Abstract
Background:
Previous epidemiologic and genetic studies have suggested a link between Parkinson disease (PD), essential tremor (ET), and restless legs syndrome (RLS).
Methods:
We describe the clinical, PET, and pathologic characteristics of an extensive kindred from Arkansas with hereditary PD, ET, and RLS. The pedigree contains 138 individuals. Sixty-five family members were examined neurologically up to 3 times from 2004 to 2010. Clinical data were collected from medical records and questionnaires. Genetic studies were performed. Five family members underwent multitracer PET. Two individuals with PD were examined postmortem.
Results:
Eleven family members had PD with generally mild and slowly progressive symptoms. Age at onset was between 39 and 74 years (mean 59.1, SD 13.4). All individuals treated with l-dopa responded positively. Postural or action tremor was present in 6 individuals with PD, and in 19 additional family members. Fifteen persons reported symptoms of RLS. PET showed reduced presynaptic dopamine function typical of sporadic PD in a patient with PD and ET, but not in persons with ET or RLS. The inheritance pattern was autosomal dominant for PD and RLS. No known pathogenic mutation in PD-related genes was found. Fourteen of the family members with PD, ET, or RLS had depression. Neuropathologic examination revealed pallidonigral pigment spheroid degeneration with ubiquitin-positive axonal spheroids, TDP43-positive pathology in the basal ganglia, hippocampus, and brainstem, and only sparse Lewy bodies.
Conclusion:
Familial forms of PD, ET, RLS, and depression occur in this family. The genetic cause remains to be elucidated.
Parkinson disease (PD) is traditionally defined by the presence of tremor at rest, rigidity, bradykinesia, and impaired postural reflexes. Essential tremor (ET) is a clinical syndrome of unknown cause with tremor during voluntary activation of a body part (action tremor). The tremor usually occurs when holding a body posture against gravity (postural tremor) and may also be noted during movements (kinetic tremor). Restless legs syndrome (RLS) is defined by the presence of the 4 criteria, 1) unpleasant sensations in the legs and an urge to move the legs, 2) worsening of these symptoms during rest or inactivity, 3) relief of the symptoms by active leg movements, and 4) occurrence or worsening of the unpleasant sensations in the evening or at night.1 Families with hereditary RLS have been described, and 8 associated gene loci are identified.2,3
Thus, the definitions of PD, ET, and RLS imply that they are distinct movement disorders. However, the clinical signs and symptoms may show a considerable overlap between these diseases, and a common pathogenetic background for PD, ET, and RLS is a possibility.
We studied a large kindred from Arkansas whose members had PD, ET, RLS, and depression, or any combination thereof. We are not aware of any previous reports on kindreds where all of these phenotypes co-occur. We present detailed clinical, multitracer PET, neuropathologic, and genetic data.
METHODS
The subjects examined for this study were all from the Arkansas Family; their positions in the family tree are shown in figure 1. The pedigree was drawn with information from family members. During 2004 to 2010, affected and unaffected family members were examined by neurologists and movement disorder specialists (R.F.P., A.P., Z.K.W.) in 3 research field visits. Every participant was seen at up to 3 visits, and when there were diagnostic uncertainties, by more than one neurologist per visit. Besides freely structured notes, the following rating scales were used: Unified Parkinson's Disease Rating Scale (UPDRS), Tremor Rating Scale (TRS),4 International Restless Legs Syndrome Study Group rating scale (IRLS),5 and Mini-Mental State Examination (MMSE). Outside this study, individuals IV-1, V-1, and V-5 were followed clinically by R.F.P., and notes from regular office visits were reviewed. Olfactory function was assessed using UPSIT Smell Identification Tests™ (Sensonics, Inc., Haddon Heights, NJ).6
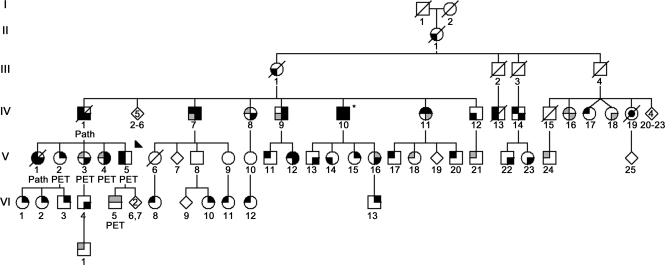
Figure 1. Simplified pedigree of the Arkansas Family.
Standard symbols were used. Round symbols indicate females, squares males, diagonal lines indicate the individual is deceased. Diamonds were used to disguise gender; numbers in diamonds indicate the number of (unaffected) siblings. The solid arrowhead indicates the proband. Black upper left quadrant denotes essential tremor (ET) and gray upper left quadrant possible ET. Black lower left quadrant signifies Parkinson disease (PD) and gray lower left quadrant possible PD. Black upper right quadrant denotes restless legs syndrome (RLS); gray, probable RLS. Black lower right quadrant signifies depression; gray, reactive depression. Individual IV-10 is the only person who had Mini-Mental State Examination below 27/30, indicating cognitive dysfunction (*). Individuals underwent multitracer PET as indicated. Individuals designated “Path” were examined neuropathologically. Individual IV-19 (black dot) had amyotrophic lateral sclerosis.
For the purpose of this study, the following criteria were used for phenotype definition:
PD.
Criteria for PD were presence of at least 3 of 4 cardinal signs (bradykinesia, resting tremor, rigidity, impaired postural reflexes) or 2 cardinal signs plus supporting features such as reduced blinking rate, hypomimia, parkinsonian gait, or falls. Alternative explanations were excluded from the available patient history, records, or investigations. Possible PD was diagnosed when signs were mild but the general impression was reminiscent of early PD.
ET.
Criteria for ET were postural tremor of the hands or forearms, with a gradual onset, present for at least 1 year. Causes of secondary tremor (hyperthyroidism, intoxication, drug or alcohol withdrawal) were excluded as far as possible. Individuals designated as possible ET had only mild postural finger and hand tremor. Diagnosis was based on the clinical examination including observing the subject perform the TRS tasks, and the patient's reports regarding the onset and the occurrence of tremor outside of the study visits. The postural tremor had to be considered clearly pathologic in order to be classified as ET or possible ET. When these methods did not allow to clearly distinguish between mild ET and physiologic tremor, this uncertainty was noted (table e-1 on the Neurology® Web site at www.neurology.org).
RLS.
Criteria for RLS were presence of all 4 essential diagnostic criteria mentioned above.1 Probable RLS was diagnosed in participants reporting 2 or 3 cardinal symptoms.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the institutional review boards (IRB) of the participating institutions. Written informed consent was obtained from all participating family members.
Genetic analysis.
DNA was extracted from peripheral leukocytes, and direct sequencing of all coding exons of LRRK2, SNCA, DCTN1, GRN, and EIF4G1 was performed as described previously.7 Triplet repeat lengths in the genes for SCA2, SCA3, and SCA12 were assessed as described previously.8 All primer sequences are available on request.
Multitracer PET.
Five members of this kindred underwent multitracer PET using 18F-6-fluoro-l-dopa (FD), 11C-(±)-α-dihydrotetrabenazine (DTBZ; vesicular monoamine transporter 2 ligand), and 11C-d-threo-methylphenidate (MP; dopamine transporter [DAT] ligand). The scanning procedure, data processing, and reconstruction have been described in detail elsewhere.9
Neuropathologic examination.
The brains of family members IV-1 and V-1 were available for neuropathologic examination. Their preparation followed a standardized dissection and staining protocol.10 Histologic stains included hematoxylin and eosin as well as thioflavin-S fluorescent microscopy. Immunohistochemistry for α-synuclein (NACP; Mayo Clinic), FUS (Sigma-Aldrich, St. Louis, MO), tau (Innogenetics, Alpharetta, GA), TDP-43 (ProteinTech Group, Chicago, IL), and ubiquitin (antibodies from EnCor Biotechnology, Alachua, FL) was performed.
RESULTS
Sixty-five individuals were examined up to 3 times in 2004, 2005, and 2010, and blood samples were obtained. Forty-three family members were affected by PD, ET, RLS, depression, or a combination of these disorders (table 1; details in table e-1, table e-2). Of these, all but 4 were examined within this study; 2 individuals who had PD according to family history (II-1 and III-1) were already deceased when the study began. Two affected individuals were not covered by this study's IRB protocol because they were younger than 18 years. One of these reportedly had postural tremor. The second was a child who had tremor, but received potentially tremorogenic medication for attention-deficit/hyperactivity disorder. In 2 family members, postural tremor was clearly visible but very mild so that the diagnosis remained uncertain despite examination by more than one study neurologist (table e-1). One family member died at age 49 years from amyotrophic lateral sclerosis (ALS).
Table 1.
Summary of the neurologic disorders in members of the Arkansas Familya
Abbreviations: ADHD = attention-deficit/hyperactivity disorder; ALS = amyotrophic lateral sclerosis; ET = essential tremor; PD = Parkinson disease; RLS = restless legs syndrome.
This table summarizes the clinical diagnoses occurring in the Arkansas Family. Fifteen family members have more than one (up to all 4) of these diagnoses. Details are provided in tables e-1 and e-2.
The average age of PD onset was 59.1 (±13.4) years. All patients with PD who received dopaminergic treatment reported good and lasting response to medication. End-of-dose wearing-off occurred after 7 years in the proband, but no patient developed dyskinesias. Dystonia was not prominent.
The majority of family members with ET or RLS were unable to state an exact year of onset, and reported that symptoms had developed very insidiously over the course of years, or in some cases, decades. Regarding tremor intensity, a fluctuating course was noted in individuals who were examined repeatedly within this study. Five of the 7 patients with both PD and ET developed postural tremor first and parkinsonism thereafter.
MMSE results were normal in all but one individual with PD after up to 15 years disease duration, and there was no history of marked cognitive problems or dementia in any family member, including those who were not examined. UPSIT smell testing was performed in 35 individuals and the average score was at the 28th percentile for 28 affected individuals and at the 31st percentile for 7 unaffected members (table e-1).
Clinical case descriptions.
In several cases, it has been difficult to classify the individual's symptom constellation as either PD or ET, despite longitudinal follow-up during 6 years' time. These individuals were clearly considered to be affected by symptoms of a movement disorder, and the majority were seen by 2 or more study neurologists independently. In all such cases, there was interrater concordance regarding the presence or absence of movement disorder symptoms. These individuals' symptoms were distinct, but mild, and in some cases were not considerably more prominent at subsequent field trips (table e-2). Detailed descriptions of affected family members are provided in appendix e-1.
Genetic analysis.
Direct sequencing of all coding exons of LRRK2, SNCA, DCTN1, GRN, and EIFG4G1 was normal. The genes for SCA2, SCA3, and SCA12 had normal triplet repeat lengths.
Multitracer PET scanning.
Five subjects (as indicated in figure 1) underwent PET scanning. Putaminal tracer binding/uptake values are shown in table e-3.
In subject V-5 (PD and ET), all 3 PET studies (FD, DTBZ, and MP) were abnormal, with asymmetric reduction of tracer uptake and a rostrocaudal gradient typical of PD. In subjects V-2 (RLS), V-3 (possible ET and depression), V-4 (possible ET, RLS, and depression), and VI-5 (possible ET and possible RLS), PET demonstrated normal uptake/binding of all 3 tracers.
Neuropathology.
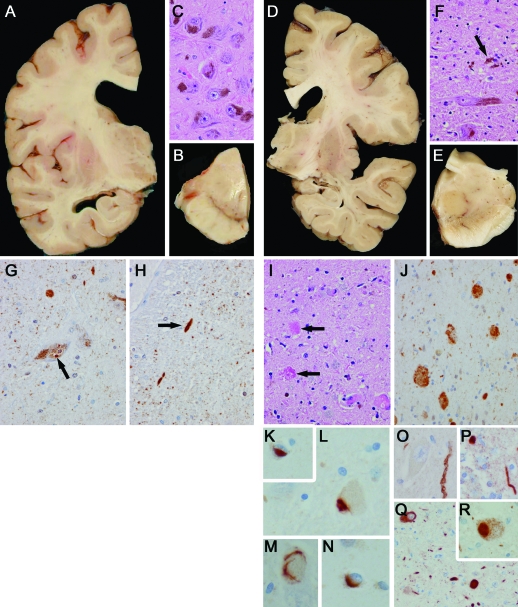
The brain of IV-1 (figure 2) weighed 1,180 g and displayed mild cortical atrophy of the frontal lobe and minimal frontal horn ventricular enlargement. There was pigment loss in the substantia nigra. The microscopic pathology was heterogeneous, with the most severe neuronal loss in the substantia nigra and correlating with the clinical history of PD.11 While there were Lewy bodies, they were sparse and relatively restricted in their distribution, including the dorsal motor nucleus of the vagus, locus ceruleus, basal forebrain, and olfactory bulb. This pattern was concordant with Braak PD stage 2,12 which is typical of incidental Lewy body disease.13 The most striking pathology in the substantia nigra was pigment-spheroid degeneration with similar pathology in the globus pallidus. It was associated with ubiquitin-positive spheroids. There were neuronal inclusions with TDP-43 immunohistochemistry. The TDP-43 pathology had a restricted distribution with most of the pathology in the basal ganglia, hippocampus, and brainstem. Alzheimer-type cortical plaques were seen predominantly in the posterior cortical areas. Medial temporal tauopathy was minimal and had some features that fit with a 4R tauopathy, including sparse tau-positive glia. There was sparse tau pathology in the basal ganglia, thalamus, and subthalamic nucleus of uncertain significance.14 Finally, there was arteriosclerotic small vessel disease with isolated cortical microinfarcts and mild leukoencephalopathy.
Figure 2. Neuropathologic examination of family members IV-1 and V-1.
V-1 (left column): (A) Frontal horn more than temporal horn ventricular enlargement. (B) Normal pigmentation of substantia nigra. (C) Normal cell density in pars compacta of substantia nigra. (G, H) Age-related ubiquitin-positive structures in substantia nigra including Marinesco body (G, arrow) and neurites (H, arrow). Neurites were negative for tau, α-synuclein, TDP-43, and FUS. IV-1 (right column): (D) Minimal frontal horn ventricular enlargement. (E) No pigmentation of substantia nigra. (F) Neuronal loss, gliosis, and neuronophagia (arrow) in substantia nigra. (I) Many granular foamy axonal spheroids (arrows) in substantia nigra, which are immunopositive for ubiquitin (J). TDP-43 pathology in the form of neuronal cytoplasmic (L, M), intranuclear (K) and glial (N) inclusions. Synuclein pathology in the form of sparse neurites in basal nucleus of Meynert (O) and olfactory bulb (P), as well as neuronal and neuritic staining in dorsal motor nucleus of vagus (Q) and rare Lewy body in locus ceruleus (R).
The brain of V-1 (figure 2) showed marked enlargement of the frontal horn, more than of the temporal horns. However, macroscopic inspection revealed no obvious atrophy over the convexities. Otherwise, macroscopic and microscopic examination was normal.
DISCUSSION
In 5 generations of this family, a total of 43 individuals displayed signs and symptoms of PD, ET, RLS, or depression. A considerable number of the affected persons had features of more than one of these disorders. This overlap was especially prominent for the individuals with PD, 64% (7/11) of whom also had prominent ET or RLS.
Twelve individuals clearly had parkinsonism or postural tremor, but did not fully satisfy the diagnostic criteria for PD or ET. In some cases, symptoms remained unusually mild or did not worsen significantly during the study's 6-year follow-up period (table e-2, figure 3). Thus, in 12 individuals, no clinically definite diagnosis could be made despite reexamination of most cases, and they were classified as having possible PD or ET. Mild postural tremor may be physiologic tremor rather than ET. However, the high density and the distribution of family members with mild postural tremor rather suggests that this phenotype is a characteristic, inherited disease entity in this kindred.
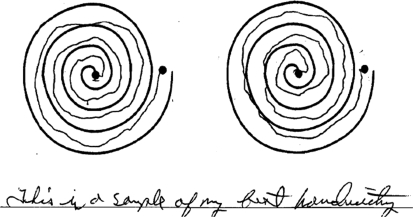
Figure 3. Mild action tremor.
Slight tremor in drawing and handwriting tasks as noted in V-11 (age 51 years), considered a typical example of several members in this family with mild action tremor but no other neurologic signs. Left: dominant hand. Right: nondominant hand. The person received 3 points on the Tremor Rating Scale.
One family member died of ALS. No signs of amyotrophy were found in other family members. Cognitive impairment was not a prominent feature of the neurologic disorders in this family. Olfactory dysfunction was an inconsistent feature (table e-1). Multitracer PET was performed in 5 individuals, representing all different diagnoses in the family, and was abnormal only in the proband with both PD and ET.
Postmortem examination was performed on 2 individuals. The proband's father, IV-1, had had episodes of severe depression, was clearly affected by PD and ET, and died at age 85 years after a disease duration of 15 years. There was slight frontal atrophy, and a heterogeneous microscopic pathology with pallidonigral pigment spheroid degeneration and TDP43-proteinopathy, reminiscent of that seen in Perry syndrome.7 There was mild Lewy body disease predominantly in the brainstem, senile changes of Alzheimer type, as well as mild vascular pathology. The proband's sister, V-1, had depressive episodes with frequent crying, but only mild motor symptoms classified as possible PD. She died of cancer at age 57, 11 years after the first symptoms. Neuropathologic examination of her brain revealed a marked enlargement of the frontal horn. Microscopic examination was normal. Thus, the only finding common to both brains, albeit to various degrees, was that of decreased frontal lobe volume.
The question arises whether the different disease phenotypes observed in this family are manifestations of the same underlying disease process and the same genetic predisposition, or if there is a coincidental accumulation of different genetic traits.
The volume loss of the frontal lobes seen in both autopsied patients would support a common cause, and the development of microscopically detectable pathology may have been interrupted by the premature death of V-1. Multitracer PET showed compromise of the striatal dopaminergic system in a person with manifest parkinsonism but did not reveal abnormalities in those with other phenotypes, which may be interpreted as evidence against a common underlying cause.
The distribution of affected family members in the pedigree clearly indicates heritability. When considering only the family members with PD and possible PD, autosomal dominant inheritance and full penetrance is suggested. The only exception is IV-13, whose parkinsonian symptoms may be secondary to severe tick-borne encephalitis and to multiple brain infarctions (appendix e-1). Similarly, considering only the family members with RLS suggests autosomal dominant inheritance. Exceptions here are III-1, IV-1, and V-5, who all had PD as well. PD symptoms or their treatment may have masked RLS.
The ET phenotype in isolation may also be inherited in an autosomal dominant manner within the Arkansas Family, but in this case, penetrance would be considerably below 100%. III-4, IV-9, IV-12, IV-15, V-6, V-9, and V-10 had children with ET but were not affected themselves. However, V-6 died at an age of only 38 years, V-9 was 45, and V-10 54 years at the time of the most recent evaluation, and future development of symptoms cannot be excluded. Considerably reduced, and age-related, penetrance has been reported in ET.15 A common, monogenetic inheritance for all of these disorders is also conceivable, but more than half of the children of IV-1, IV-9, IV-10, and IV-11 were affected by any of the diseases, more than statistically expected.
Complete analysis remains limited by the fact that some family members have not been examined. However, we carefully compiled all accounts of neurologic disease provided by the family members with whom we had contact. Furthermore, we expect that close relatives of affected individuals are more prepared to participate in research studies. Limitations of this study also include the nonblinded state of the clinical investigators and the fact that we only describe one family. The prevalence of PD and ET in the general population is high enough to allow for a number of affected individuals without genetic cause in a pedigree with 138 members. The intensive media campaigns about RLS that temporally coincided with the data collection for this study may explain why the disorder was more commonly reported by the younger generations.16
Previous studies have revealed commonalities between any 2 of PD, ET, RLS, and depression. Relatives of patients with PD are more likely to have ET than the general population.17–19 ET may transform to PD.20,21 Action tremor and tremor at rest may occur in the same individual.22 First-degree relatives of PD probands from 303 Greek (Cretan) kindreds with hereditary PD were approximately 4 times more likely to have ET than unrelated controls, and both diseases taken together followed autosomal dominant inheritance in several kindreds from Crete.23 In family C, which defines the PARK3 locus, most members have hereditary PD.24 However, in a few members, ET has occurred, either alone or in combination with PD, and multitracer PET was normal in the family members with ET only (unpublished data). Lewy bodies were found in the locus ceruleus of a subset of patients with ET, who had a trend toward less marked cerebellar pathology.25 Variants in the LINGO1 gene are risk factors common to PD and ET.26
Similarly, a causal relationship between PD and RLS has been suggested, based on the fact that both conditions improve with dopaminergic treatment.27 While the symptoms of RLS may be attributed to alterations in spinal dopaminergic modulation, there is now pathologic and clinical evidence that patients with RLS also display changes in the striatonigral dopaminergic system, and the associative loop involved in decision-making.28,29 Prior imaging studies in RLS have suggested either normal30 or mildly impaired31–33 presynaptic dopamine function, compatible to the findings reported here.
Depression is not uncommon in the general population and may have manifold causes. The depressive episodes of 3 family members coincided with life events. Conversely, depression occurs more frequently among patients with PD,34 RLS,29 and ET.35 Interestingly, certain symptoms of depression improve with dopaminergic drugs, and there is increasing evidence for a role of dopamine in depression.36 Hypothetically, a factor that affects the molecular machinery in the dopamine system may cause PD, problems with decision-making, depressive symptoms, or RLS, and the obvious clinical manifestation may only be influenced by the predominant localization of the monoaminergic deficit.
Also, ET and RLS may be connected. Among consecutive patients at a movement disorder clinic, one-third of patients with ET also had RLS, and conversely, more than half of patients with RLS had mild action or essential tremor in their hands.37 Serotonergic medications can attenuate the symptoms of both ET and RLS.38,39
PD, ET, RLS, and depression occur in members of the Arkansas Family. The clinical course of PD seen in this family was similar in all affected members. It was usually tremor-dominant, mild, progressed only slowly, and did not entail dementia. Neuropathology revealed volume loss of the frontal lobes of both autopsied patients. In light of recent findings regarding the epidemiology, genetics, and pathophysiology of PD, ET, RLS, and depression, a common cause for their manifestations within the Arkansas Family appears possible.
Testing for known gene mutations in PD-associated genes was negative. DNA from 65 individuals from members of the Arkansas Family has been collected. Samples are currently analyzed with exome sequencing technology. This will hopefully elucidate the genetic causes of the neurologic disorders in this family, adding new aspects to our understanding of the pathomechanisms of neurologic disease. Further studies may reveal whether the phenotypes encountered in this family share a common cause or are separate entities, and if the disease process is primarily functional, inhibiting neurotransmitter systems, or primarily neurodegenerative.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the members of the family who participated in this study.
Supplemental data at www.neurology.org
- ALS
- amyotrophic lateral sclerosis
- ET
- essential tremor
- IRB
- institutional review board
- IRLS
- International Restless Legs Syndrome Study Group rating scale
- MMSE
- Mini-Mental State Examination
- PD
- Parkinson disease
- RLS
- restless legs syndrome
- TRS
- Tremor Rating Scale
- UPDRS
- Unified Parkinson's Disease Rating Scale
AUTHOR CONTRIBUTIONS
A.J. Puschmann: drafting and revising the manuscript, analysis and interpretation of data, acquisition of data. R.F. Pfeiffer: revising the manuscript, study design, acquisition of data. A.J. Stoessl: revising the manuscript, analysis and interpretation of data, acquisition of data. R. Kuriakose: revising the manuscript, analysis or interpretation of data, acquisition of data. J.L. Lash: analysis of data, acquisition of data, other: organization of field trips. J.A. Searcy: analysis of data, acquisition of data, other: organization of field trips. A.J. Strongosky: analysis of data, acquisition of data, other: organization of field trips. C. Vilariño-Güell: revising the manuscript, analysis and interpretation of data, acquisition of data. M.J. Farrer: revising the manuscript, interpretation of data. O.A. Ross: revising the manuscript. D.W. Dickson: revising the manuscript, analysis and interpretation of data, acquisition of data. Z.K. Wszolek: revising the manuscript, study design, analysis and interpretation of data, study supervision and coordination, obtaining funding.
DISCLOSURE
Dr. Puschmann has received funding for travel from Lundbeck Inc. and receives research support from the Swedish Parkinson Academy, AFA Insurance, and the Swedish Parkinson Foundation. Dr. Pfeiffer serves on the scientific advisory board for the National Parkinson Foundation; served as a consultant for Solvay and Ipsen; serves on the speakers' bureaus of Novartis, GlaxoSmithKline, Boehringer Ingelheim, Teva, and UCB/Schwarz; served on the scientific advisory boards for Kyowa, Solvay, Ipsen, and Theravance; and served as a legal consultant for Spriggs & Hollingsworth and Davis Graham & Stubbs as a legal consultant. Dr. Pfeiffer serves as Co-Editor-in-Chief for Parkinsonism and Related Disorders; receives royalty payments from Neurogastroenterology (Butterworth Heinemann, 2008), Parkinson's Disease (CRC Press, 2008, 2009), and Parkinson's Disease and Nonmotor Dysfunction (Humana Press, 2008); and has received research support from Kyowa, Novartis, Boehringer Ingelheim, Eisai, UCB/Schwarz, and Santhera. Dr. Stoessl serves on a scientific advisory board for Biovail Corporation/Medgenesis; has received funding for travel and speaker honoraria from Novartis; serves on the editorial boards of Annals of Neurology, Lancet Neurology, and Parkinsonism & Related Disorders; and receives research support from CIHR, the Michael Smith Foundation for Health Research, and the Pacific Alzheimer Research Foundation. Dr. Kuriakose, J.L. Lash, J.A. Searcy, A.J. Strongosky, and Dr. Vilariño-Güell report no disclosures. Dr. Farrer serves on a scientific advisory board for the Michael J. Fox Foundation; serves/has served on the editorial boards of Neurobiology of Disease and Parkinsonism and Related Disorders; is coinventor on patents re: LRRK2 gene and mutations; receives institutional research support from Lundbeck Inc.; has received research support from the NIH, the Pacific Alzheimer Research Foundation, and the Michael J. Fox Foundation; and his institution receives annual royalties from Lundbeck Inc. from the licensing of the technology related to PARK8/LRRK2. Dr. Ross serves on the editorial boards of Open Longevity Science and PLoS ONE. Dr. Dickson serves on the editorial boards of the American Journal of Pathology, Journal of Neuropathology and Experimental Neurology, Brain Pathology, Neurobiology of Aging, Journal of Neurology Neurosurgery and Psychiatry, Annals of Neurology, and Neuropathology; and receives research support from the NIH and Cure PSP/Society for PSP. Dr. Wszolek serves as Co-Editor-in-Chief of Parkinsonism and Related Disorders, Regional Editor of the European Journal of Neurology, and on the editorial boards of Neurologia i Neurochirurgia Polska, Advances in Rehabilitation, the Medical Journal of the Rzeszow University, and Clinical and Experimental Medical Letters; holds and has contractual rights for receipt of future royalty payments from patents re: A novel polynucleotide involved in heritable Parkinson's disease; receives royalties from publishing Parkinsonism and Related Disorders (Elsevier, 2007, 2008, 2009) and the European Journal of Neurology (Wiley-Blackwell, 2007, 2008, 2009); and receives research support from Allergan, Inc., the NIH, the Pacific Alzheimer Research Foundation (Canada), the CIHR, the Mayo Clinic Florida Research Committee CR program, and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch.
REFERENCES
- 1. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology: a report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 2003; 4:101–119 [DOI] [PubMed] [Google Scholar]
- 2. Young JE, Vilarino-Guell C, Lin SC, Wszolek ZK, Farrer MJ. Clinical and genetic description of a family with a high prevalence of autosomal dominant restless legs syndrome. Mayo Clin Proc 2009; 84:134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol 2010; 6:337–346 [DOI] [PubMed] [Google Scholar]
- 4. Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, eds. Parkinson's Disease and Movement Disorders. Baltimore: Williams & Wilkins; 1993: 225–234 [Google Scholar]
- 5. Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 2003; 4:121–132 [DOI] [PubMed] [Google Scholar]
- 6. Doty RL. The Smell Identification TestTM Administration Manual: 3rd ed. Philadelphia: Sensonics, Inc.; 1995 [Google Scholar]
- 7. Farrer MJ, Hulihan MM, Kachergus JM, et al. DCTN1 mutations in Perry syndrome. Nat Genet 2009; 41:163–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gwinn-Hardy K, Chen JY, Liu HC, et al. Spinocerebellar ataxia type 2 with parkinsonism in ethnic Chinese. Neurology 2000; 55:800–805 [DOI] [PubMed] [Google Scholar]
- 9. Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal progression of sporadic Parkinson's disease: a multi-tracer positron emission tomography study. Brain 2009; 132:2970–2979 [DOI] [PubMed] [Google Scholar]
- 10. Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord 2003; 18:1018–1026 [DOI] [PubMed] [Google Scholar]
- 11. Dickson DW, Fujishiro H, Orr C, et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 2009; 15 (suppl 3): S1–S5 [DOI] [PubMed] [Google Scholar]
- 12. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003; 24:197–211 [DOI] [PubMed] [Google Scholar]
- 13. Dickson DW, Uchikado H, Fujishiro H, Tsuboi Y. Evidence in favor of Braak staging of Parkinson's disease. Mov Disord 2010; 25 (suppl 1): S78–S82 [DOI] [PubMed] [Google Scholar]
- 14. Mattila P, Togo T, Dickson DW. The subthalamic nucleus has neurofibrillary tangles in argyrophilic grain disease and advanced Alzheimer's disease. Neurosci Lett 2002; 320:81–85 [DOI] [PubMed] [Google Scholar]
- 15. Louis ED, Ford B, Frucht S, Ottman R. Mild tremor in relatives of patients with essential tremor: what does this tell us about the penetrance of the disease? Arch Neurol 2001; 58:1584–1589 [DOI] [PubMed] [Google Scholar]
- 16. Woloshin S, Schwartz LM. Giving legs to restless legs: a case study of how the media helps make people sick. PLoS Med 2006; 3:e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rocca WA, Bower JH, Ahlskog JE, et al. Increased risk of essential tremor in first-degree relatives of patients with Parkinson's disease. Mov Disord 2007; 22:1607–1614 [DOI] [PubMed] [Google Scholar]
- 18. Tan EK, Lee SS, Fook-Chong S, Lum SY. Evidence of increased odds of essential tremor in Parkinson's disease. Mov Disord 2008; 23:993–997 [DOI] [PubMed] [Google Scholar]
- 19. Costello S, Bordelon Y, Bronstein J, Ritz B. Familial associations of Alzheimer disease and essential tremor with Parkinson disease. Eur J Neurol 2010; 17:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson's disease. Parkinsonism Relat Disord 2007; 13:67–76 [DOI] [PubMed] [Google Scholar]
- 21. Minen MT, Louis ED. Emergence of Parkinson's disease in essential tremor: a study of the clinical correlates in 53 patients. Mov Disord 2008; 23:1602–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen O, Pullman S, Jurewicz E, Watner D, Louis ED. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol 2003; 60:405–410 [DOI] [PubMed] [Google Scholar]
- 23. Spanaki C, Plaitakis A. Essential tremor in Parkinson's disease kindreds from a population of similar genetic background. Mov Disord 2009; 24:1662–1668 [DOI] [PubMed] [Google Scholar]
- 24. Wszolek ZK, Cordes M, Calne DB, Munter MD, Cordes I, Pfeifer RF. [Hereditary Parkinson disease: report of 3 families with dominant autosomal inheritance.] Nervenarzt 1993; 64:331–335 [PubMed] [Google Scholar]
- 25. Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007; 130:3297–3307 [DOI] [PubMed] [Google Scholar]
- 26. Vilarino-Guell C, Wider C, Ross OA, et al. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics 2010; 11:401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moller JC, Unger M, Stiasny-Kolster K, Oertel WH. Restless legs syndrome (RLS) and Parkinson's disease (PD): related disorders or different entities? J Neurol Sci 2010; 289:135–137 [DOI] [PubMed] [Google Scholar]
- 28. Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 2009; 132:2403–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bayard S, Yu H, Langenier MC, Carlander B, Dauvilliers Y. Decision making in restless legs syndrome. Mov Disord 2010; 25:2634–2640 [DOI] [PubMed] [Google Scholar]
- 30. Trenkwalder C, Walters AS, Hening WA, et al. Positron emission tomographic studies in restless legs syndrome. Mov Disord 1999; 14:141–145 [DOI] [PubMed] [Google Scholar]
- 31. Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology 1999; 52:932–937 [DOI] [PubMed] [Google Scholar]
- 32. Ruottinen HM, Partinen M, Hublin C, et al. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology 2000; 54:502–504 [DOI] [PubMed] [Google Scholar]
- 33. Cervenka S, Palhagen SE, Comley RA, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain 2006; 129:2017–2028 [DOI] [PubMed] [Google Scholar]
- 34. Reichmann H, Schneider C, Lohle M. Non-motor features of Parkinson's disease: depression and dementia. Parkinsonism Relat Disord 2009; 15 (suppl 3): S87–S92 [DOI] [PubMed] [Google Scholar]
- 35. Louis ED. Essential tremor as a neuropsychiatric disorder. J Neurol Sci 2010; 289:144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008; 358:55–68 [DOI] [PubMed] [Google Scholar]
- 37. Ondo WG, Lai D. Association between restless legs syndrome and essential tremor. Mov Disord 2006; 21:515–518 [DOI] [PubMed] [Google Scholar]
- 38. Hargrave R, Beckley DJ. Restless leg syndrome exacerbated by sertraline. Psychosomatics 1998; 39:177–178 [DOI] [PubMed] [Google Scholar]
- 39. Arshaduddin M, Al Kadasah S, Biary N, Al Deeb S, Al Moutaery K, Tariq M. Citalopram, a selective serotonin reuptake inhibitor augments harmaline-induced tremor in rats. Behav Brain Res 2004; 153:15–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.