Abstract
Objective:
To devise a rapid, sensitive method to quantify tactile threshold of finger pads for early detection and staging of peripheral neuropathy and for use in clinical trials.
Methods:
Subjects were 166 healthy controls and 103 patients with, or at risk for, peripheral neuropathy. Subjects were screened by questionnaire. The test device, the Bumps, is a checkerboard-like smooth surface with 12 squares; each square encloses 5 colored circles. The subject explores the circles of each square with the index finger pad to locate the one circle containing a small bump. Bumps in different squares have different heights. Detection threshold is defined as the smallest bump height detected. In some subjects, a 3-mm skin biopsy from the tested finger pad was taken to compare density of Meissner corpuscles (MCs) to bump detection thresholds.
Results:
The mean (±SEM) bump detection threshold for control subjects was 3.3 ± 0.10 μm. Threshold and test time were age related, older subjects having slightly higher thresholds and using more time. Mean detection threshold of patients with neuropathy (6.2 ± 0.35 μm) differed from controls (p < 0.001). A proposed threshold for identifying impaired sensation had a sensitivity of 71% and specificity of 74%. Detection threshold was higher when MC density was decreased.
Conclusions:
These preliminary studies suggest that the Bumps test is a rapid, sensitive, inexpensive method to quantify tactile sensation of finger pads. It has potential for early diagnosis of tactile deficiency in subjects suspected of having neuropathy, for staging degree of tactile deficit, and for monitoring change over time.
Reduced tactile sensation (numbness) is a common symptom of patients with peripheral neuropathy. A need exists for efficient techniques to diagnose and quantify tactile deficiency early, when treatment has the greatest potential of success. Furthermore, evaluation of therapeutic benefits from advances in molecular biology, genetics, pharmacology, and other sciences will require more precise measurements than are currently available.
Meissner corpuscles (MCs) are sensitive organs for touch recognition on glabrous skin of the hands and fingers. Early studies showed lower MC density in older persons1–3 and in neuropathy.4 More recently, reduced MC density, distorted MC structure, focal thinning or loss of myelin, and short myelin internodes coupled with decreased sensitivity to touch have been reported.5–7
Functional studies of touch conveyed by cutaneous mechanoreceptors in glabrous skin found that detection of small raised dots (bumps) on a smooth surface using the finger pad was signaled by MCs.8,9 Based on these studies, we devised a simple device called the Bumps to quantify tactile sensitivity on the finger pads. The Bumps test determines the tactile detection threshold by having the subject rub the finger pad over a smooth surface to locate single, coin-shaped bumps of different heights. Using this device, we found a difference in detection thresholds between control subjects and patients with a diagnosis of neuropathy. We contend that the Bumps device has potential utility for early diagnosis, staging degrees of severity of neuropathy, and measuring its progression.
METHODS
Study subjects.
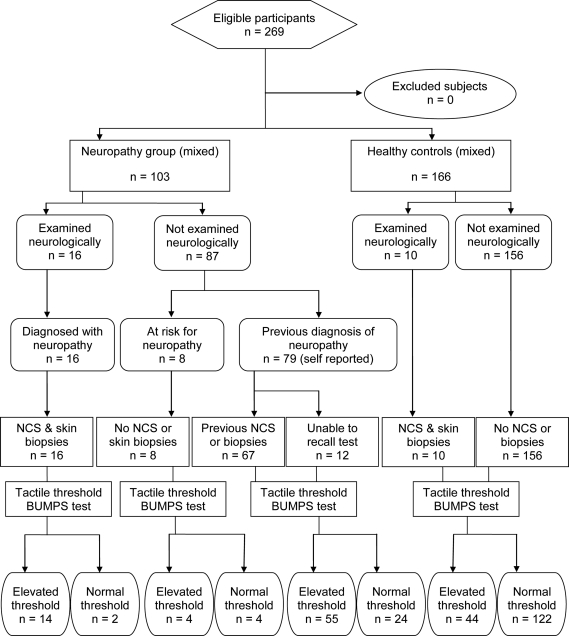
A total of 269 subjects (figure 1) were enrolled (June 2008–August 2009): 166 healthy volunteers and 103 subjects with or at risk for peripheral neuropathy recruited from the Minnesota Neuropathy Association (79), the Salvatore Maugeri Foundation (16), and University of Minnesota clinics (8). MN control subjects were screened using a general health assessment questionnaire for systemic diseases or use of drugs associated with nerve damage, but were not examined neurologically. MN neuropathy subjects were interviewed and completed the same questionnaire to determine methods of diagnosis and location of sensory deficits (hands, feet, or both). Subjects from the Salvatore Maugeri Foundation were assessed by neurologic examination, motor (median, ulnar, and peroneal), and sensory (median, ulnar, and sural) nerve conduction studies and skin biopsies.
Figure 1. Study flow chart.
NCS = nerve conduction studies.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the University of Minnesota's Institutional Review Board in accordance with ethical guidelines for conducting research on human subjects. Subjects gave written informed consent to participate in the research study in a manner compatible with Health Insurance Portability and Accountability Act regulations.
Bumps device and testing procedure.
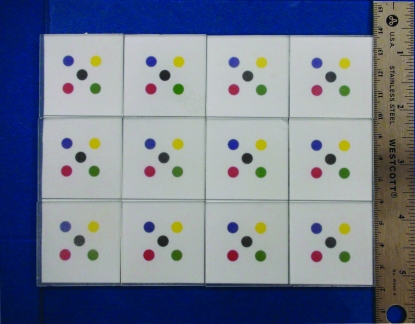
The Bumps device was produced by photolithography in the University of Minnesota's nanofabrication center. It is a checkerboard-like smooth surface divided into 12 squares (figure 2). Each square contains 5 colored circles; one randomly selected circle contains a coin-shaped bump. All bumps are 550 μm in diameter, but of different heights. We used 2 such devices that were identical except for the bump heights: bumps on plate A were 2.5 to 8 μm whereas bumps on plate B were 8.5 to 14 μm; bump heights on each plate increased in 0.5-μm increments.
Figure 2. The Bumps device.
Each square on the plate contains 5 colored circles; one randomly selected colored circle in each square contains a bump. The 12 bumps have different heights.
Testing was performed in a quiet, distraction-free room. Participants were read standard instructions and each trial (plate B followed by plate A) was timed by stopwatch as they searched the 5 circles on each square of the plates with the index finger pad (not fingernail) to locate the bump. Best results were obtained by rubbing lightly side-to-side or in a circular motion to strike the bump with the fingerprint ridges at approximately 90°. A successful second try to identify incorrectly located bumps received full credit. Most subjects completed 2 trials during the same visit.
Biopsy procedures and MC quantification.
In 10 control subjects and 16 subjects with neuropathy of various etiologies (diabetes, small fiber sensory neuropathy, Ross syndrome), a 3-mm punch biopsy was removed from the finger pad used to detect bumps, after intradermal injection of 1% Xylocaine. Biopsies were fixed in Zamboni solution for 24 hours and then cryoprotected.10,11 Thick, 50-μm sections were cut vertical to the skin surface, at 90° to the fingerprint ridges, processed for indirect immunofluorescence, and MCs were quantified as described.12
Data analyses.
Bump detection threshold for a trial (plate B followed by plate A) was defined as the lowest bump such that it and the next 2 highest bumps were successfully detected. This criterion was chosen because for a given set of 3 consecutive bump heights, the chance of guessing all 3 correctly is under 1%. Subjects who did not detect 3 consecutive bump heights received detection threshold 13.5 μm if they detected the highest 2 bumps and 14.0 μm otherwise. For subjects who completed 2 trials, the better (lower) of the 2 detection thresholds was used.
Analyzing control subjects, the sexes were compared using a t test (checked with Wilcoxon signed-rank test), association of detection threshold with age was tested using linear regression, and age decades were compared according to average time to complete the test using one-way analysis of variance (ANOVA).
Control subjects were compared to neuropathy patients for detection threshold and MC density using t tests (checked with Wilcoxon signed-rank test). Neuropathy patients grouped by symptom location were compared according to detection threshold using one-way ANOVA with the Tukey-Kramer posthoc test; age-adjusted comparison used linear regression.
RESULTS
Subjects.
Control subjects ranged in age from 20 to 88 years (M/F = 80/86); neuropathy subjects were older, from 36 to 89 years (M/F = 49/54). Neuropathy subjects were either diagnosed (95) or at risk for neuropathy (8). The latter were asymptomatic subjects with diabetes. Members of the MN Neuropathy Association (79), at interview and by questionnaire, confirmed a previous diagnosis of neuropathy by a neurologist supplemented by EMG (61), nerve conduction study (61), or skin biopsy (6). All Salvatore Maugeri Foundation neuropathy subjects (16) had abnormal neurologic examination, nerve conduction studies, and skin biopsies.
Detection thresholds in control group.
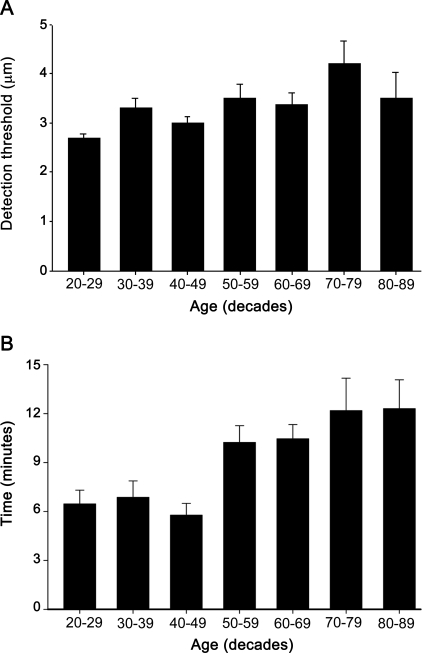
Although mean detection thresholds as a function of age (decades) were similar in control subjects (figure 3A), linear regression showed detection threshold tended to increase with age (r = 0.22, p = 0.004). Male and female control subjects did not differ significantly in mean detection threshold (females 3.1 ± 0.10 μm, males 3.5 ± 0.17 μm, p = 0.06).
Figure 3. Bump detection thresholds in the control group.
(A) Mean (±SEM) detection thresholds (μm) as a function of age (decades) in control subjects. Mean detection thresholds did not change much with age for subjects between the ages of 20 and 89 years, though linear regression showed that older subjects had a modest tendency to higher thresholds. (B) Time taken to complete the test as a function of age; the 7 decade groups tested different.
Time to complete the test.
The time taken for control subjects to complete 2 trials increased with age (figure 3B; 1 way ANOVA, p < 0.001). Control subjects under age 40 completed 2 trials in 6.5 minutes on average; those aged 70 and older averaged 12.2 minutes; 90% of all control subjects completed 2 trials in under 14 minutes. Patients with neuropathy took longer, averaging 13.6 minutes (90% completed 2 trials in under 23 minutes); those over age 70 completed 2 trials in 13.9 minutes (90% completed 2 trials in under 24 minutes). Thus, the Bumps as administered in this study (each plate tested twice) typically takes 11 minutes on average to complete and uncommonly requires more than 15 minutes even in older subjects with neuropathy. A single trial is expected to take half of the reported times.
Detection threshold comparison between groups.
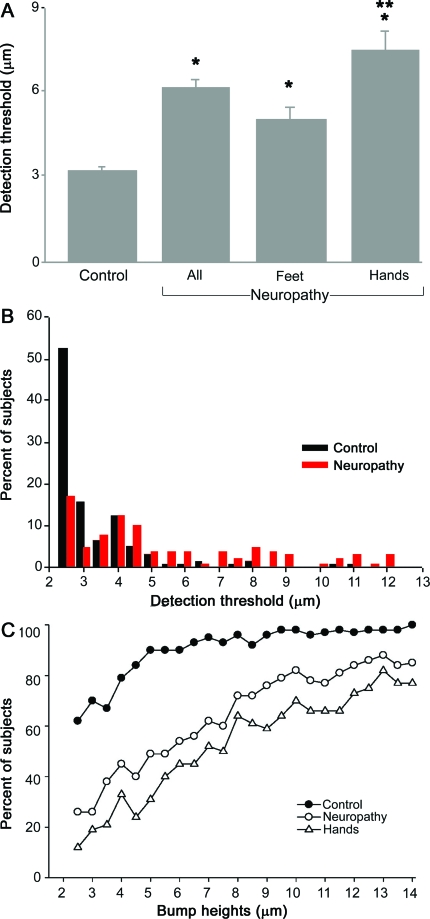
Since the mean detection thresholds in the control group varied modestly with age and sex, the mean (±SEM) detection threshold for all control subjects was computed as 3.3 ± 0.10 μm. The mean detection threshold for all patients with previously diagnosed neuropathy or a condition placing them at risk for neuropathy was 6.2 ± 0.35 μm, higher than the control group (p < 0.001; figure 4A). Detection thresholds were higher in patients with symptoms that included the hands compared to patients with symptoms restricted to the feet, which in turn were higher than detection thresholds in control subjects (p < 0.05, Tukey-Kramer post hoc test). The age of neuropathy patients started at 36 years compared to 20 for the control group; adjusting for age using linear regression produced results similar to the unadjusted comparisons.
Figure 4. Tactile sensitivity of control and neuropathy subjects using the Bumps test.
(A) Mean (±SEM) bump detection thresholds for all control subjects (control) and all patients (all) with neuropathy. Patients were subdivided according to those with sensory symptoms in the feet only (feet) and those in whom symptoms were also reported in the hands (hands). *Significant difference from control; **significant difference from feet, p < 0.001. (B) The percent of control subjects and patients who exhibited specific bump detection thresholds (μm). A greater proportion of control subjects had thresholds <4 μm whereas a greater proportion of neuropathy patients had thresholds >4 μm. (C) The percent of control subjects (no neuropathy), all neuropathy patients (neuropathy), and the subgroup of patients with sensory symptoms in the hands (hands) that identified individual bumps on their first trial.
Figure 4B shows the proportion of control subjects and patients with specific detection thresholds. More than 50% of control subjects had detection thresholds of 2.5 μm, with 91% at 4.5 μm or lower. By contrast, under 20% of patients detected the 2.5-μm bump and just over half had detection thresholds of 5.0 μm or higher. The proportion of subjects who detected individual bumps also differed between control and neuropathy subjects (figure 4C). For example, only 50% of all neuropathy patients were able to locate the 5-μm bump, which was located by 90% of the control group. Control and neuropathy subjects differed most for the lowest bumps (under 8 μm) and differed considerably less for higher bumps. Collectively, these results show dramatic differences in tactile sensitivity between control subjects and neuropathy patients. Using a receiver operator characteristic curve, we propose a cutoff threshold as follows: a threshold of 4.0 μm or lower is considered normal, whereas a threshold greater than 4.0 μm indicates sensory dysfunction; this gives 74% specificity and 71% sensitivity.
Comparison of Meissner corpuscle density and bump detection threshold.
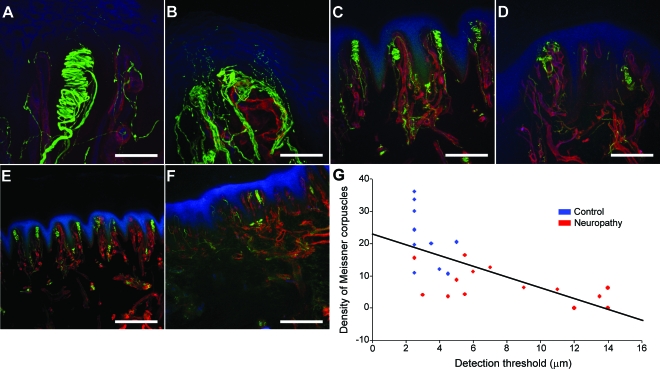
We compared bump detection thresholds with MC density in biopsies from 10 control and 16 neuropathy subjects. For these control subjects the mean detection threshold was 3.1 ± 0.3 μm and mean MC density was 21.2 ± 2.7 MCs/mm2 (skin surface area). Detection thresholds were higher for this group of neuropathy patients (8.7 ± 1.1 μm, p < 0.001) and MC density was lower (6.1 ± 1.4 MCs/mm2, p < 0.001). Figure 5 shows representative examples of the structure and number of MCs from a control subject (figure 5, A, C, and E) and a subject with diabetic neuropathy (figure 5, B, D, and F). This control subject had a detection threshold of 2.5 μm and MC density of 30 MC/mm2. In contrast, the patient had a detection threshold of 7 μm and MC density of 12.6 MC/mm2. This patient had fewer MCs and the innervation of the remaining MCs was absent and presumably had degenerated. Figure 5G shows that for all 26 subjects that bump detection threshold increased as MC density decreased (r = −0.71, p < 0.001).
Figure 5. Representative confocal images of the structure and density of Meissner corpuscles (MCs) and correlation to tactile threshold.
MCs and nerve fibers, labeled with antibody to protein gene product 9.5, appear green; blood vessels and basement membrane, labeled with type IV collagen antibody, appear red; epidermis and endothelium were labeled with Ulex europaeus agglutinin I and appear blue. Images (A–F) are projections of confocal z stacks acquired from 50-μm-thick sections. Images (A, C, E) represent a control subject and (B, D, F) represent a subject with diabetic neuropathy. (A, B) High magnification (40×) of the structure and innervation of a single MC; scale bar equals 50 μm. (A) Well-organized and intact MC in a control subject. (B) A comparable image from a subject with diabetic neuropathy where MC is degenerated and the nerve fibers are disorganized. (C, D) Lower magnification (10×) showing density of MCs; scale bar equals 200 μm. (C) MCs are present along each ridge in the control subject whereas (D) the patient has fewer MCs and the remaining MCs have an abnormal structure. (E, F) Lower magnification (5×; scale bar equals 400 μm) showing the dramatic reduction in the number of MCs in this patient (F) compared to the control subject (E). (G) The density of MCs was quantified from skin biopsies obtained from 10 control and 16 neuropathy subjects. Linear regression was used to test for an association between the number of MCs and detection thresholds. Subjects with lower bump detection threshold tended to have a high density of MCs while elevated thresholds were accompanied by a decrease in the number of MCs.
DISCUSSION
The Bumps is an elegantly simple, easy to understand and administer method to determine touch sensitivity on the finger pad. The size and portable design make it useful in the clinic or informal settings. Tactile detection thresholds are quantitative into the low micrometer range. In this initial preliminary study, thresholds of control subjects averaged 3.3 μm, increasing modestly with age. Females had slightly lower thresholds. Testing times were longer in older subjects.
A perceived study weakness is that diagnosis of neuropathy was by different neurologists and questionnaire, not by preplanned protocol, except for Maugeri Foundation subjects. The questionnaire screened for methods of diagnosis, including nerve conduction tests, and carpal tunnel syndrome. Nevertheless, elevated thresholds were common in this heterogeneous group and many subjects who reported sensory symptoms confined to their feet had elevated finger tactile thresholds. Although values of 71% sensitivity and 74% specificity obtained in the present study may not indicate the power needed for a diagnostic tool, these values were obtained from the finger pad where sensory deficits in neuropathy are usually absent or less severe than in the toes. This suggests that the Bumps can often identify tactile dysfunction in the fingers, whether generalized or focal, before the patient becomes aware of a deficit. Also, the distribution of detection thresholds and the proportion of subjects who detected each bump differed between patients and controls. Together, these measures demonstrate the ability of the Bumps not only to detect impaired touch sensation, but to possess potential to aid diagnosis of peripheral neuropathy at an early stage. Validation studies of well-characterized patients with neuropathies of different severities are needed to evaluate the effectiveness of the Bumps as an early diagnostic tool. Another advantage is that the Bumps are easily used in children. In preliminary studies, over 100 children ages 8–12 understood the procedure and 86% detected the 2.5-μm bump.
We chose 2.5 μm as the smallest bump height to test because we speculated that detection thresholds for control subjects would be in the range reported for young subjects.8,9 Surprisingly, 51% of control subjects detected the 2.5-μm bump. Because this was the smallest bump, subjects had no chance to locate smaller bumps and attain a lower threshold. This reduced the difference between control and neuropathy group averages, but does not affect sensitivity or specificity in identifying individual neuropathy vs control subjects.
Other available tests attempt to obtain quantitative data on impaired touch sensation. Presently, von Frey–like nylon monofilaments are used to measure tactile thresholds in research studies. A series of nylon filament probes with calibrated bending forces are applied repeatedly to the skin. The subject reports if the stimulus is detected (forced choice method). The smallest force detected in over 50% of trials is accepted as the touch threshold. Because the examiner cannot objectively verify that the subject detected a stimulus, numerous applications are required, limiting routine use in the clinic. Electromechanical instruments exist that obtain accurate evaluations of touch threshold. However, examination time, cost, and nonportability discourage common usage of these instruments.
The Bumps test provides a fast, quantitative measure of tactile function. Total time to test a subject twice with each plate was usually well under 10 minutes in controls and seldom required 15 minutes, even in the older neuropathy patients. Although additional studies are needed to determine the extent of practice effects on detection threshold, we believe that administering one trial (each plate tested once), approximately halving the reported test time, will be adequate since mean detection thresholds improved by only 0.3 μm with the second trial (data not shown). Requiring subjects to demonstrate that they felt the tactile stimulus by locating the bump in one of 5 circles avoids the problems of the forced choice method. Although it is possible to identify the correct bump by chance, the odds against a correct guess are 4:1 for each square. Defining threshold as the smallest bump height of 3 consecutive correct locations increases odds against guessing correctly to approximately 100:1. We considered other definitions of threshold, e.g., requiring only 2 consecutive correct detections or threshold derived from a sophisticated statistical model. Thresholds derived from these variations differed only slightly in performance (data not shown).
The Bumps device is modeled after stimuli used by Johansson and LaMotte8 and LaMotte and Whitehouse9 to study peripheral neural mechanisms of tactile detection of a raised dot (bump) on a smooth surface. Young, healthy humans detected raised dots of varying heights that were stroked passively across the finger pad with controlled force and velocity or actively stroked by the finger. Detection thresholds were 2–4 μm height for a 550-μm diameter dot. Threshold varied with dot diameter from 1 μm height for a 600 μm diameter dot to 6 μm height for a 40 μm dot. Neither the amount of pressure by the finger (3 to 45 g weight) nor velocity of movement across the finger pad (10 to 40 mm/s) influenced thresholds.9 We measured thresholds for 550-μm bumps similar to those described and estimate that finger pressure and velocity were within the wide ranges described.9 Our experience with the present device will lead to redesign with bump heights more closely reflecting these experimental results.
We devised the first Bumps device for fingers rather than toes because easy access to fingers and the ability of fingers to accommodate to various devices increased design options. Although we tested the index finger, the device can be used with other fingers. Because MC density varies with finger volume,1 touch thresholds can vary from those reported. This may explain the slightly lower thresholds we reported for females. Another consideration was that fingers are more sensitive to touch than toes and our goal is to measure small degrees of sensory deficiency, which are important for early detection of neuropathy. Whereas the symptoms of decreased touch sensitivity in neuropathy patients are usually first manifest in the feet, the discovery of structural abnormalities of MCs in feet of most normal persons (personal communication) suggests it may be difficult to distinguish changes due to neuropathy from those already present.
Patients with neuropathy who underwent finger biopsy tended to have lower MC density and elevated detection threshold compared to control subjects. This is consistent with studies showing that detection of bumps at or near threshold is signaled by MCs.8,9 Earlier studies of patients with hereditary neuropathy demonstrated that patients with the greatest sensory loss had lower MC concentrations in the toes and fingers.4
Bump detection thresholds for elderly control subjects were similar to thresholds of younger subjects. This was surprising since the increasingly irregular distribution1 and decrease of MC density with age1,3 is accompanied by a progressive decline in tactile acuity.13 MC density is highest in young children; it decreases with digit enlargement during the first 2 to 3 decades, then further decreases by approximately 50% by the eighth decade.1 We recorded testing time because without a time limit older subjects searched the 5 circles for prolonged times before successfully locating a bump. We speculate that prolonged searching allows more contacts between the bump stimulus and the reduced number of MCs. Combined with temporal and spatial summation, this facilitates bump detection.
Correlation of MC function measured by the Bumps with a noninvasive morphologic means to quantify MC density would be useful to diagnose and track peripheral neuropathy. MC quantification by reflectance confocal microscopy is a possible method14,15 if the imaged number of MCs is proven to be accurate.16
The Bumps device provides clinicians and researchers with a simple method to quickly quantify the sensation of touch. Ongoing validation studies of the Bumps as a tool for diagnosing and tracking peripheral neuropathy include comparing results from the Bumps test with those obtained using von Frey monofilaments, and comparisons of bump detection with the density of MCs. Our data show that decreased touch sensitivity measured by the Bumps indicates reduced MC density, just as reduced pinprick and heat sensitivity are related to decreased epidermal nerve fiber density.17,18 Thus, the Bumps has potential as a biomarker that may be useful for early detection, for tracking changes in touch perception in patients with neuropathy, or in clinical trials.
ACKNOWLEDGMENT
The authors thank the volunteers, including members of the Minnesota Neuropathy Association, especially Lois Martin (president), for their participation in our research.
Footnotes
- ANOVA
- analysis of variance
- MC
- Meissner corpuscle
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. James S. Hodges.
DISCLOSURE
Dr. Kennedy receives research support from the NIH, the University of Minnesota, the Institute for Engineering in Medicine, and the Juvenile Diabetes Research Foundation (JDRF). Dr. Selim receives research support from the NIH and the JDRF. Dr. Brink has received research support the NIH/NIDCR. Dr. Hodges has served on a data safety monitoring board for Kaiser Permanente and receives research support from the NIH, the JDRF, the American Diabetes Association, the US Department of Veterans Affairs, and the Minneapolis Heart Institute Foundation. G. Wendelschafer-Crabb receives research support from the NIH, the University of Minnesota, the Institute for Engineering in Medicine, and the JDRF. S.X.Y.-L. Foster receives research support from the NIH, the University of Minnesota, and the Institute for Engineering in Medicine. Dr. Nolano and Dr. Provitera report no disclosure. Dr. Simone receives research support from the NIH and Fogarty International Foundation.
REFERENCES
- 1. Bolton CF, Winkelmann RK, Dyck PJ. A quantitative study of Meissner's corpuscles in man. Neurology 1966; 16:1–9 [DOI] [PubMed] [Google Scholar]
- 2. Dickens WN, Winkelmann RK, Dyck PJ. Cholinesterase demonstration of dermal nerve endings in patients with impaired sensation: a clinical and pathologic study of 41 patients and 37 control subjects. Neurology 1963; 13:91–100 [DOI] [PubMed] [Google Scholar]
- 3. Martinez Perez R. Contribution a l'etude des terminaisons nerveuses dans la peau de la main. Trav Lab Rech Biol 1931; 27:187–226 [Google Scholar]
- 4. Dyck PJ, Winkelmann RK, Bolton CF. Quantitation of Meissner's corpuscles in hereditary neurologic disorders: Charcot-Marie-Tooth disease, Roussy-Levy syndrome, Dejerine-Sottas disease, hereditary sensory neuropathy, spinocerebellar degenerations, and hereditary spastic paraplegia. Neurology 1966; 16:10–17 [DOI] [PubMed] [Google Scholar]
- 5. Nolano M, Provitera V, Crisci C, et al. Small fibers involvement in Friedreich's ataxia. Ann Neurol 2001; 50:17–25 [DOI] [PubMed] [Google Scholar]
- 6. Nolano M, Provitera V, Perretti A, et al. Ross syndrome: a rare or a misknown disorder of thermoregulation? A skin innervation study on 12 subjects. Brain 2006; 129:2119–2131 [DOI] [PubMed] [Google Scholar]
- 7. Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain 2008; 131:1903–1911 [DOI] [PubMed] [Google Scholar]
- 8. Johansson RS, LaMotte RH. Tactile detection thresholds for a single asperity on an otherwise smooth surface. Somatosens Res 1983; 1:21–31 [DOI] [PubMed] [Google Scholar]
- 9. LaMotte RH, Whitehouse J. Tactile detection of a dot on a smooth surface: peripheral neural events. J Neurophysiol 1986; 56:1109–1128 [DOI] [PubMed] [Google Scholar]
- 10. Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology 1996; 47:1042–1048 [DOI] [PubMed] [Google Scholar]
- 11. Kennedy WR, Wendelschafer-Crabb G, Polydefkis M, McArthur J. Pathology and quantitation of cutaneous nerves. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy, 4th ed Philadelphia: Saunders; 2005: 869–896 [Google Scholar]
- 12. Nolano M, Provitera V, Crisci C, et al. Quantification of myelinated endings and mechanoreceptors in human digital skin. Ann Neurol 2003; 54:197–205 [DOI] [PubMed] [Google Scholar]
- 13. Ronge H. Altersveränderungen der Meissnerschen Korperchen in der Fingerhaut. Z mikr-anat Forsch 1943; 54:167–177 [Google Scholar]
- 14. Herrmann DN, Boger JN, Jansen C, Alessi-Fox C. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology 2007; 69:2121–2127 [DOI] [PubMed] [Google Scholar]
- 15. Dyck PJ. Enumerating Meissner corpuscles: future gold standard of large fiber sensorimotor polyneuropathy? Neurology 2007; 69:2116–2118 [DOI] [PubMed] [Google Scholar]
- 16. Nolano M, Provitera V, Santoro L, et al. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology 2008; 71:536–537 [DOI] [PubMed] [Google Scholar]
- 17. Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci 1998; 18:8947–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain 1999; 81:135–145 [DOI] [PubMed] [Google Scholar]