pathology. 2005;166(2):399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feghali CA, Wright TM. Identification of multiple, differentially expressed messenger RNAs in dermal fibroblasts from patients with systemic sclerosis. Arthritis and Rheumatism. 1999;42(7):1451–1457. doi: 10.1002/1529-0131(199907)42:7<1451::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 49.Huang X, Wu J, Zhu W, Pytela R, Sheppard D. Expression of the human integrin β6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in β6 knockout mice. American Journal of Respiratory Cell and Molecular Biology. 1998;19(4):636–642. doi: 10.1165/ajrcmb.19.4.3293. [DOI] [PubMed] [Google Scholar]
- 50.Sureshbabu A, Okajima H, Yamanaka D, et al. IGFBP-5 induces epithelial and fibroblast responses consistent with the fibrotic response. Biochemical Society Transactions. 2009;37(4):882–885. doi: 10.1042/BST0370882. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen TQ, Goldschmeding R. Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? Pharmaceutical Research. 2008;25(10):2416–2426. doi: 10.1007/s11095-008-9548-9. [DOI] [PubMed] [Google Scholar]
- 52.Inoki I, Shiomi T, Hashimoto G, et al. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB Journal. 2002;16(2):219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 53.Abreu JG, Ketpura NI, Reversade B, de Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β . Nature Cell Biology. 2002;4(8):599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abrass CK, Hansen KM. Insulin-like growth factor-binding protein-5-induced laminin γ1 transcription requires filamin A. Journal of Biological Chemistry. 2010;285(17):12925–12934. doi: 10.1074/jbc.M109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwa V, Oh Y, Rosenfeld RG. Insulin-like growth factor binding protein-3 and -5 are regulated by transforming growth factor-β and retinoic acid in the human prostate adenocarcinoma cell line PC-3. Endocrine. 1997;6(3):235–242. doi: 10.1007/BF02820498. [DOI] [PubMed] [Google Scholar]
- 56.LeRoith D, Adamo ML, Shemer J, et al. Retinoic acid inhibits growth of breast cancer cell lines: the role of insulin-like growth factor binding proteins. Growth Regulation. 1993;3(1):78–80. [PubMed] [Google Scholar]
- 57.Nickerson T, Huynh H. Vitamin D analogue EB1089-induced prostate regression is associated with increased gene expression of insulin-like growth factor binding proteins. Journal of Endocrinology. 1999;160(2):223–229. doi: 10.1677/joe.0.1600223. [DOI] [PubMed] [Google Scholar]