Abstract
Objective:
Previous studies have estimated that wake-up strokes comprise 8%to 28% of all ischemic strokes, but these studies were either small or not population-based. We sought to establish the proportion and event rate of wake-up strokes in a large population-based study and to compare patients who awoke with stroke symptoms with those who were awake at time of onset.
Methods:
First-time and recurrent ischemic strokes among residents of the Greater Cincinnati/Northern Kentucky region (population 1.3 million) in 2005 were identified using International Classification of Diseases–9 codes 430–436 and verified via study physician review. Ischemic strokes in patients aged 18 years and older presenting to an emergency department were included. Baseline characteristics were ascertained, along with discharge modified Rankin Scale scores and 90-day mortality.
Results:
We identified 1,854 ischemic strokes presenting to an emergency department, of which 273 (14.3%) were wake-up strokes. There were no differences between wake-up strokes and all other strokes with regard to clinical features or outcomes except for minor differences in age and baseline retrospective NIH Stroke Scale score. The adjusted wake-up stroke event rate was 26.0/100,000. Of the wake-up strokes, at least 98 (35.9%) would have been eligible for thrombolysis if arrival time were not a factor.
Conclusions:
Within our population, approximately 14% of ischemic strokes presenting to an emergency department were wake-up strokes. Wake-up strokes cannot be distinguished from other strokes by clinical features or outcome. We estimate that approximately 58,000 patients with wake-up strokes presented to an emergency department in the United States in 2005.
IV tissue plasminogen activator (tPA) remains the only medication approved by the Food and Drug Administration for the treatment of ischemic stroke. The original National Institute of Neurological Disorders and Stroke study restricted tPA use to 3 hours following known symptom onset,1 and the recent European Cooperative Acute Stroke Study 3 study extended that window to 4.5 hours in selected patients.2 Patients who go to sleep healthy and wake up with symptoms beyond this time window are not eligible for treatment based on these time restrictions. Previous studies have estimated that wake-up strokes comprise 8% to 28% of all ischemic strokes,3–14 but these studies either were not population-based or were relatively small. Some of these studies have suggested differences between wake-up strokes and non-wake-up strokes, reporting that wake-up strokes had greater initial stroke severity6,13 and were more likely to have a poor outcome,10,13 while other studies have found no appreciable differences.8,9 We sought to establish the proportion and event rate of wake-up strokes within a large biracial population and to evaluate possible differences between wake-up and non-wake-up strokes. We also sought to establish how many patients with wake-up strokes would have been eligible for tPA if time were not a factor.
METHODS
The Greater Cincinnati/Northern Kentucky region includes 2 southwestern Ohio counties and 3 contiguous Kentucky counties bordering the Ohio River. Its biracial population of approximately 1.3 million is largely representative of the United States in terms of age, proportion of black subjects, and economic status.15 There were 17 hospitals active in this area in 2005. While residents of surrounding counties may seek care at these hospitals, only residents of the 5 study counties were eligible for this analysis. Previous studies have shown that residents of these 5 counties who have a stroke seek care at these hospitals rather than going to more distant hospitals.15
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Board of all participating hospitals.
A detailed description of the epidemiologic methods employed for the Greater Cincinnati/Northern Kentucky Stroke Study has been described previously.15–17 Briefly, study nurses abstracted the medical records of all area residents who were either inpatients or discharged from an emergency department (ED) with primary or secondary stroke-related International Classification of Disease, ninth revision, discharge diagnoses 430–436 at the 17 area hospitals. Strokes not found through this methodology were ascertained via screening of all stroke-related visits to public health clinics, hospital-based outpatient clinics, and family practice centers. Further monitoring involved examination of records of potential stroke cases in a random sample of 51 of the 832 primary care physicians' offices and 25 of the 126 nursing homes in the Greater Cincinnati/Northern Kentucky region.
To qualify as a case, a patient must have met clinical criteria adapted from the Classification of Neurological Disorders III18 and from previous epidemiologic studies of stroke in Rochester, MN.19 Charts were screened for an additional 60 days beyond the end of the study period to capture patients with a stroke during the study period but not yet discharged from the hospital. A study physician reviewed every abstract and all available neuroimaging studies to determine whether a stroke had occurred.
We restricted the present study group to adult patients (age 18 years and older) with acute ischemic stroke who presented to an ED because thrombolysis outside an ED setting is difficult. Both first-time and recurrent ischemic stroke events were included. Transient ischemic attacks, defined as symptoms lasting less than 24 hours without neuroradiologic evidence of infarction, were not included in this analysis. The onset of stroke symptoms had to occur within the calendar year 2005.
The abstractors recorded the date and time of stroke onset, if known. If onset time was unknown, the following mutually exclusive options were available for estimation of onset: “awoke with symptoms,” “presentation >24 hours after onset time,” “after midnight” (defined as 00:01 am to 06:00 am), “morning” (06:01 am to noon), “afternoon” (12:01 pm to 18:00 pm), and “evening” (18:01 pm to midnight). Stroke severity was represented by using a validated method20,21 for estimating the NIH Stroke Scale Score retrospectively (rNIHSS) by review of the physician examination as documented in the emergency department evaluation. Clinical outcome was assessed using hospital discharge modified Rankin Scale scores (mRS) and 90-day mortality rates.
The abstractors also recorded data corresponding to non-time-related exclusion criteria for IV thrombolysis. Patients were considered eligible for tPA if they met the standard inclusion/exclusion criteria as per the current standard of care.22 Variables pertinent to tPA exclusion that were not uniformly gathered in our abstracting process include history of head trauma, history of gastrointestinal or urinary tract hemorrhage within 3 months, arterial puncture at a noncompressible site in the previous 7 days, and evidence of active bleeding or acute trauma on examination.
Data were managed and analyzed using SAS®, versions 8.02 and 9.2, respectively (SAS Institute, Cary, NC). Population estimates were obtained by including sampling weights in all analyses. The relationships of demographic and clinical characteristics with stroke type (wake-up and non-wake-up) were analyzed with generalized estimating equations (GEE) to account for multiple stroke occurrences in some patients as well as for the sampling weights for the cases ascertained from physicians' offices and nursing homes.23 The working correlation structure that gave the best model fit was obtained. A binary or multinomial distribution was specified for categorical variables, as appropriate. Multiple logistic regression analysis with GEE was used to examine outcome differences between wake-up strokes and strokes that occurred when the patient was awake after adjusting for age, sex, race, prestroke mRS, rNIHSS, and prior atrial fibrillation. Covariates were determined a priori, as having been shown to be associated with the outcome in other studies, or if differences were observed between wake-up and non-wake-up strokes at a p value <0.25. We also calculated an event rate for wake-up strokes presenting to an ED with age, sex, and race adjustment to the 2000 US Census. The event rate was calculated for adults only (age ≥18). Extrapolation to the US population was based on an estimated total population of 296 million in 2005,24 with approximately 25% of the population younger than 18.25 Data are reported as raw quantiles, raw frequencies with weighted percentages, and weighted means and standard errors.
RESULTS
We identified 1,778 adult area residents, with 1,854 first-ever and recurrent ischemic strokes that were first evaluated in an ED in 2005; 273 wake-up strokes (14.3%, 95% confidence interval [CI] 12.7–15.9) occurred in 271 subjects. Seventy-three patients had more than one stroke identified during 2005; 70 patients had 2 strokes reported during the year and 3 patients had 3 strokes. Eighteen patients had both a wake-up stroke and a non-wake-up stroke during the year. The exact times of stroke onset and arrival to the ED were recorded for 637 non-wake-up strokes, of which 428 presented to the ED within 3 hours of onset and 209 presented beyond 3 hours of stroke onset. Of the 944 non-wake-up strokes without a documented time of onset to arrival, 362 had an estimated time of stroke in one of the 6-hour windows, 358 presented to the ED greater than 24 hours after onset, 219 had no estimate of stroke onset, and 5 had no recorded time of arrival to the ED. There were 11 cases ascertained through out-of-hospital surveillance. The adjusted event rate of wake-up strokes presenting to an ED was 26.0/100,000 (95% CI 22.9–29.1).
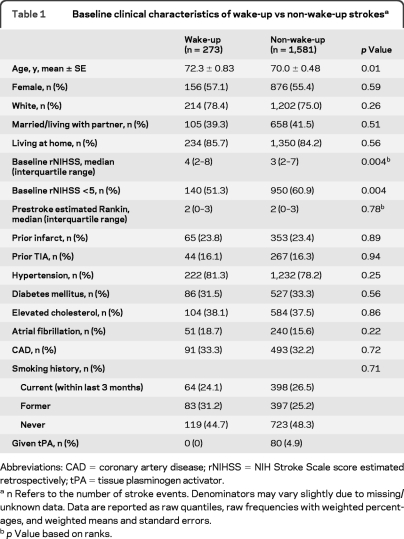
A comparison of the baseline characteristics of wake-up stroke patients with all others is found in table 1 . Compared with the non-wake-up stroke group, wake-up stroke patients were older (72.3 ± 0.83 years vs 70.0 ± 0.48 years; p = 0.01) and had higher baseline rNIHSS scores (median [IQR] = 4 [2, 8] vs 3 [2, 7]; p = 0.004). There were no differences between the groups with regard to sex, race, marital/partner status, residence at home, stroke risk factors, or estimated prestroke mRS. None of the patients in the wake-up group received tPA, while 80 (4.9%) of all non-wake-up strokes received tPA.
Table 1.
Baseline clinical characteristics of wake-up vs non-wake-up strokesa
Abbreviations: CAD = coronary artery disease; rNIHSS = NIH Stroke Scale score estimated retrospectively; tPA = tissue plasminogen activator.
n Refers to the number of stroke events. Denominators may vary slightly due to missing/unknown data. Data are reported as raw quantiles, raw frequencies with weighted percentages, and weighted means and standard errors.
p Value based on ranks.
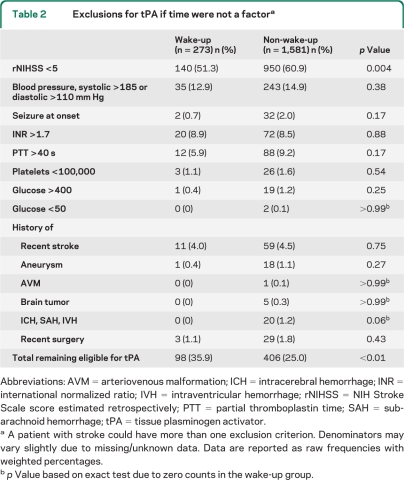
Table 2 compares the eligibility for thrombolysis, with time eliminated as an exclusion criterion, for the wake-up and non-wake-up strokes. The most common exclusion in both groups was a mild stroke, as more than half of the patients in each group had rNIHSS scores <5. The number with mild stroke was significantly higher in the non-wake-up strokes (p = 0.004). Extreme hypertension and coagulopathies were also common exclusions. In the wake-up group, 98 (35.9%, 95% CI 30.4–41.8) patients would have been eligible for thrombolysis, compared with 406 (25.0%, 95% CI 22.8–27.4) in the non-wake-up group (p < 0.01).
Table 2.
Exclusions for tPA if time were not a factora
Abbreviations: AVM = arteriovenous malformation; ICH = intracerebral hemorrhage; INR = international normalized ratio; IVH = intraventricular hemorrhage; rNIHSS = NIH Stroke Scale score estimated retrospectively; PTT = partial thromboplastin time; SAH = subarachnoid hemorrhage; tPA = tissue plasminogen activator.
A patient with stroke could have more than one exclusion criterion. Denominators may vary slightly due to missing/unknown data. Data are reported as raw frequencies with weighted percentages.
p Value based on exact test due to zero counts in the wake-up group.
With regard to short-term outcomes, there were no significant differences in the discharge mRS scores or 90-day mortality after adjusting for age, sex, race, prestroke mRS, rNIHSS, and prior atrial fibrillation between the wake-up and non-wake-up groups. Ninety-day mortality rates were 15.8% in the wake-up group and 15.6% in all others (p = 0.57). Median discharge mRS was 3 in both the wake-up group and all others (p = 0.94).
The previous analysis assumed that the 219 strokes with no estimate of stroke onset time were non-wake-up strokes. In order to evaluate the potential for misclassification, the data were reanalyzed with the assumption that the 219 were wake-up strokes. With this reclassification, the only change was that baseline rNIHSS was no longer significantly different between the 2 groups (median rNIHSS [IQR] was 4 [2, 8] for wake-up and 3 [2, 7] for non-wake-up, p = 0.70; the percentage with rNIHSS <5 was 57% for wake-up and 61% for non-wake-up, p = 0.20). Therefore, potential misclassification of strokes with unknown stroke onset times did not affect the conclusions drawn from this study.
DISCUSSION
Wake-up strokes comprised approximately 14% of ischemic stroke cases that presented to an ED in the Greater Cincinnati/Northern Kentucky population in 2005. Aside from minor differences in age and baseline rNIHSS, we did not find significant differences between wake-up strokes and all other ischemic strokes with regard to baseline demographics, risk factor profiles, or clinical outcome. Based upon extrapolation from our data, we estimate that approximately 58,000 patients with wake-up ischemic strokes presented to an ED in the United States in 2005. Our data suggest that at least a third of the wake-up strokes would have been eligible for IV tPA if time were not a factor and thus represent a group of patients that could be a focus for future trials of acute therapy.
In the past 20 years, several large studies have reported that the proportion of wake-up stroke ranges from 14% to 28%.3,5,–10,13,14 The CASPR investigators reported in their survey of 11 hospitals in 5 different population regions of California that 8% of strokes were wake-up.11 A few smaller community-based studies have also been performed. Investigators included 375 patients from a single Italian territory with a population of about 50,000. The authors found that wake-up strokes occurred in 18% of their patients but noted that many of these patients did not have a head CT, and therefore it is unclear how many of these cases were true acute ischemic strokes.4 Investigators in the Netherlands found that 48 of 263 patients (18%) had wake-up strokes over the course of 3 months in a population of 520,000.12 Our study reports a similar proportion of wake-up strokes with the advantage of a larger, biracial population of 1.3 million.
The limitations of time-based therapy and the possibility that some patients with wake-up strokes may benefit from treatment have prompted investigations into possible interventions for this group of patients. The AbESTT investigators initially included wake-up strokes as part of the eligible study population but stopped enrolling these patients because the rate of symptomatic ICH with study treatment was unacceptably high.26 Other investigators have reported better results in small, retrospective studies.27,28 Current trials utilizing multimodal imaging to better select patients beyond the standard time window, including those who wake up with their symptoms, are ongoing.29–32
A significant strength of this article is the population-based methodology. Our study is the largest population-based study of stroke incidence in the United States, and our largely representative population allows us to quantify the problem of wake-up strokes on a national scale. Studies from single centers or major academic centers are subject to referral bias.
There are some limitations to this study. The study is retrospective in nature and, despite best efforts at complete case ascertainment, it is possible that some cases were missed despite the surveillance methods in place. Furthermore, this study included only acute ischemic stroke patients who presented to an ED and did not include those evaluated only in other settings. Another potential problem is misclassification bias with regard to timing. Approximately 12% of the strokes in our population did not have a clear or estimated time of onset, and if some or all of these cases were wake-up strokes the event rate could increase substantially. Underdocumentation bias is a potential problem in an ED setting because subjects who were clearly not treatment candidates might not have had as thorough neurologic examinations or rigorous documentation of treatment exclusions as potential candidates. Finally, our estimation of patients eligible for treatment is a conservative underestimate of those eligible, since many treating physicians regard NIHSS <5 and elevated blood pressure (among others) as relative rather than absolute contraindications for tPA treatment. Indeed, the 2007 acute stroke treatment guidelines do not recommend using a strict NIHSS cutoff for thrombolysis but rather recommend treatment for disabling symptoms.
Our study indicates that there are no obvious distinguishing features between wake-up and non-wake-up strokes. Wake-up strokes constitute a significant percentage of ischemic strokes and are ineligible for thrombolytic therapy due to the current time-based restrictions, which is unfortunate because it is likely that some of these events occurred immediately prior to awakening. Efforts are ongoing to develop better methods of identifying those patients most likely to benefit from treatment while at the same time minimizing exposure to undue risk.
ACKNOWLEDGMENT
The authors thank the following for their participation in the Greater Cincinnati/Northern Kentucky Stroke Study: University Hospital; Good Samaritan Hospital; Bethesda North Hospital; Christ Hospital; St. Elizabeth Edgewood, Covington, Florence, and Ft. Thomas Hospitals; Mercy Anderson, Clermont, Fairfield, Mt. Airy, and Western Hills Hospitals as well as the Jewish Hospital; Deaconess Hospital; Cincinnati Children's Medical Center; and the Cincinnati Veteran Affairs Medical Center.
Footnotes
- CI
- confidence interval
- ED
- emergency department
- GEE
- generalized estimating equations
- mRS
- modified Rankin Scale
- rNIHSS
- NIH Stroke Scale score estimated retrospectively
- tPA
- tissue plasminogen activator
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Drs. Sucharew, Khoury, and Hornung.
DISCLOSURE
Dr. Mackey receives research support from the NIH. Dr. Kleindorfer has served on a scientific advisory board for Boehringer Ingelheim; serves on the speakers' bureau for Genentech, Inc.; serves on the editorial board of Stroke; receives research support from the NIH/NINDS and AAMC/CDC; and has provided medico-legal case review. Dr. Sucharew receives research support from the NIH. Dr. Moomaw receives research support from the NIH/NINDS. Dr. Kissela has served on scientific advisory boards for Allergan, Inc. and Northstar Neuroscience, Inc.; has received funding for travel and speaker honoraria from Allergan, Inc.; serves as a consultant for NexStim, receives research support from the NIH; and has provided medico-legal case review. K. Alwell receives research support from the NIH/NINDS. Dr. Flaherty has served as a consultant to Boehringer Ingelheim; receives research support from the NIH/NINDS and is PI of an NIH-funded study for which study drug is supplied by Novo Nordisk; and has provided medico-legal case review. Dr. Woo receives research support from the NIH/NINDS. Dr. Khatri has served on a scientific advisory board for Otsuka Pharmaceutical Co., Ltd.; serves as an Associate Editor for Frontiers in Clinical Trials in Neurology; receives publishing royalties from Informa Inc. and for The Stroke Center Handbook (Informa, 2006); receives research support from the NIH/NINDS; and serves on the Executive Committee of the IMS III Trial NINDS U01NS052220, for which drugs and devices are provided by EKOS Corporation, Concentric Medical, Penumbra, Inc., and Genentech, Inc. Dr. Adeoye serves on speakers' bureaus for and has received speaker honoraria from Genentech, Inc. and EKR Therapeutics, Inc.; and receives research support from EKR Therapeutics, Inc. and the NIH (NINDS, NIBIB). Dr. Ferioli reports no disclosures. Dr. Khoury receives research support from the NIH and has served as a consultant for Mead-Johnson. Dr. Hornung serves on a scientific advisory board for and has received funding for travel and speaker honoraria from the National Cancer Institute (NCI); and serves as an Associate Editor for Public Health Reports and Plos Medicine. Dr. Broderick has served on scientific advisory boards for Johnson & Johnson, Wyeth, and PhotoThera; holds patents re: Method for controlling the lysis of coagulated blood with apolipoprotein e4 phenotype; has served as a consultant for Novo Nordisk and Genentech, Inc. (all consulting fees and honoraria are placed in an education/research fund in Dr. Broderick's department of his institution); has received research support in the form of materials from Genentech, Inc., Novo Nordisk, Schering-Plough Corp., Concentric, EKOS Corporation, and Johnson & Johnson; and receives institutional research support from Boehringer Ingelheim, Genentech, Inc., Schering-Plough Corp., and the NIH/NINDS.
REFERENCES
- 1. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333:1581–1588 [DOI] [PubMed] [Google Scholar]
- 2. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329 [DOI] [PubMed] [Google Scholar]
- 3. Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20:473–476 [DOI] [PubMed] [Google Scholar]
- 4. Ricci S, Celani MG, Vitali R, La Rosa F, Righetti E, Duca E. Diurnal and seasonal variations in the occurrence of stroke: a community-based study. Neuroepidemiology 1992; 11:59–64 [DOI] [PubMed] [Google Scholar]
- 5. Lago A, Geffner D, Tembl J, Landete L, Valero C, Baquero M. Circadian variation in acute ischemic stroke: a hospital-based study. Stroke 1998; 29:1873–1875 [DOI] [PubMed] [Google Scholar]
- 6. Bornstein NM, Gur AY, Fainshtein P, Korczyn AD. Stroke during sleep: epidemiological and clinical features. Cerebrovasc Dis 1999; 9:320–322 [DOI] [PubMed] [Google Scholar]
- 7. Chaturvedi S, Adams HP, Jr, Woolson RF. Circadian variation in ischemic stroke subtypes. Stroke 1999; 30:1792–1795 [DOI] [PubMed] [Google Scholar]
- 8. Fink JN, Kumar S, Horkan C, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke 2002; 33:988–993 [DOI] [PubMed] [Google Scholar]
- 9. Serena J, Dávalos A, Segura T, Mostacero E, Castillo J. Stroke on awakening: looking for a more rational management. Cerebrovasc Dis 2003; 16:128–133 [DOI] [PubMed] [Google Scholar]
- 10. Nadeau JO, Fang J, Kapral MK, Silver FL, Hill MD. Outcome after stroke upon awakening. Can J Neurol Sci 2005; 32:232–236 [DOI] [PubMed] [Google Scholar]
- 11. Johnston SC. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology 2005; 64:654–659 [DOI] [PubMed] [Google Scholar]
- 12. Boode B, Welzen V, Franke C, Van Oostenbrugge R. Estimating the number of stroke patients eligible for thrombolytic treatment if delay could be avoided. Cerebrovasc Dis 2007; 23:294–298 [DOI] [PubMed] [Google Scholar]
- 13. Jiménez-Conde J, Ois A, Rodríguez-Campello A, Gomis M, Roquer J. Does sleep protect against ischemic stroke? Less frequent ischemic strokes but more severe ones. J Neurol 2007; 254:782–788 [DOI] [PubMed] [Google Scholar]
- 14. Silva GS, Lima FO, Camargo ECS, et al. Wake-up stroke: clinical and neuroimaging characteristics. Cerebrovasc Dis 2010; 29:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke 1998; 29:415–421 [DOI] [PubMed] [Google Scholar]
- 16. Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 2004; 35:426–431 [DOI] [PubMed] [Google Scholar]
- 17. Kleindorfer D, Broderick J, Khoury J, et al. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke 2006; 37:2473–2478 [DOI] [PubMed] [Google Scholar]
- 18. Special report from the National Institute of Neurological Disorders and Stroke Classification of Cerebrovascular Diseases III. Stroke 1990; 21:637–676 [DOI] [PubMed] [Google Scholar]
- 19. Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: Secular trends in Rochester, Minnesota, through 1989. Stroke 1996; 27:373–380 [PubMed] [Google Scholar]
- 20. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke 2000; 31:858–862 [DOI] [PubMed] [Google Scholar]
- 21. Lindsell CJ, Alwell K, Moomaw CJ, et al. Validity of a retrospective National Institutes of Health Stroke Scale scoring methodology in patients with severe stroke. J Stroke Cerebrovasc Dis 2005; 14:281–283 [DOI] [PubMed] [Google Scholar]
- 22. Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007; 38:1655–1711 [DOI] [PubMed] [Google Scholar]
- 23. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42:121–130 [PubMed] [Google Scholar]
- 24.US Census Bureau. National and state population estimates. [Accessed September 9, 2010.]. Available at: http://www.census.gov/popest/states/NST-ann-est.html.
- 25.US Census Bureau. 2005–2007 American community survey 3-year estimates. [Accessed September 9, 2010.]. Available at: http://factfinder.census.gov/servlet/STTable?_bm=y&-geo_id=01000US&-qr_name=ACS_2007_3YR_G00_S0101&-ds_name=ACS_2007_3YR_G00_&-_lang=en&-redoLog=false.
- 26. Adams HP, Jr, Leira EC, Torner JC, et al. Treating patients with ‘wake-up' stroke: the experience of the AbESTT-II trial. Stroke 2008; 39:3277–3282 [DOI] [PubMed] [Google Scholar]
- 27. Barreto AD, Martin-Schild S, Hallevi H, et al. Thrombolytic therapy for patients who wake up with stroke. Stroke 2009; 40:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Natarajan SK, Snyder KV, Siddiqui AH, Ionita CC, Hopkins LN, Levy EI. Safety and effectiveness of endovascular therapy after 8 hours of acute ischemic stroke onset and wake-up strokes. Stroke 2009; 40:3269–3274 [DOI] [PubMed] [Google Scholar]
- 29. University of California, Los Angeles, National Institute of Neurological Disorders and Stroke. Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE). In: ClinicalTrials.gov (NLM Identifier: NCT00389467). Available at: http://clinicaltrials.gov/ct2/show/NCT00389467 Accessed June 22, 2010.
- 30. National Stroke Research Institute, Australia: Commonwealth Scientific & Industry Research Organisation, Brain Research Institute, University of Melbourne, Melbourne Health. Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND). In: Clinical Trials.gov (NLM Identifier: NCT00887328). Available at: http://clinicaltrials.gov/ct2/show/NCT00887328. Accessed June 22, 2010.
- 31. Nogueira RG, Liebeskind D, Gupta R, et al. DWI/PWI and CTP assessment in the triage of wake-up and late presenting strokes undergoing neurointervention: the DAWN trial: presented at the International Stroke Conference, February 17–20, 2009, San Diego, CA Available at: http://asa.scientificposters.com/epsabstract.cfm?id=1 Accessed July 2, 2010. [Google Scholar]
- 32. The University of Texas Health Science Center, Houston: Genentech. Safety of intravenous thrombolysis for wake-up stroke. In: ClinicalTrials.gov (NLM Identifier: NCT01183533). Available at: http://clinicaltrials.gov/ct2/show/NCT01183533 Accessed December 3, 2010.