Abstract
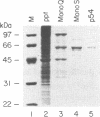
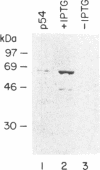
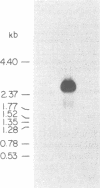
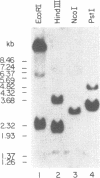
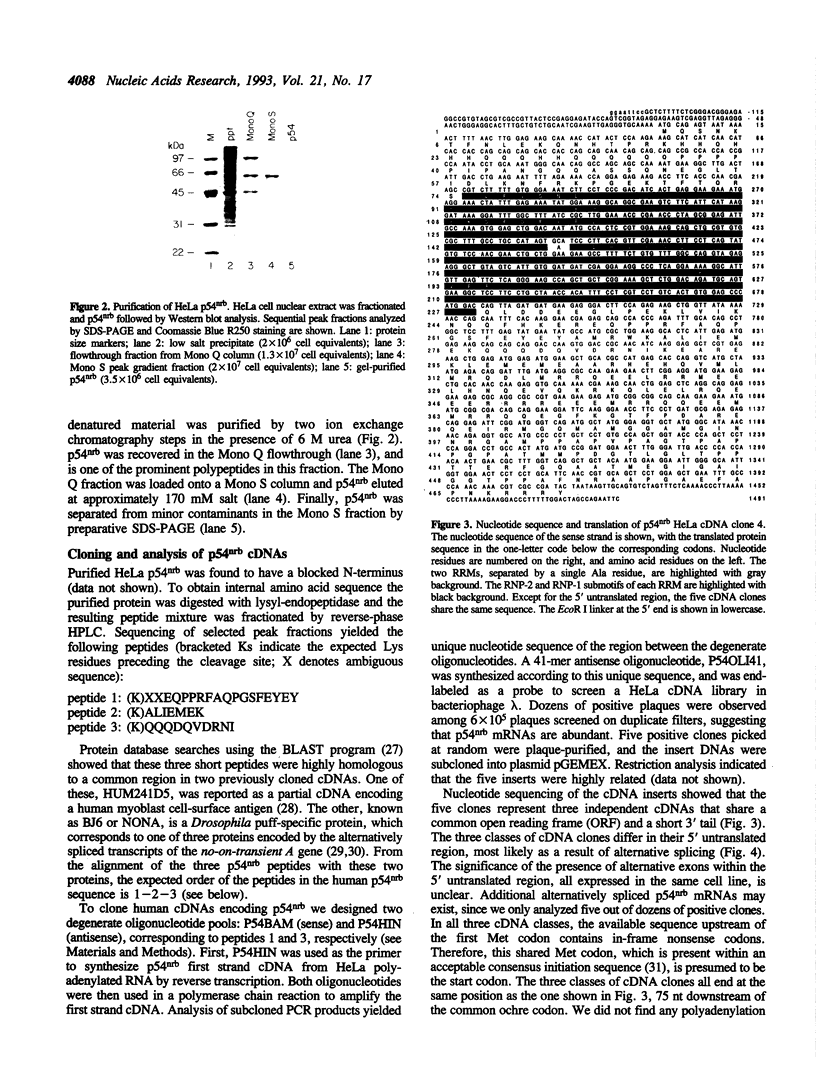
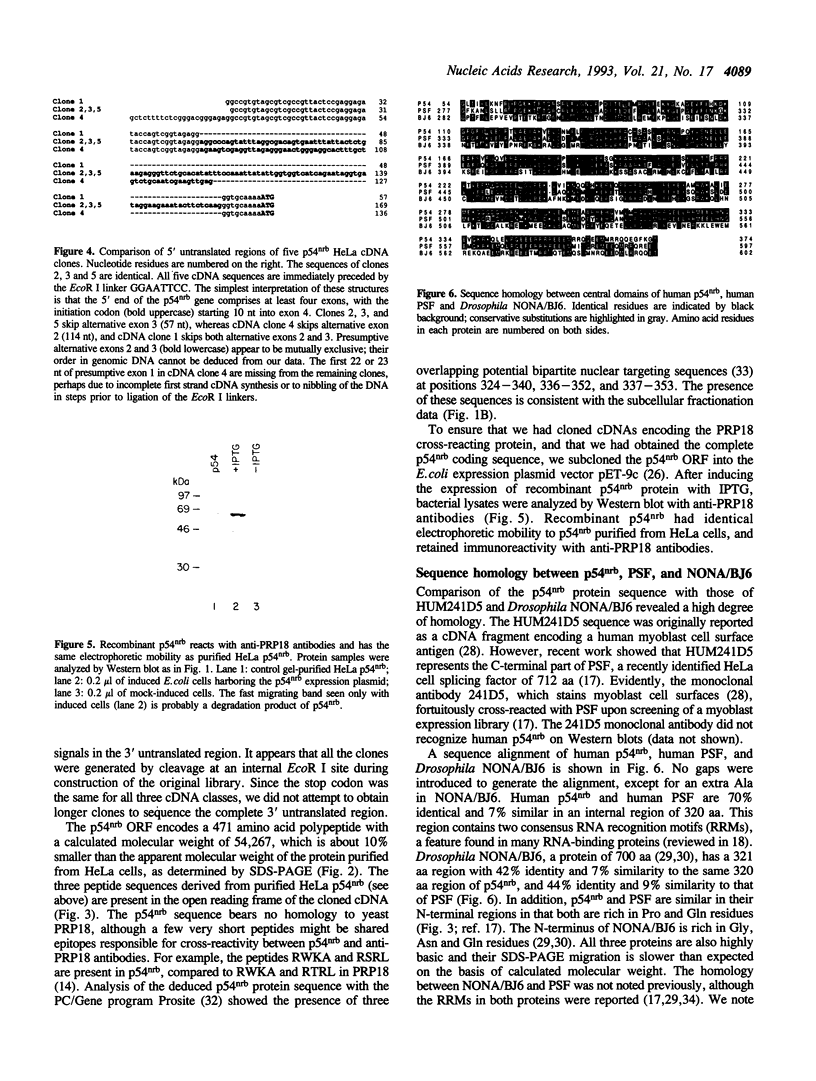
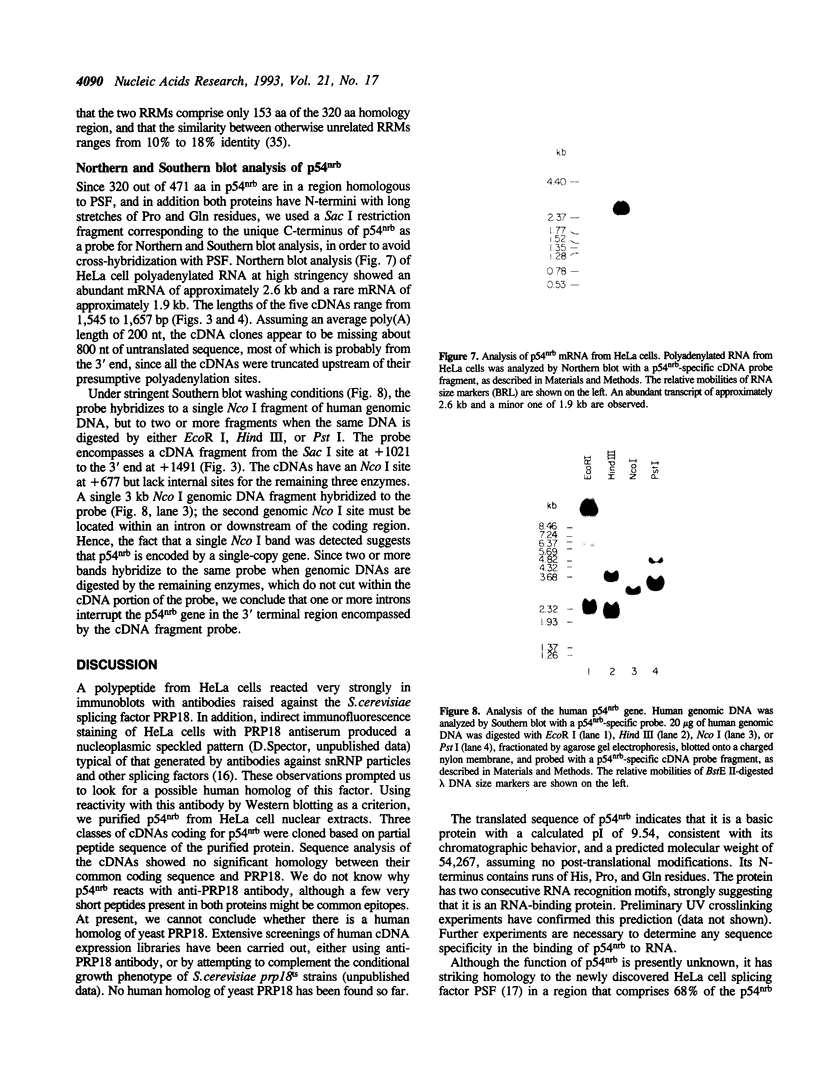
While searching for a human homolog of the S.cerevisiae splicing factor PRP18, we found a polypeptide that reacted strongly with antibodies against PRP18. We purified this polypeptide from HeLa cells using a Western blot assay, and named it p54nrb (for nuclear RNA-binding protein, 54 kDa). cDNAs encoding p54nrb were cloned with probes derived from partial sequence of the purified protein. These cDNAs have identical coding sequences but differ as a result of alternative splicing in the 5' untranslated region. The cDNAs encode a 471 aa polypeptide that contains two RNA recognition motifs (RRMs). Human p54nrb has no homology to yeast PRP18, except for a common epitope, but is instead 71% identical to human splicing factor PSF within a 320 aa region that includes both RRMs. In addition, both p54nrb and PSF are rich in Pro and Gln residues outside the main homology region. The Drosophila puff-specific protein BJ6, one of three products encoded by the alternatively spliced no-on-transient A gene (nonA), which is required for normal vision and courtship song, is 42% identical to p54nrb in the same 320 aa region. The striking homology between p54nrb, PSF, and NONA/BJ6 defines a novel phylogenetically conserved protein segment, termed DBHS domain (for Drosophila behavior, human splicing), which may be involved in regulating diverse pathways at the level of pre-mRNA splicing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson G. J., Bach M., Lührmann R., Beggs J. D. Conservation between yeast and man of a protein associated with U5 small nuclear ribonucleoprotein. Nature. 1989 Dec 14;342(6251):819–821. doi: 10.1038/342819a0. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992 May 11;20 (Suppl):2013–2018. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A. L., Osheim Y. N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988 Jun;2(6):754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- Birney E., Kumar S., Krainer A. R. A putative homolog of U2AF65 in S. cerevisiae. Nucleic Acids Res. 1992 Sep 11;20(17):4663–4663. doi: 10.1093/nar/20.17.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champlin D. T., Frasch M., Saumweber H., Lis J. T. Characterization of a Drosophila protein associated with boundaries of transcriptionally active chromatin. Genes Dev. 1991 Sep;5(9):1611–1621. doi: 10.1101/gad.5.9.1611. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Frasch M., Saumweber H. Two proteins from Drosophila nuclei are bound to chromatin and are detected in a series of puffs on polytene chromosomes. Chromosoma. 1989 Jan;97(4):272–281. doi: 10.1007/BF00371966. [DOI] [PubMed] [Google Scholar]
- Fu X. D., Mayeda A., Maniatis T., Krainer A. R. General splicing factors SF2 and SC35 have equivalent activities in vitro, and both affect alternative 5' and 3' splice site selection. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11224–11228. doi: 10.1073/pnas.89.23.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Blanco M. A., Anderson G. J., Beggs J., Sharp P. A. A mammalian protein of 220 kDa binds pre-mRNAs in the spliceosome: a potential homologue of the yeast PRP8 protein. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3082–3086. doi: 10.1073/pnas.87.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower H. J., Moore S. E., Dickson G., Elsom V. L., Nayak R., Walsh F. S. Cloning and characterization of a myoblast cell surface antigen defined by 24.1D5 monoclonal antibody. Development. 1989 Apr;105(4):723–731. doi: 10.1242/dev.105.4.723. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Horowitz D. S., Abelson J. A U5 small nuclear ribonucleoprotein particle protein involved only in the second step of pre-mRNA splicing in Saccharomyces cerevisiae. Mol Cell Biol. 1993 May;13(5):2959–2970. doi: 10.1128/mcb.13.5.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz D. S., Abelson J. Stages in the second reaction of pre-mRNA splicing: the final step is ATP independent. Genes Dev. 1993 Feb;7(2):320–329. doi: 10.1101/gad.7.2.320. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. Will the real splicing sites please light up? Curr Biol. 1992 Apr;2(4):188–190. doi: 10.1016/0960-9822(92)90516-d. [DOI] [PubMed] [Google Scholar]
- Jones K. R., Rubin G. M. Molecular analysis of no-on-transient A, a gene required for normal vision in Drosophila. Neuron. 1990 May;4(5):711–723. doi: 10.1016/0896-6273(90)90197-n. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Emori Y., Suzuki K. Production and separation of peptides from proteins stained with Coomassie brilliant blue R-250 after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1990 Dec;191(2):332–336. doi: 10.1016/0003-2697(90)90227-z. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Suzuki K. Separation of peptides dissolved in a sodium dodecyl sulfate solution by reversed-phase liquid chromatography: removal of sodium dodecyl sulfate from peptides using an ion-exchange precolumn. Anal Biochem. 1990 May 1;186(2):264–268. doi: 10.1016/0003-2697(90)90077-m. [DOI] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Zuo P., Manley J. L., Baker B. S. The Drosophila RNA-binding protein RBP1 is localized to transcriptionally active sites of chromosomes and shows a functional similarity to human splicing factor ASF/SF2. Genes Dev. 1992 Dec;6(12B):2569–2579. doi: 10.1101/gad.6.12b.2569. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liao X. C., Tang J., Rosbash M. An enhancer screen identifies a gene that encodes the yeast U1 snRNP A protein: implications for snRNP protein function in pre-mRNA splicing. Genes Dev. 1993 Mar;7(3):419–428. doi: 10.1101/gad.7.3.419. [DOI] [PubMed] [Google Scholar]
- Mayeda A., Zahler A. M., Krainer A. R., Roth M. B. Two members of a conserved family of nuclear phosphoproteins are involved in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1301–1304. doi: 10.1073/pnas.89.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. G., Porro E. B., Galceran J., Tempst P., Nadal-Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev. 1993 Mar;7(3):393–406. doi: 10.1101/gad.7.3.393. [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Steitz J. A. The mammalian analogue of the yeast PRP8 splicing protein is present in the U4/5/6 small nuclear ribonucleoprotein particle and the spliceosome. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8742–8746. doi: 10.1073/pnas.86.22.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendahl K. G., Jones K. R., Kulkarni S. J., Bagully S. H., Hall J. C. The dissonance mutation at the no-on-transient-A locus of D. melanogaster: genetic control of courtship song and visual behaviors by a protein with putative RNA-binding motifs. J Neurosci. 1992 Feb;12(2):390–407. doi: 10.1523/JNEUROSCI.12-02-00390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby S. W., Abelson J. Pre-mRNA splicing in yeast. Trends Genet. 1991 Mar;7(3):79–85. doi: 10.1016/0168-9525(91)90276-V. [DOI] [PubMed] [Google Scholar]
- Rymond B. C. Convergent transcripts of the yeast PRP38-SMD1 locus encode two essential splicing factors, including the D1 core polypeptide of small nuclear ribonucleoprotein particles. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):848–852. doi: 10.1073/pnas.90.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass H., Pederson T. Transcription-dependent localization of U1 and U2 small nuclear ribonucleoproteins at major sites of gene activity in polytene chromosomes. J Mol Biol. 1984 Dec 25;180(4):911–926. doi: 10.1016/0022-2836(84)90263-8. [DOI] [PubMed] [Google Scholar]
- Shuster E. O., Guthrie C. Human U2 snRNA can function in pre-mRNA splicing in yeast. Nature. 1990 May 17;345(6272):270–273. doi: 10.1038/345270a0. [DOI] [PubMed] [Google Scholar]
- Smith V., Barrell B. G. Cloning of a yeast U1 snRNP 70K protein homologue: functional conservation of an RNA-binding domain between humans and yeast. EMBO J. 1991 Sep;10(9):2627–2634. doi: 10.1002/j.1460-2075.1991.tb07805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan U., Abelson J. PRP18, a protein required for the second reaction in pre-mRNA splicing. Mol Cell Biol. 1990 Jan;10(1):324–332. doi: 10.1128/mcb.10.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan U., Company M., Abelson J. Isolation and characterization of pre-mRNA splicing mutants of Saccharomyces cerevisiae. Genes Dev. 1989 Aug;3(8):1206–1216. doi: 10.1101/gad.3.8.1206. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Lane W. S., Stolk J. A., Roth M. B. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992 May;6(5):837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- von Besser H., Schnabel P., Wieland C., Fritz E., Stanewsky R., Saumweber H. The puff-specific Drosophila protein Bj6, encoded by the gene no-on transient A, shows homology to RNA-binding proteins. Chromosoma. 1990 Dec;100(1):37–47. doi: 10.1007/BF00337601. [DOI] [PubMed] [Google Scholar]