Abstract
Background:
Hypokalemic periodic paralysis (HypoPP) is associated with mutations in either the CaV1.1 calcium channel or the NaV1.4 sodium channel. Some NaV1.4 HypoPP mutations have been shown to cause an anomalous inward current that may contribute to the attacks of paralysis. Herein, we test whether disease-associated NaV1.4 mutations in previously untested homologous regions of the channel also give rise to the anomalous current.
Methods:
The functional properties of mutant NaV1.4 channels were studied with voltage-clamp techniques in an oocyte expression system.
Results:
The HypoPP mutation NaV1.4-R1132Q conducts an anomalous gating pore current, but the homologous R1448C mutation in paramyotonia congenita does not.
Conclusions:
Gating pore currents arising from missense mutations at arginine residues in the voltage sensor domains of NaV1.4 are a common feature of HypoPP mutant channels and contribute to the attacks of paralysis.
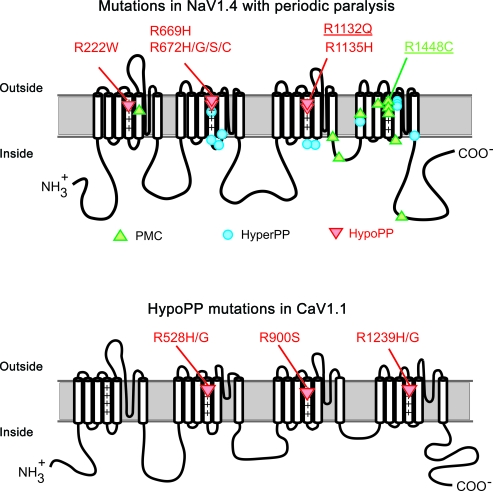
Hypokalemic periodic paralysis (HypoPP) is a channelopathy of skeletal muscle for which the causative mutation may be either in CACNA1S1,2 encoding the CaV1.1 l-type calcium channel (∼60% of families) or in SCN4A3,4 encoding the NaV1.4 sodium channel (∼20% of families). Fourteen of the 15 identified HypoPP mutations are missense substitutions at arginine residues in the voltage-sensor domains of either CaV1.1 or NaV1.4 (figure 1). This remarkable convergence suggests there may be a common functional defect produced by these voltage-sensor mutations that would explain why a mutation in either channel causes HypoPP.5
Figure 1. Mutations of the sodium channel (NaV1.4) or calcium channel (CaV1.1) associated with periodic paralysis.
Diagrams show the membrane folding topology and location of missense mutations associated with periodic paralysis. Three allelic disorders with periodic paralysis occur in NaV1.4 (paramyotonia congenita [PMC], hyperkalemic periodic paralysis[HyperPP], hypokalemic periodic paralysis [HypoPP]). HypoPP mutations (red triangles) occur at arginine residues (R) in the voltage-sensor domains of NaV1.4 or CaV1.1. The NaV1.4 mutations investigated in this study are underlined.
Expression studies of HypoPP mutant sodium channels have revealed an anomalous “gating pore” current that is active at the resting potential.6–8 This small current (<1% of the peak Na+ current during an action potential) may cause the aberrant depolarization during attacks of weakness.9,10 Prior studies have demonstrated that all 5 missense mutations at the arginines of the domain II voltage sensor in NaV1.4 support a gating pore current.6–8 An arginine to glutamine mutation in the domain III voltage sensor (R1132Q) was recently reported in HypoPP.11 We now show that R1132Q creates a gating pore current, thereby increasing the number of HypoPP NaV1.4 mutants with this anomalous current to 6 of all 6 tested, and also extending the affected sites to the domain III voltage sensor. Moreover, we show that a homologous arginine to cysteine mutation in the domain IV voltage sensor (R1448C) associated with paramyotonia congenita (PMC) does not produce a gating pore current.
METHODS
Construction of NaV1.4 mutants.
The rat adult skeletal muscle sodium channel α subunit cDNA (rNaV1.4) was subcloned into the vector pGEMHE, which is optimized for high-level protein expression in Xenopus oocytes.12 The R1125Q missense mutation of rNaV1.4 (ortholog of the human HypoPP mutation R1132Q) was introduced using the QuikChange Mutagenesis kit (Strategene, LA Jolla, CA) and confirmed by sequencing the entire cDNA. The rNaV1.4-R666G mutant in pGEMHE (ortholog of human HypoPP mutation R672G) was generated previously in our laboratory.8 The rat NaV1.4-R1441C mutant (ortholog of human R1448C) was provided by F. Bezanilla (University of Chicago) in the pBSTA vector. Throughout this article, the rat NaV1.4 mutants are referred to by their corresponding human NaV1.4 numbering for ease of comparison to the literature on human HypoPP mutations. cRNA was synthesized by in vitro transcription using the mMessage mMachine kit (Ambion, Austin TX).
Expression in oocytes.
Frogs (Xenopus laevis) were housed in an AAALAC-accredited facility, and all procedures were performed within guidelines established by the UTSW Institutional Animal Care and Use Committee. Stage V oocytes were injected with 50 ng of cRNA encoding either WT or mutant rNaV1.4, along with 50 ng (10-fold molar excess) of cRNA encoding the human sodium channel β1 subunit.13
Electrophysiology.
Currents were recorded from oocytes voltage-clamped in the cut-open configuration, using the CA-1B amplifier (Dagan Corporation, Minneapolis, MN), as described previously.8 The recording chamber was modified to allow gravity-flow perfusion to exchange the solutions on both the extracellular and intracellular faces of the oocyte membrane. The external bath to approximate the mammalian physiologic monovalent cation gradient contained (in mM) 112 NaOH, 3 KOH, 1.5 CaOH2, 4.5 BaOH2, 10 HEPES, pH 7.4 with methane sulfonic acid. For ionic substitution experiments, NaOH was replaced by KOH or N-methyl-d-glucamine (NMDG+). All external solutions contained 1 μM tetrodotoxin (TTX, Tocris Chemicals) to block ionic currents through the NaV1.4 pore. The internal solution contained (in mM) 103 KOH, 7 NaOH, 10 EGTA, 10 HEPES, pH 7.4. For the ionic selectivity experiments in figure 3B, the internal KOH and NaOH were replaced by 110 mM NMDG+. Currents were filtered at 5 kHz and sampled at 100 kHz for measurement of gating charge displacement, or 1 kHz and 10 kHz respectively for measurement of steady-state currents. Application of command pulse sequences and data acquisition were performed with pClamp 7.0 (Molecular Devices, Sunnyvale, CA). A −P/8 subtraction protocol was used to remove the linear leakage and capacitance currents from the gating charge displacement measurements. No on-line leak subtraction was performed during the measurement of ionic gating pore currents. Recordings were performed at room temperature (22°C).
Figure 3. Gating pore current for the hypokalemic periodic paralysis (HypoPP) R1132Q mutant.
(A) Steady-state current recorded at the end of a 400-msec pulse for test potentials between −140 mV and +30 mV. The HypoPP R1132Q mutant passes an inward gating pore current at membrane potentials less than −60 mV (inward rectification), whereas WT and the paramyotonia congenita (PMC) R1448C mutant do not (n = 4 oocytes for WT and 5 for each mutant). Linear background currents have been subtracted by fitting the I-V response between −20 mV and +20 mV. (B) Selectivity of theR1132Q gating pore by measuring currents for a variety of test cations. The permeability sequence is K+> Na+> NMDG+ (N-methyl-d-glucamine). Under physiologic conditions, the primary charge carrier would be inward movement of Na+ ions.
RESULTS
A sustained inward (negative) current was observed in oocytes injected with cRNA encoding the HypoPP mutant R1132Q, but not for those injected with WT or the PMC mutant R1448C. The top row of figure 2 shows currents elicited in response to a series of voltage steps from −140 to +40 mV from a holding potential of −100 mV. The small-amplitude proportionately spaced current responses for WT and the PAM mutant R1448C show the passive background leakage currents. For the HypoPP mutant R1132Q, however, progressively larger amplitude current responses were observed for test potentials more negative than −60 mV. These features are typical of anomalous gating pore currents, as reported for HypoPP mutations at arginines in positions R669 and R672.6,7
Figure 2. Currents recorded from voltage-clamped oocytes expressing NaV1.4 constructs.
(A) The currents elicited by a series of step depolarizations of 400 msec duration over a range of −140 mV to 40 mV, in 10-mV increments from a holding potential of −100 mV. No leak subtraction has been performed; rapid transients are clipped. Only the HypoPP mutant R1132Q (black, middle trace) has large inward currents elicited at negative test potentials. Notice the R1132Q current offset at the holding potential of −100 mV. Wild-type (blue) and PMC R1448C mutants (red) have only small background leakage currents. (B) Current responses for a series of brief 15-msec depolarizations from −140 mV for the same oocytes. The linear background currents (leakage and capacitance) have been subtracted digitally to yield the gating charge displacement current. The area under the rapid current transient is an index of channel expression, and these data show that all 3 NaV1.4 constructs had comparable levels of expression at the surface membrane.
The absence of a detectable gating pore current in WT or PMC-R1448C channels is not caused by a failure of channel expression at the plasma membrane. The expression level of functional sodium channels can be quantified by recording the small current transients that arise from movement of the channel voltage sensors in response to step changes in membrane potential. These so-called gating charge displacement currents are revealed by linear subtraction of background capacitance current and linear ionic leakage currents, as shown in the tracings on the bottom row of figure 2. The area under these current transients reflects the number of charges that moved, and will saturate for large-amplitude voltage steps. The maximal charge displacement, Qmax in nanocoulombs, is proportional to the channel expression level. Measurement of Qmax gives experimental verification of channel expression at the membrane and can be used to normalize the ionic currents recorded from different oocytes, thereby adjusting for variations in expression level and allowing data to be pooled from many oocytes.
The average gating pore current, normalized by expression level Qmax, is shown as a function of test potential in figure 3A. In these summary plots, the background nonspecific leakage currents have been removed by subtraction of a linear fit to the passive currents observed over a voltage range from −10 mV to +10 mV. Robust gating pore currents are observed for the HypoPP-R1132Q mutant, but not for WT or PMC-R1448C channels. To gain insight on which ions are permeating the channel to produce the gating pore current, we substituted the extracellular K+ by Na+ or the large organic molecule N-methyl-d-glucamine (NMDG+). The current amplitude was reduced by about 30% in Na+, figure 3B, but was still detectable when the only major extracellular cation was NMDG+. In contrast, the gating pore currents are insensitive to cation substitution for the histidine HypoPP mutations in domain II (R669H and R672H) because those gating pore currents are carried by protons. These data imply a rank order in relative permeability of K+> Na+≫ NMDG+. Under physiologic conditions in vivo, the electrochemical gradient would favor inward Na+ flow as the major charge carrier for the anomalous gating pore current in HypoPP R1132Q channels.
Small molecules or ions that could block the anomalous gating pore current would be ideal therapeutic agents for reducing the severity and frequency for attacks of weakness in HypoPP. Consequently, much interest has been generated by reports that Ca2+ is a low-affinity blocker of gating pore currents.6,14 In principle, this effect could underlie the beneficial effect of elevated extracellular Ca2+ to stabilize the resting membrane potential of HypoPP fibers studied in vitro,15 although clinical improvement by the administration of calcium gluconate has been reported only in hyperkalemic periodic paralysis.16 We measured the current-voltage response for gating pore currents from HypoPP-R1132Q mutant channels in a range of external Ca2+ from 0.2 mM to 10 mM. As shown in figure 4, external Ca2+ did not appreciably block the R1132Q gating pore current, although there was an apparent rightward (depolarized) shift in the I-V relation at high Ca2+ as would be expected for surface charge screening effects by the divalent cation.17 For comparison to prior reports,14 we also tested for Ca2+ block of gating pore currents in HypoPP-R672G channels, but again detected none (data not shown). We tested for block of HypoPP-R1132Q by other divalent cations at a concentration of 3.5 mM and a test potential of −100 mV. No block was detected for Ba2+ (relative current of 99 ± 3.2%, n = 3), and modest block was observed for Ni2+ (65 ± 4.3%, n = 3) and Zn2+ (53 ± 4.1%, n = 3). The carbonic anhydrase inhibitor acetazolamide is the preferred prophylactic agent for reducing the risk of an attack of weakness in HypoPP.18 We therefore tested for block of gating pore currents in HypoPP-R1132Q and HypoPP-R672G mutant channels by acetazolamide. As shown in figure 5, 100 μM acetazolamide did not block gating pore currents, across all voltages tested from −140 mV to +40 mV.
Figure 4. Calcium does not block the R1132Q gating pore.
Hypokalemic periodic paralysis (HypoPP) R1132Q gating pore currents were recorded in high external K+ (100 mM) as the Ca2+ was varied from 0.2 to 10 mM. Each oocyte served as its own control for expression level, as exposure to all 4 Ca2+ levels was performed and current amplitudes were normalized to the response at a test potential of −110 mV in 1.5 mM Ca2+ (n = 3 oocytes). The rightward shift of the I-V curve for higher [Ca2+] is caused by an apparent shift in the voltage dependence of channel gating due to screening of surface charges on the plasma membrane.
Figure 5. Acetazolamide (ACTZ) does not block gating pore currents in NaV1.4 hypokalemic periodic paralysis (HypoPP) mutants.
Current-voltage plots show exposure to 100 μM acetazolamide did not block the gating pore current for the HypoPP mutants NaV1.4-R1132Q (A) or NaV1.4-R672G (B). The current-voltage relation for the R672G mutant did not show saturation as we reported before,8 because the oocyte was maintained at a holding potential of −30 mV between test pulses.
DISCUSSION
A growing set of experimental data and modeling studies support the notion that an anomalous gating pore current is a fundamental mechanism for the loss of sarcolemmal excitability during attacks of weakness in HypoPP (reviewed in5). All 8 of the mutations in NaV1.4 associated with HypoPP are missense substitutions at arginine residues in the voltage sensor domains.19 Prior studies have shown that all 5 mutations in the voltage sensor of domain II are capable of producing a gating pore current.6–8 The present study now extends these observations to an arginine in the domain III voltage sensor (R1132Q). Moreover, we show specificity by demonstrating that a PMC mutation of an arginine in a homologous position for the voltage sensor of domain IV (R1448C) does not result in the creation of a gating pore. Rather, the PMC-R1448C mutation has a gain-of-function defect that is manifest as a slower rate of inactivation for the Na current through the conventional pore.20 Additional missense mutations at R1448 associated with PMC (leucine, proline, or serine) were tested, and we did not detect gating pore currents. The confidence was lower, however, to exclude these mutations as permissive for conducting gating pore currents because the expression level was lower (Qmax <0.5 nC, data not shown). Nevertheless, we propose it is unlikely that any PMC mutations at R1448 produce gating pore currents. This conclusion is based on the clear absence of gating pore currents for R1448C and the fact that we have never encountered a mixed setting wherein some disease-associated substitutions at a particular S4 arginine produce a gating pore current while others do not. Curiously, a set of 3 missense mutations at the third arginine in domain II produce gating pore currents that are activated by depolarization and are closed at the resting potential.21 These mutations (R657G/Q/W) cause potassium-sensitive normokalemic periodic paralysis,22 for which the mechanistic link to a depolarization-activated gating pore current remains to be established. A variety of gain-of-function changes for the Na+ current (impaired inactivation or enhanced activation) have been observed for more than 20 PMC and hyperkalemic periodic paralysis mutations studied to date,23 but conversely have never been observed in studies of NaV1.4-HyppoPP mutants.24,25
Computer simulations9 and recordings from dissociated muscle fibers10 have demonstrated that the gating pore current is sufficient to cause the instability of the muscle resting potential that produces a susceptibility to attacks of weakness in HypoPP. The gating pore current is active at the resting potential, and thereby depolarizes the fiber, but only by a few mV in normal external [K+]. More importantly, when external [K+] is reduced to ∼3.0 mM or lower, then the affected fiber undergoes a sustained depolarization that renders the muscle inexcitable.5,9 This depolarization in the setting of reduced external [K+] is paradoxical, in that normal fibers would hyperpolarize. For normal fibers, this paradoxical depolarized shift of Vrest can occur, but only at an extremely low [K+] of ∼1.5 mM.9 The mechanism for the paradoxical depolarization in low [K+] is a failure of the inward rectifier K conductance to balance the depolarizing currents from the gating pore and leakage conductance.5 As a result, the fiber depolarizes until the delayed rectifier K channels become active at about −50 mV.
In principle, a drug that could block the gating pore but otherwise not affect Na channel function would be an ideal therapy for HypoPP. We tested 2 agents, Ca2+ and acetazolamide, that have been used to abort or prevent attacks of weakness in periodic paralysis. Our voltage-clamp studies did not reveal significant block of the gating pore current by either, and so presumably they must act through other mechanisms. Others have reported low-affinity block by Ca2+ of the gating pore current for the HypoPP-R672G mutant.6,14 We attribute this difference to experimental methodology. We used a rapid perfusion system to add and wash out test reagents, for which no discernable block occurred (figure 4). In prior studies, however, the high Ca2+ was added to the external media in an equal-volume 2× concentration and the currents were recorded after minutes of equilibration.6,14 We have often observed a nonspecific rundown of the gating current amplitude and propose that this time-dependent effect produced an apparent block.
The most commonly affected gene in HypoPP is CACNA1S,3 which encodes the CaV1.1 calcium channel that serves as the voltage sensor for excitation-contraction coupling. Six of the 7 mutations in CaV1.1-HypoPP are missense mutations at arginines in the voltage sensor domains. Sodium and calcium channels share many structural similarities (figure 1), including the S4 voltage-sensor domains. The possibility that mutations in the voltage-sensor domains of CaV1.1 also create gating pore currents provides a unifying hypothesis for how mutations of 2 channels with very different functions in skeletal muscle, NaV1.4 and CaV1.1, may result in a common clinical phenotype of HypoPP. Experimental demonstration of gating pore currents for HypoPP CaV1.1 mutations is needed, since important functional differences exist between the homologous voltage-sensor domains, even within the NaV1.4 channel. The domain IVS4, for example, is coupled to inactivation gating and R1448C does not support a gating pore current, whereas domain IIS4 is coupled to activation and HypoPP mutations at 2 arginines in this S4 (R669, R672) are permissive for gating pore currents. Attempts to detect a gating pore current in CaV1.1-HypoPP mutant channels have been hampered by the relatively poor expression level of the channel at the membrane in heterologous systems.
ACKNOWLEDGMENT
Hillery Gray provided technical assistance with oocyte preparation.
- HypoPP
- hypokalemic periodic paralysis
- PMC
- paramyotonia congenita
Editorial, page 1614
AUTHOR CONTRIBUTIONS
David Francis performed the experiments, data analysis, and edited the manuscript. Volodymyr Rybalchenko performed the studies on channel blockers. Arie Struyk assisted with the experimental design and performed the mutagenesis. Stephen Cannon assisted with experimental design, data analysis, and wrote the manuscript.
DISCLOSURE
Dr. Francis and Dr. Rybalchenko report no disclosures. Dr. Struyk is an employee of Merck & Co., Inc. and receives compensation in the form of salary and stock; his spouse is an employee of Johnson & Johnson and receives compensation in the form of salary and stock; and receives research support from Merck & Co., Inc., Johnson & Johnson, and Muscular Dystrophy Association. Dr. Cannon serves on the scientific advisory board for Telethon (Italy); serves on the editorial advisory board of the Journal of General Physiology; and receives research support from the NIH/NIAMS.
REFERENCES
- 1. Ptacek LJ, Tawil R, Griggs RC, et al. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell 1994; 77:863–868 [DOI] [PubMed] [Google Scholar]
- 2. Jurkat-Rott K, Lehmann-Horn F, Albaz A, et al. A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet 1994; 3:1415–1419 [DOI] [PubMed] [Google Scholar]
- 3. Sternberg D, Maisonobe T, Jurkat-Rott K, et al. Hypokalemic periodic paralysis type 2 caused by mutations at codon 672 in the muscle sodium channel gene SCN4A. Brain 2001; 124:1091–1099 [DOI] [PubMed] [Google Scholar]
- 4. Bulman DE, Scoggan KA, van Oene MD, et al. A novel sodium channel mutation in a family with hypokalemic periodic paralysis. Neurology 1999; 53:1932–1936 [DOI] [PubMed] [Google Scholar]
- 5. Cannon SC. Voltage-sensor mutations in channelopathies of skeletal muscle. J Physiol 2010; 588:1887–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature 2007; 446:76–78 [DOI] [PubMed] [Google Scholar]
- 7. Struyk AF, Cannon SC. A Na+ Channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J Gen Physiol 2007; 130:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Struyk AF, Markin VS, Francis D, Cannon SC. Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: saturation of ion flux and implications for disease pathogenesis. J Gen Physiol 2008; 132:447–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Struyk AF, Cannon SC. Paradoxical depolarization of Ba2+-treated muscle exposed to low extracellular K+: insights into resting potential abnormalities in hypokalemic paralysis. Muscle Nerve 2008; 37:326–337 [DOI] [PubMed] [Google Scholar]
- 10. Jurkat-Rott K, Weber MA, Fauler M, et al. K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks. Proc Natl Acad Sci USA 2009; 106:4036–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carle T, Lhuillier L, Luce S, et al. Gating defects of a novel Na+ channel mutant causing hypokalemic periodic paralysis. Biochem Biophys Res Commun 2006; 348:653–661 [DOI] [PubMed] [Google Scholar]
- 12. Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 1992; 9:861–871 [DOI] [PubMed] [Google Scholar]
- 13. McClatchey AI, Cannon SC, Slaugenhaupt SA, Gusella JF. The cloning and expression of a sodium channel beta 1-subunit cDNA from human brain. Hum Mol Genet 1993; 2:745–749 [DOI] [PubMed] [Google Scholar]
- 14. Sokolov S, Scheuer T, Catterall WA. Ion permeation and block of the gating pore in the voltage sensor of NaV1.4 channels with hypokalemic periodic paralysis mutations. J Gen Physiol 2010; 136:225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hofmann WW, Smith RA. Hypokalemic periodic paralysis studies in vitro. Brain 1970; 93:445–474 [DOI] [PubMed] [Google Scholar]
- 16. Van Der Meulen JP, Gilbert GJ, Kane CA. Familial hyperkalemic paralysis with myotonia. N Engl J Med 1961; 264:1–6 [DOI] [PubMed] [Google Scholar]
- 17. Hille B. Charges and potentials at the nerve surface: divalent ions and pH. J Gen Physiol 1968; 51:221–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Resnick JS, Engle WK, Griggs RC, Stam AC. Acetazolamide prophylaxis in hypokalemic periodic paralysis. N Engl J Med 1968; 278:582–586 [DOI] [PubMed] [Google Scholar]
- 19. Matthews E, Labrum R, Sweeney MG, et al. Voltage sensor charge loss accounts for most cases of hypokalemic periodic paralysis. Neurology 2009; 72:1544–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang N, Ji S, Zhou M, et al. Sodium channel mutations in paramyotonia congenita exhibit similar biophysical phenotypes in vitro. Proc Nat Acad Sci USA 1994; 91:12785–12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sokolov S, Scheuer T, Catterall WA. Depolarization-activated gating pore current conducted by mutant sodium channels in potassium-sensitive normokalemic periodic paralysis. Proc Natl Acad Sci USA 2008; 105:19980–19985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vicart S, Sternberg D, Fournier E, et al. New mutations of SCN4A cause a potassium-sensitive normokalemic periodic paralysis. Neurology 2004; 63:2120–2127 [DOI] [PubMed] [Google Scholar]
- 23. Cannon SC. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu Rev Neurosci 2006; 29:387–415 [DOI] [PubMed] [Google Scholar]
- 24. Struyk AF, Scoggan KA, Bulman DE, Cannon SC. The human skeletal muscle Na channel mutation R669H associated with hypokalemic periodic paralysis enhances slow inactivation. J Neurosci 2000; 20:8610–8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jurkat-Rott K, Mitrovic N, Hang C, et al. Voltage-sensor sodium channel mutations cause hypokalemic periodic paralysis type 2 by enhanced inactivation and reduced current. Proc Natl Acad Sci USA 2000; 97:9549–9554 [DOI] [PMC free article] [PubMed] [Google Scholar]