Glycogen is the largest soluble cytosolic macromolecule, containing up to 55,000 glucoses per molecule. It is formed by glycogen synthase (GS) and branching enzyme (BE), which acting coordinately lead to branching after every sixth glucose and ultimately to a spherical shape that allows solubility. Polyglucosans are malformed glycogen molecules containing much less branching. They precipitate and accumulate into polyglucosan bodies (PB). PB characterize adult polyglucosan body disease (APBD) and Lafora disease (LD). In APBD, PB form in and often obstruct axons. APBD is an axonopathy with upper and lower motor neuron signs and no epilepsy. In LD, PB, called Lafora bodies (LB), occupy neuronal perikarya and dendrites. LD is a progressive myoclonus epilepsy, with no long tract or peripheral nerve deficits. APBD is caused by BE deficiency. LD is caused by mutations in the EPM2A and EPM2B genes encoding respectively the laforin phosphatase and the malin ubiquitin E3 ligase, which regulates laforin. In LD, glycogen becomes progressively phosphorylated, a pathologic process normally prevented by laforin. The charged phosphates unfold glycogen, expose its hydrophobic regions, and lead it to precipitate. GS precipitates with glycogen, but BE does not. Subsequent extension by GS unchecked by branching would convert the glycogen to polyglucosan.1,2
The next step in LD research is to understand the basis of the progressive phosphorylation. What is known is that the phosphates accumulate not on but within glycogen,1 indicating that they are added during cycles of glycogen breakdown and resynthesis, i.e., during glycogen metabolism, as it would be impossible to incorporate phosphate within the formed extremely dense glycogen sphere. While glycogen is ubiquitous, its conversion to polyglucosan in LD is not, occurring in only some cell types. Understanding particularities of glycogen metabolism in these cell types could provide valuable clues into the origin of the phosphorylation. In brain, LB form in neurons and not glia, even though astrocytes possess far more glycogen than neurons. In liver, LB form in periportal and not perivenous hepatocytes.3 Skeletal muscle is composed of slow-twitch type I and fast-twitch type II fibers, the latter divided into IIA, IID, and IIB, where IIA are the slowest fast-twitch fibers, IIB the fastest, and IID intermediate. It is not known whether LB form in all myofiber types. We address this question in the present work.
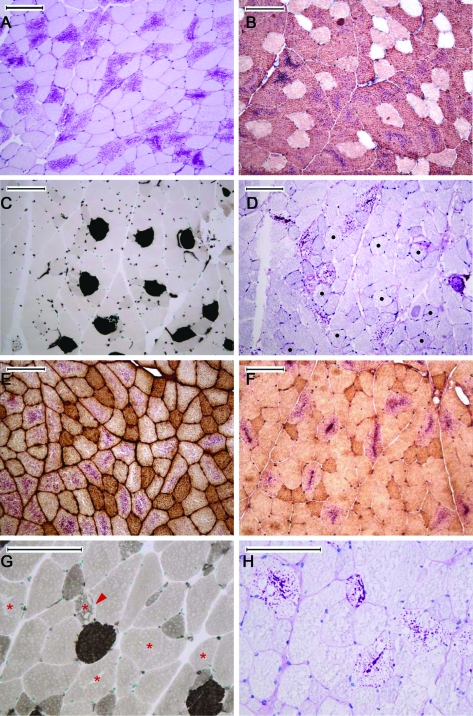
Each myofiber type has a unique myosin ATPase. We fiber-typed quadriceps muscle from 12 month-old Epm2a−/− and Epm2b−/− mice (6 animals per genotype) using histochemical and immunohistochemical staining of mATPase isoforms. Results were similar in all animals: 97% of LB were in type IIB fibers (37.7% of type IIB fibers contained LB). A total of 2% of LB were in type IID fibers (<2% of IID fibers contained LB). A total of 1% in of LB were in type IIA fibers (<1% of IIA fibers contained LB). No type I fibers contained LB (figure).
Figure. Lafora bodies (LB) are predominantly in type IIB myofibers.
(A) LB are not in all myofibers; stain, PAS-D; LB are the purple contents within fibers. (B) LB are in type IIB fibers; type IIB antibody BF-F3 and PAS-D costaining; LB are the purple contents of antibody-positive (brown) cells. (C, D) LB are not in type I fibers; (C) mATPase histochemical stain at pH 4.3; darkly stained cells are type I fibers. (D) PAS-D-stained serial section; black dots are the type I cells in C. (E) The vast majority of type IIA fibers (brown) do not contain LB; type IIA antibody SC-71. (F) The vast majority of type IID fibers (darker color) do not contain LB; type IID antibody 6H1. (G, H) Five LB-containing fibers shown, 4 type IIB (asterisks) and 1 type IID (arrowheads); (G) mATPase histochemical stain at pH 4.6; the darkest fibers are type I, second-darkest IID, and the large pale fibers IIB. (H) PAS-D-stained serial section to G. With the exception of (A), all staining was on frozen sections. Techniques were standard.e1 (Reference e1 is available on the Neurology® Web site at www.neurology.org.) All bars equal 100 μm.
Are there aspects of glycogen metabolism that are unique to type IIB fibers to potentially explain their near-exclusive LB formation? Glycogen metabolism in type IIB differs from other types in 2 ways. It is glyconeogenic,4 i.e., its glucoses derive from lactate through gluconeogenesis, not from serum. Secondly, its rates of glycogen synthesis and breakdown are much higher than in other fiber types.5 Interestingly, in liver, glycogen metabolism in the LB-containing periportal hepatocytes is glyconeogenic and has high turnover, which is not the case in perivenous hepatocytes.3 Cardiomyocytes, the other major glycogen-metabolizing cell type, contain abundant LB in LD. Cardiomyocytes, again, are characterized by being glyconeogenic and possessing very high glycogen turnover. In fact, 50% of glucose consumed in each cardiac contraction cycles through glycogen,6 which allows the heart to always have a supply of glucose (55,000 per glycogen molecule) in case of need and at the same time have the glycogen-digesting enzymes constantly primed and engaged. Neurons are gluconeogenic, supplied with lactate from astrocytes.7 The amount of glycogen they maintain is small. Whether it has high turnover has not been studied, but it would not be surprising if neurons, like cardiomyocytes, constantly cycle glucose through glycogen to maintain a ready supply of concentrated glucose for neuroprotection at times of energy shortfall.
Our results suggest 2 possible entry points for the phosphate that drives glycogen conversion to polyglucosan, the enzymatic steps of gluconeogenesis, and possible errors or other mechanisms related to high and rapid glycogen breakdown and resynthesis. Whether these entry points prove true or not, any mechanism proposed to explain the abnormal glycogen phosphorylation and polyglucosan formation in LD needs to encompass an explanation for the specificity of LB to type IIB myofibers.
Supplementary Material
ACKNOWLEDGMENT
Fiber type-specific monoclonal antibodies used in this study were developed by S. Schiaffino or C. Lucas and colleagues and obtained from the Developmental Studies Hybridoma Bank (under the auspices of the NICHD) maintained by The Department of Biology of The University of Iowa.
Footnotes
Disclosure: J. Turnbull is supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Canada Graduate Scholarship. Dr. Girard, N. Pencea, and X. Zhao report no disclosures. Dr. Graham is Editor of Applied Physiology, Nutrition and Metabolism. Dr. Wang reports no disclosures. Dr. Ackerley receives research support from the CIHR. Dr. Minassian has received speaker honoraria from Athena Diagnostics, Inc.; is listed as inventor on, and has received license fees and royalties for patents re: Diagnostic testing of four genes; and receives research support from the CIHR.
Supplemental data at www.neurology.org.
References
- 1. Tagliabracci VS, Girard JM, Segvich D, et al. Abnormal metabolism of glycogen phosphate as a cause for Lafora disease. J Biol Chem 2008; 283: 33816–33825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turnbull J. Glycogen hyperphosphorylation underlies Lafora body formation. Ann Neurol 2010; 68: 925–933 [DOI] [PubMed] [Google Scholar]
- 3. Andrade DM, Ackerley CA, Minett TS, et al. Skin biopsy in Lafora disease: genotype-phenotype correlations and diagnostic pitfalls. Neurology 2003; 61: 1611–1614 [DOI] [PubMed] [Google Scholar]
- 4. McDermott JC, Bonen A. Glyconeogenic and oxidative lactate utilization in skeletal muscle. Can J Physiol Pharmacol 1992; 70: 142–149 [DOI] [PubMed] [Google Scholar]
- 5. Kernell D, Lind A, van Diemen AB, De Haan A. Relative degree of stimulation-evoked glycogen degradation in muscle fibres of different type in rat gastrocnemius. J Physiol 1995; 484: 139–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taegtmeyer H. Glycogen in the heart: an expanded view. J Mol Cell Cardiol 2004; 37: 7–10 [DOI] [PubMed] [Google Scholar]
- 7. Velasquez ZD, Perez M, Moran MA, et al. Ultrastructural localization of fructose-1,6-bisphosphatase in mouse brain. Microsc Res Tech Epub 2010 Aug 4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.