Abstract
Management of first trimester pregnancy loss has conventionally involved two options: expectant management or dilation and curettage in the operating room. New options in the outpatient setting are providing women with alternatives that can be less expensive and performed in more private settings. This review discusses the available approaches to expectant, medical, and surgical management of first trimester loss and the comparative efficacy of each method.
Key Words: Miscarriage, Dilation and curettage, Pregnancy loss, Office procedures
Spontaneous pregnancy loss occurs in 25% to 50% of pregnancies prior to 14 weeks of gestation. Description of first trimester losses can be somewhat confusing, due to nonstandardized terminology. The term blighted ovum has been largely abandoned, although debate lingers concerning terms such as anembryonic pregnancy and missed abortion, which are still commonly used. Jauniax and colleagues1 have attempted to simplify the descriptions of first trimester losses by characterizing pregnancy loss according to the stage of the process the patient is in at the time of presentation to the practitioner. Simplified recommendations use the terms complete, incomplete, and delayed pregnancy loss (Table 1). A complete pregnancy loss is characterized by complete passage of the intrauterine tissue. The cervix is closed and the remaining endometrial thickness is typically less that 15 mm by ultrasound. An incomplete pregnancy loss is characterized by partial passage of the products of conception with clinical or ultrasonographic evidence of retained pregnancy tissue. The term delayed pregnancy loss includes those failed pregnancies previously called missed abortions, blighted ova, or anembryonic1 and differs from incomplete pregnancy loss in that it precedes the passage of tissue and the onset of significant vaginal bleeding.
Table 1.
Classification of First Trimester Pregnancy Loss
| Diagnosis | Characteristic |
| Complete pregnancy loss | Complete passage of products of conception |
| Cervix closed | |
| Endometrial thickness typically < 15 mm | |
| Incomplete pregnancy loss | Partial passage of products of conception |
| Cervix open or closed | |
| Any endometrial thickness | |
| Tissue distorting central endometrial echo is heterogeneous and may contain a gestational sac | |
| Delayed pregnancy loss | No tissue passage |
| (includes what were previously called blighted ovum, anembryonic pregnancies, missed abortions) | Cervix closed |
| Gestational sac > 20 mm without evidence of fetal pole or yolk sac | |
| Gestational sac < 20 mm with no change in size over 7 days | |
| Fetal pole > 6 mm with no detectable fetal heart activity | |
| Fetal pole < 6 mm with no change over 7 days |
Adapted from Jauniaux E et al.1
Although the etiologies of first trimester loss are multifactorial and often remain unknown, certain risk factors increase the likelihood of pregnancy loss. Arck and colleagues2 enrolled 1098 women with gestations between 4 and 12 weeks and identified risk factors for subsequent pregnancy loss. Risk of pregnancy loss was significantly higher among women of advanced maternal age (> 35 years), women with a low body mass index (BMI; < 20 kg/m2) regardless of age, and women with elevated cortisol levels (suggestive of elevated stress) and low progesterone levels (< 12 ng/mL) prior to 7 weeks of gestation.2 Other associated risk factors include smoking, nonsteroidal anti-inflammatory drug (NSAID) use, poor glycemic control, obesity, thyroid dysfunction, acute infections (Listeria, toxoplasmosis, parvovirus B19, rubella, herpes), and hypercoagulable states.
Detection of First Trimester Loss
No single parameter is highly sensitive for predicting an impending loss, although certain clinical and ultrasound findings are suggestive of pregnancies that will not reach viability. The most reassuring marker of a healthy pregnancy is the presence of cardiac activity, which should be evident after 5 weeks of gestation or once the embryo reaches 2 mm in length. If cardiac activity is present, the risk of spontaneous loss declines to 3% to 6%.3 Prior to detection of a heartbeat, findings such as a gestational sac smaller than expected for dates or an abnormal-appearing yolk sac that is large, irregular, free floating, or calcified may suggest subsequent pregnancy loss, and should be followed by repeat ultrasonographic evaluation.4 Although they have poor reproducibility, abnormalities such as increased placental echogenicity, increased thickness of the trophoblast, or intrauterine hematoma (subchorionic hemorrhage) have been hypothesized as potential markers for subsequent pregnancy loss.1
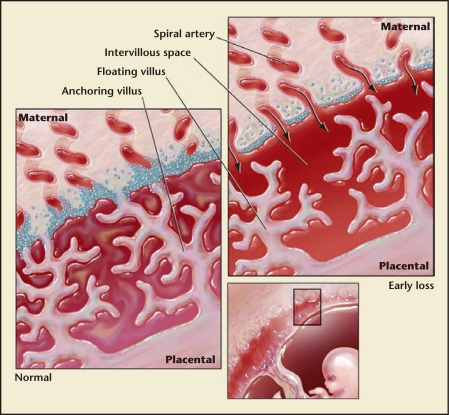
Although placental abnormalities can be difficult to identify ultrasonographically during the first trimester, a subchorionic hemorrhage may result in clinical presentation with vaginal bleeding. Vaginal bleeding at any time and in any amount during early pregnancy typically causes great concern for patients, but the timing of the bleeding has the most important prognostic implications. If bleeding occurs before 6 weeks of gestation, there does not seem to be an increase in adverse pregnancy outcomes.1 Bleeding after 7 weeks of gestation, even in the presence of cardiac activity, indicates a risk for pregnancy loss or later pregnancy complications approaching 10%.3 Most often, subchorionic hemorrhages escape clinical detection. However, it has been hypothesized that use of Doppler ultrasound to assess placental blood flow may serve as a possible screening tool for pregnancy viability. Surprisingly, maternal blood does not circulate within the placenta until about 9 weeks of gestation. Prior to this time, trophoblastic cells serve to “plug” maternal spiral arteries, preventing flow to these arteries and into the intervillous space until the villi mature and begin to grow into the myometrium1 (Figure 1). It is critical to the have minimal exposure to the high levels of oxygen in maternal blood during the first trimester of human pregnancy. Both the fetus and the placental villi are exquisitely sensitive to oxidative stress and, if exposed prematurely, pregnancy loss may result. Premature intervillous blood flow has been noted in histopathologic studies of fetal and placental tissue after pregnancy loss. Still, the measurement of blood flow in the intervillous space by Doppler ultrasound cannot reliably predict whether a pregnancy will progress.5 Although Doppler flow may not be sensitive enough for assessing pregnancy outcome, it has shown promise in predicting success of expectant management with incomplete pregnancy loss. Finding blood flow in the intervillous space using color Doppler during evaluation of a threatened first trimester pregnancy loss increases by fourfold the chance for subsequent complete pregnancy loss with expectant management.1
Figure 1.
Placental development in normal early pregnancy and in early pregnancy loss. Normal: The maternal spiral arteries are plugged with trophoblast cells until approximately 9 weeks of gestation in normal early pregnancies. This inhibits maternal blood flow into the intervillous space and any associated oxidative stress. Rather, the intervillous space is filled with serum transudate and nutrients to support the developing fetus. Early loss: Poor placentation associated with early pregnancy loss is characterized by limited trophoblast plug formation in the maternal spiral arteries and free flow of maternal blood into the intervillous space. This allows for oxidative damage to the extremely vulnerable placental villi.
Management Options
Once a spontaneous pregnancy loss has been diagnosed, there are three forms of management: expectant, medical, or surgical. The optimal mode of management is determined by gestational age, whether the pregnancy loss is delayed or incomplete, maternal hemodynamic stability, the presence of infection, and, most importantly, patient preference.
Expectant Management
Expectant management is often the initial treatment choice for patients experiencing a spontaneous pregnancy loss. However, women who choose this option should be counseled that complete expulsion may take up to 1 month. By day 7 postdiagnosis, approximately 50% of women request surgical management; 70% do so by day 14.6 The emotional toll of prolonging completion of the pregnancy loss process can be significant. Often, making expedient intervention is a more appealing alternative. The likelihood of spontaneous expulsion declines rapidly after 1 week of expectant management. Therefore, it may be reasonable to offer 1 week without intervention to a patient with an early spontaneous loss prior to exploring alternative management options. Stage of pregnancy loss must also be considered when offering expectant management. Women with an incomplete pregnancy loss respond better to expectant management than those with a delayed pregnancy loss (85% vs 33% completion).6
Medical Management
Medical management may be an excellent alternative for women with delayed pregnancy loss and those desiring minimal intervention. Medical treatment typically begins with misoprostol, a prostaglandin E1 analog, although the standard dose and route of administration of this medication has not been definitively established. Misoprostol successfully completes pregnancy expulsion in approximately 66% to 99% of women with incomplete or delayed pregnancy loss in the first trimester. Some regimens for medical management of early pregnancy loss include mifepristone (a progesterone receptor antagonist) in combination with misoprostol. Winikoff and colleagues7 found that mifepristone, 200 mg, given 24 to 36 hours before one dose of misoprostol, 800 µg, resulted in an overall expulsion success rate of 91% to 96% when given up to 9 weeks of gestation.7 There is some debate on the utility of progesterone inhibition in a failing pregnancy. Insufficient progesterone has been postulated as a possible contributor to first trimester loss; therefore, the use of further progesterone suppression with mifepristone is of questionable utility.8,9 However, when used for elective termination of pregnancy, mifepristone does appear to increase expulsion rates.7
The American College of Obstetrics and Gynecology (ACOG) endorses a protocol for medical management of women with an incomplete pregnancy loss and a uterus less than 12 weeks in size that utilizes misoprostol, 600 µg orally or 400 µg sublingually.10 For delayed pregnancy losses, misoprostol can be increased to 800 µg vaginally or 600 µg sublingually. Doses can be repeated every 3 hours for up to three total doses.10 Alternative regimens have also been studied. Overall, misoprostol, 800 µg, produces the highest expulsion rate, with little additional benefit noted after the third dose.11 In women with gestations at 7 to 17 weeks, the 800-µg vaginal misoprostol regimen resulted in an 80% success rate when measured by complete expulsion within 3 days of treatment.12 The efficacy is similar among all modes of administration, although gastrointestinal (GI) side effects (nausea, diarrhea) are more common when misoprostol is administered orally or sublingually.13 In multiple-dose regimens, administration every 3 to 4 hours results in more pronounced GI side effects and 6- to 12-hour spacing is often more tolerable to most patients. The optimal dosing schedule should be determined by the route of administration. Vaginally administered misoprostol has the longest half-life, with serum metabolites persisting longer than 6 hours as compared with approximately 2 hours after oral or sublingual delivery.14 Peak serum absorption is optimized via sublingual administration, which avoids the first-pass metabolism by the liver that occurs after oral administration and the variable absorption across the vaginal epithelium due to the presence of cervical mucus or blood in the genital tract.14 Ultimately, the optimal route of administration should be guided by patient preference because the efficacy of each approach is similar.15
When counseling women about expectations with misoprostol use, it is important to discuss common side effects. GI distress is the most frequently reported side effect, but patients also commonly note fever or chills. The bleeding pattern experienced with tissue passage is typically described as heavier than the patient’s usual menstrual flow and lasts approximately 3 or 4 days. This is followed by a transition to vaginal spotting that may last for a week or longer. One common concern among providers who are considering treating outpatients with misoprostol is the possibility of acute blood loss resulting in hemodynamic instability and return to the emergency room. Although that is a legitimate concern that increases with gestational age, investigators have reported a greater decrease in hemoglobin among those treated by curettage when compared with those treated with misoprostol.16
Surgical Management
Surgical treatment of spontaneous first trimester pregnancy loss can involve sharp curettage, electric vacuum aspiration (EVA), manual vacuum aspiration (MVA), or a combination of vacuum aspiration and sharp curettage. EVA is the conventional procedure. It is performed in the operating room with an electric suction device and a rigid curette and typically involves general, intravenous, or spinal anesthesia. Its efficacy and complications have been extensively studied. MVA is performed with a flexible curette attached to a 60-mL syringe that can apply negative pressure equal to that of EVA.17 MVA devices are recommended for use in pregnancies at less than 12 weeks of gestation.
Cervical ripening agents aid in patient comfort and reduce the difficulty of uterine evacuation procedures. Available cervical ripening agents include misoprostol, mifepristone, and osmotic dilators. A recent meta-analysis compared the efficacy of each approach and concluded that laminaria, vaginal, or sublingual mifepristone (200 mg) and misoprostol (400 µg) were equally effective in reducing the difficulty of manual dilation.18 When given at least 2 to 3 hours prior to procedure, misoprostol produced better results with fewer side effects when administered vaginally or sublingually rather than orally. However, when compared with mifepristone, 200 mg, given 24 hours prior to the evacuation procedure, the use of misoprostol by any route was less effective.18
Anesthesia for MVA typically includes use of a preprocedural NSAID and a paracervical block containing 10 to 20 cc of 1% lidocaine.17 Addition of agents such as ropivacaine and fentanyl to the lidocaine has been used to expand and prolong anesthetic benefit. The use of minimal and moderate oral and intravenous (IV) sedation protocols for additional pain control has also been promoted. In a study comparing the use of oral oxycodone, 10 mg, and sublingual lorazepam, 1 mg, to IV fentanyl, 100 µg, and IV midazolam, 2 mg, patients reported significantly better pain control with IV sedation.19 Anxiolytics such as lorazepam are often used in sedation protocols for uterine evacuation in the office setting, but they do not appear to significantly reduce patient-reported pain. In fact, Allen and colleagues reported a decrease in patient satisfaction when lorazepam was added to sedation protocols.20
A uterotonic agent can be given postoperatively to reduce vaginal bleeding with both forms of surgical treatment. Typically methylergonovine is used, but regimens vary among providers. Rh-immune globulin should be administered if patient is Rh negative. A dose of 50 µg is effective through the 12th week of gestation. Table 2 outlines the protocol most commonly used for office-based MVA in our clinic at the University of Missouri.
Table 2.
University of Missouri Protocol for Manual Vacuum Aspiration
| Pregnancy Loss Diagnosed by Ultrasound Evaluation |
| Patient counseling |
| Consents signed, procedure education, prescriptions provided for preprocedure preparation and postoperative care |
| Patient preparation 1 day prior to procedure |
| 800 mg ibuprofen PO q 8 h scheduled |
| 100 mg doxycycline PO bid (continue for 7-day course) |
| 400 mcg misoprostol PO × 1 in the evening |
| Patient preparation day of procedure |
| Presedation medications 2 h before scheduled start time: |
| Diazepam 10 mg PO |
| Meperidine 100 mg PO |
| Promethazine 50 mg PO |
| (ibuprofen and doxycycline continued as previously noted) |
| Patient to surgical suite |
| Patient positioned in dorsolithotomy for administration of paracervical block containing 22 cc total volume of the following components: |
| 10 cc ropivacaine 0.5% |
| 10 cc lidocaine 1% (without epinephrine) |
| 2 cc fentanyl 50 mcg/ml |
| *allow 15 min prior to proceeding for optimal analgesia* |
| Cervical dilation followed by uterine evacuation using manual vacuum aspiration |
| If patient’s pain is not well controlled by oral sedation agents, consider adjunct use of IV midazolam and/or meperidine |
| Intramuscular injection of methylergonovine maleate, 0.2 mg, at the end of procedure and Rh-immune globulin if indicated |
| Patient transferred to recovery; monitored according to conscious sedation protocol |
| Postoperative care (begin 4 h after procedure) |
| 50 mg meperidine and 25 mg promethazine PO q 4–6 h (continue for the next 12–18 h) |
| 0.2 mg methylergonovine maleate PO q 8 h × 3 doses |
| 800 mg ibuprofen PO q 8 h (convert to prn day after procedure) |
| Bleeding and infection precautions reviewed 2-week postoperative visit scheduled, nothing per vagina (intercourse, douches, tampons, etc), until seen for follow-up Patient to avoid pregnancy × 2 cycles |
Comparison of Surgical Approaches
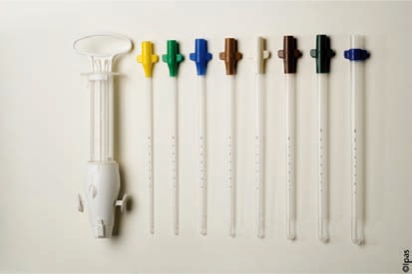
Several investigators have compared MVA to EVA for the treatment of early pregnancy loss and there is now a growing consensus that use of the former has multiple benefits. These include decreases in expense, time of procedure, procedural complications, and blood loss in the absence of a detriment in procedural completion rates. Reported rates for complete pregnancy loss with use of these techniques range from 95% to 98% for MVA and 97% to 98% for EVA.17,21,22 The use of MVA in the clinical setting was predicted to decrease procedural costs because it can be performed without the use of general or spinal anesthesia and uses inexpensive, reusable instrumentation that can be autoclaved (Figure 2). The time involved in completing the procedure itself is also less with MVA than with EVA. Dalton and colleagues23 found that the average total patient time for MVA was 97 minutes and the specific procedural time was 10 minutes. This compared with 290 minutes total time and 19 minutes of specific procedural time with the use of EVA.23 The increased cost of EVA is largely due to the use of an operating room and more invasive anesthesia and analgesia protocols. In 2006, it was estimated that nearly $1000 per procedure savings could be obtained by performing evacuations in clinic.23
Figure 2.
Ipas manual vacuum aspirator with cannulae. Product may be autoclaved and has been approved for use in up to 12 weeks of gestation. Image courtesy of WomanCare Global, Inc. http://www.womancareglobal.com.
For many patients, MVA results in less postoperative pain, particularly those with losses at early gestational ages. In a meta-analysis by Wen and colleagues21 combining over 800 participants, perioperative pain was lower during MVA compared with EVA in the subgroup of women at 7 weeks of gestation or less (relative risk [RR], 0.04; 95% confidence interval [CI], 0.01–0.12; P < .0001).21 Women at 7 to 11 weeks of gestation reported no significant differences in perioperative pain.21
Surgical complications, including uterine perforation, appear to be equally common with the use of EVA when compared with MVA.24 In the largest study to date, Goldberg and colleagues reported no significant difference in complication rates between the two approaches when performed at 10 weeks of gestation or earlier (Table 3).24 Although a study of 165 patients in 2006 found a significant decrease in blood loss (70 cc vs 311 cc; P < .001) during MVA compared with EVA,23 subsequent studies have not consistently supported a significant reduction in blood loss with MVA.21
Table 3.
Immediate Operative Complications of MVA Versus EVA
| Complication | MVA (%) (n = 1002) | EVA (%) (n = 724) | Significance |
| Repeat uterine aspiration | 2.2 | 1.7 | NS |
| Need for transfusion | 0 | 0 | NS |
| Uterine perforation | 0.3 | 0.3 | NS |
| Cervical laceration | 0 | 0 | NS |
| Hospital admission | 0 | 0.14 | NS |
| All complications | 2.5 | 2.1 | NS |
EVA, electric vacuum aspiration; MVA, manual vacuum aspiration; NS, not significant.
Adapted from Goldberg AB et al.24
The use of intraoperative ultrasound during evacuation procedures has been shown to decrease the creation of false passages within the endocervix during cervical dilation, perforation of the uterus during sounding or dilation, and retained products of conception postprocedure.25 Sharp curettage, often performed after vacuum aspiration to ensure complete evacuation, is associated with an increased risk for uterine perforation and Asherman syndrome. In a large Swedish observational study (n = 84,850) involving 145 recognized perforations during uterine evacuation in the first trimester, 31% occurred during the sharp curettage.26 There are no data that have shown a decrease in retained products with use of sharp curettage. Ultrasonographic guidance during uterine evacuation in the first trimester of pregnancy is a safe and effective alternative to sharp curettage in ensuring complete uterine evacuation. It is also associated with less intraoperative and postoperative blood loss, a shorter operative time, and less postoperative NSAID use.25
In the United States, pelvic infections occur in 0.5% to 5% of patients following suction curettage and infection rates are not associated with the evacuation approach (MVA or EVA).27 A meta-analysis of 12 studies involving women with surgical evacuation prior to 16 weeks of gestation reported the overall RR for postprocedure infections in women receiving antibiotics was 0.58 (95% CI, 0.47–0.71) when compared with those receiving placebo.27 In the ACOG practice bulletin on antibiotic prophylaxis prior to gynecologic procedures, the authors recommend the administration of doxycycline, 100 mg, orally 1 hour prior to dilation and evacuation followed by 200 mg after the procedure.28
Although several studies have found no significant reduction in postprocedure pelvic inflammatory disease (PID) with the use of antibiotic prophylaxis, the cost of treating a single case of postabortal PID as an outpatient far exceeds the cost of doxycycline prophylaxis. 29 Some advocate risk stratification to reduce overuse of antibiotic prophylaxis due to the low prevalence of postabortal infection. However, even in low-risk groups (no history of PID, negative chlamydial cultures), only 35 women need to be treated to prevent one case of postabortal PID, the ramifications of which most clinicians would consider significant enough to implement uniform prophylaxis. 27 ACOG recommends that uniform screening for Chlamydia trachomatis be implemented at the time of uterine evacuation.28
Overall Comparison of Expectant, Medical, and Surgical Management
Few investigators have compared expectant, medical, and surgical management of first trimester pregnancy loss in a single study. In a meta-analysis performed in 2005 by Sotiriadis and colleagues,30 medical management with misoprostol resulted in a 2.77-fold greater success than expectant management (Table 4). When medical management was compared with surgical management using EVA in the operating room, surgical treatment resulted in a 1.5-fold greater rate of completion.30 A 2001 cost comparison of expectant, medical, and surgical management showed expenses of approximately $1172, $1000, and $2007, respectively.9 It should be noted that these figures were reported at a time when nearly all dilation and evacuation procedures performed in the United States for first trimester pregnancy loss involved EVA in an operating room. The cost of using MVA in an office setting was not represented in this study.
Table 4.
Comparison of Expectant, Medical, and Surgical Management
| Parameter | Medical vs Expectant | Surgical vs Expectant | Surgical vs Medical |
| Success | 2.77 | 1.21 | 1.44 |
| Satisfaction | NA | 1.05 | 0.87 |
| PID | 1.0 | 1.33 | 1.23 |
| Blood transfusion | 0.62 | 0.59 | 1.0 |
| Emergency curettage | 0.94 | 0.86 | 0.4 |
| Bleeding | 0.31 | 0.95 | 0.77 |
| Nausea | 1.38 | NA | 0.63 |
| Vomiting | 1.4 | NA | 0.52 |
| Cost | 1.54 | NA | NA |
Values are expressed as odds ratios.
NA, no data available; PID, pelvic inflammatory disease.
Adapted from Sotiriadis A et al.30
Although it is the most rapid mode of treatment, surgical treatment may rarely result in uterine perforation requiring additional surgery, intrauterine adhesions and scarring, or cervical trauma with subsequent cervical incompetence. However, women who have undergone dilation and curettage can be reassured by studies showing that endometrial thickness at 6 months postprocedure does not differ when women who chose expectant management are compared with those who chose surgical evacuations.31
Postpregnancy Loss Care
At least 2 weeks of pelvic rest should be recommended for women who have experienced a pregnancy loss of any type. Because ovulation may resume as early as day 21 after a pregnancy loss, a visit with a practitioner should be scheduled within this time frame to allow for discussion and initiation of contraception. Menses typically returns by approximately 6 weeks after evacuation of a spontaneous loss. If not, the patient should be evaluated for a new pregnancy, gestational trophoblastic disease, or Asherman syndrome.32
When, if, and how often to follow serum β-human chorionic gonadotropin hormone (β-hCG) levels after a spontaneous pregnancy loss is not clear. Some practitioners routinely follow the β-hCG value until it has dropped to below the detectable level (usually < 5 mIU/mL) to ensure complete expulsion of the pregnancy and absence of gestational trophoblastic disease. The average amount of time necessary for serum β-hCG levels to become undetectable depends on the initial level at the time of pregnancy loss and the mode of management. The mean time for serum β-hCG levels to drop to < 2 mIU/mL has been reported as 37.5 days after suction curettage and 27.4 days after misoprostol administration.33 In a study by Perriera and colleagues,34 27 out 116 women opting for medical management had a positive urine pregnancy test (detection level of 25 mIU/mL) 1 month after treatment. None of these women had a continuing pregnancy or gestational trophoblastic disease with further follow-up.34
When minimal tissue is obtained during surgical evacuation of a pregnancy loss or after a trial of medical management, a repeat serum β-hCG level test 24 hours after treatment may predict an ongoing pregnancy, either intrauterine or ectopic. For all others, it seems reasonable to check a urine pregnancy test 4 weeks following pregnancy extraction or expulsion. If positive, a transvaginal ultrasound should be considered to determine the presence of an ongoing intrauterine pregnancy, retained products of conception, or ectopic pregnancy. Once the urine pregnancy test is negative and products of conception have been obtained, following serum β-hCG levels to < 5 mIU/mL does not appear to have clinical benefit.
Contraception and Preconceptional Considerations
Patients who have experienced an early spontaneous pregnancy loss frequently want to know the optimal timing for attempts at the next pregnancy, a question that is difficult to definitively answer. All conceptions following a pregnancy loss are more likely to result in a subsequent pregnancy loss irrespective of the interval between the pregnancies: 20% after one loss, 28% after two losses, and 43% after more than two spontaneous losses.35 However, when stratified for inter-pregnancy interval, DaVanzo and colleagues35 reported that subsequent pregnancies at intervals of < 6 months were 31 times more likely to begin with a repeat spontaneous loss when compared with intervals of 27 to 50 months. An informed balance among increased risk, advancing maternal age, and patient preference must be discussed in detail when the couple experiencing a recent early pregnancy loss is considering future pregnancies.
Practitioners should discuss and implement reliable contraception for those couples wishing to defer subsequent pregnancy. Two of the most reliable methods can be placed at the time of treatment: medroxyprogesterone acetate injection and a progestin-containing intrauterine device (IUD). Medroxyprogesterone acetate injection can be administered at the time of surgical evacuation or as early as when the pregnancy loss is recognized. Immediate medroxyprogesterone acetate administration reduced subsequent pregnancy by 10% compared with injection at follow-up visit.36 IUDs can be safely inserted immediately following a uterine evacuation procedure. Reeves and colleagues reported that 28 pregnancies per 1000 women can be prevented with immediate placement as opposed to the follow- up visit. Risk of expulsion with immediate placement was 5% in the Reeves study, compared with 4% reported by the manufacturer under guidelines that recommend placement later than 6 weeks postpartum.37
Summary
Management of first trimester pregnancy loss should be primarily driven by the wishes of the well-informed patient. Expectant or medical management may be ideal for hemodynamically stable women with incomplete pregnancy loss. Among several effective regimens for medical management of delayed loss, the use of vaginal misoprostol, 800 µg, is one of the most successful, showing 80% to 90% completion rates. Surgical management should be considered in patients who choose it primarily and in those with failed expectant and/or medical management. MVA, particularly when performed in the office setting, is less expensive and more efficient than EVA in the operating room, but maintains equal or improved safety and efficacy. The addition of ultrasound guidance and antibiotic prophylaxis has been shown to decrease operative complications for either operative approach.
Main Points.
Terminology for early pregnancy loss can be simplified to the terms complete pregnancy loss, incomplete pregnancy loss, and delayed pregnancy loss.
The presence of fetal cardiac activity after 5 to 6 weeks of gestational age, or once the embryo reaches 2 mm in length, is associated with a less than 3% to 6% risk of spontaneous pregnancy loss.
Vaginal bleeding after 7 weeks of gestation indicates an approximate 10% risk for pregnancy loss or later pregnancy complications, even in the presence of fetal cardiac motion.
The optimal mode for management of spontaneous first trimester pregnancy loss is usually determined by patient preference.
With expectant management, expulsion of a delayed pregnancy loss may take up to 1 month from the time of diagnosis.
Women with incomplete first trimester pregnancy loss respond better to expectant management than those with delayed pregnancy loss.
Misoprostol is very successful in completing expulsion in women with incomplete or delayed first trimester pregnancy loss.
The efficacy of misoprostol is similar when used orally, sublingually, or vaginally. Vaginal administration is associated with fewer gastrointestinal side effects.
Office-based manual vacuum aspiration (MVA) under conscious sedation decreases the expense, time of procedure, procedural complications, and procedural blood loss when compared with use of hospital-based electric vacuum aspiration (EVA) for the treatment of early pregnancy loss.
MVA and EVA have similar completion success rates when used for the treatment of incomplete or delayed early pregnancy loss.
Use of intraoperative ultrasound guidance during uterine evacuation procedures decreases complication rates and rates of retained products of conception postprocedure.
Medroxyprogesterone acetate injection and progestin-containing intrauterine devices can be safely and effectively initiated at the time of treatment of first trimester pregnancy loss.
References
- 1.Jauniaux E, Johns J, Burton GJ. The role of ultrasound imaging in diagnosing and investigating early pregnancy failure. Ultrasound Obstet Gynecol. 2005;25:613–624. doi: 10.1002/uog.1892. [DOI] [PubMed] [Google Scholar]
- 2.Arck PC, Rücke M, Rose M, et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed Online. 2008;17:101–113. doi: 10.1016/s1472-6483(10)60300-8. [DOI] [PubMed] [Google Scholar]
- 3.Juliano M, Dabulis S, Heffner A. Characteristics of women with fetal loss in symptomatic first trimester pregnancies with documented fetal cardiac activity. Ann Emerg Med. 2008;52:143–147. doi: 10.1016/j.annemergmed.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Rowling SE, Coleman BG, Langer JE, et al. Firsttrimester US parameters of failed pregnancy. Radiology. 1997;203:211–217. doi: 10.1148/radiology.203.1.9122395. [DOI] [PubMed] [Google Scholar]
- 5.Mercé LT, Barco MJ, Alcázar JL, et al. Intervillous and uteroplacental circulation in normal early pregnancy and early pregnancy loss assessed by 3-dimensional power Doppler angiography. Am J Obstet Gynecol. 2009;200:315. doi: 10.1016/j.ajog.2008.10.020. e1-e8. [DOI] [PubMed] [Google Scholar]
- 6.Bagratee JS, Khullar V, Regan L, et al. A randomized controlled trial comparing medical and expectant management of first trimester miscarriage. Hum Reprod. 2004;19:266–271. doi: 10.1093/humrep/deh049. [DOI] [PubMed] [Google Scholar]
- 7.Winikoff B, Dzuba IG, Creinin MD, et al. Two distinct oral routes of misoprostol in mifepristone medical abortion: a randomized controlled trial. Obstet Gynecol. 2008;112:1303–1310. doi: 10.1097/AOG.0b013e31818d8eb4. [DOI] [PubMed] [Google Scholar]
- 8.Ledger WL, Sweeting VM, Chatterjee S. Rapid diagnosis of early ectopic pregnancy in an emergency gynaecology service-are measurements of progesterone, intact and free beta human chorionic gonadotropin helpful? Hum Reprod. 1994;9:157–160. doi: 10.1093/oxfordjournals.humrep.a138307. [DOI] [PubMed] [Google Scholar]
- 9.Creinin MD, Schwartz JL, Guido RS, Pymar HC. Early pregnancy failure-current management concepts. Obstet Gynecol Surv. 2001;56:105–113. doi: 10.1097/00006254-200102000-00024. [DOI] [PubMed] [Google Scholar]
- 10.American College of Obstetricians and Gynecologists, authors. ACOG committee opinion no. 427. Misoprostol for postabortion care. Obstet Gynecol. 2009;113:465–468. doi: 10.1097/AOG.0b013e31819930f9. [DOI] [PubMed] [Google Scholar]
- 11.Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks) Cochrane Database Syst Rev. 2006;3:CD002253. doi: 10.1002/14651858.CD002253.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstet Gynecol. 2002;99:563–566. doi: 10.1016/s0029-7844(01)01765-3. [DOI] [PubMed] [Google Scholar]
- 13.Tang OS, Lau WN, Ng EH, et al. A prospective randomized study to compare the use of repeated doses of vaginal with sublingual misoprostol in the management of first trimester silent miscarriages. Hum Reprod. 2003;18:176–181. doi: 10.1093/humrep/deg013. [DOI] [PubMed] [Google Scholar]
- 14.Tang OS, Ho PC. The pharmacokinetics and different regimens of misoprostol in early first-trimester medical abortion. Contraception. 2006;74:26–30. doi: 10.1016/j.contraception.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108:582–590. doi: 10.1097/01.AOG.0000230398.32794.9d. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Gilles JM, Barnhart K, et al. National Institute of Child Health Human Development (NICHD) Management of Early Pregnancy Failure Trial. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761–769. doi: 10.1056/NEJMoa044064. [DOI] [PubMed] [Google Scholar]
- 17.Edwards S, Tureck R, Fredrick M, et al. Patient acceptability of manual versus electric vacuum aspiration for early pregnancy loss. J Womens Health (Larchmt) 2007;16:1429–1436. doi: 10.1089/jwh.2007.0362. [DOI] [PubMed] [Google Scholar]
- 18.Kapp N, Lohr PA, Ngo TD, Hayes JL. Cervical preparation for first trimester surgical abortion. Cochrane Database Syst Rev. 2010;2:CD007207. doi: 10.1002/14651858.CD007207.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen RH, Fitzmaurice G, Lifford KL, et al. Oral compared with intravenous sedation for first-trimester surgical abortion: a randomized controlled trial. Obstet Gynecol. 2009;113:276–283. doi: 10.1097/AOG.0b013e3181938758. [DOI] [PubMed] [Google Scholar]
- 20.Allen RH, Kumar D, Fitzmaurice G, et al. Pain management of first-trimester surgical abortion: effects of selection of local anesthesia with and without lorazepam or intravenous sedation. Contraception. 2006;74:407–413. doi: 10.1016/j.contraception.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Wen J, Cai QY, Deng F, Li YP. Manual versus electric vacuum aspiration for first-trimester abortion: a systematic review. BJOG. 2008;115:5–13. doi: 10.1111/j.1471-0528.2007.01572.x. [DOI] [PubMed] [Google Scholar]
- 22.Greenslade FC, McKay H, Wolf M, McLaurin K. Post-abortion care: a women’s health initiative to combat unsafe abortion. Adv Abort Care. 1994;4:1–4. [PubMed] [Google Scholar]
- 23.Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103–110. doi: 10.1097/01.AOG.0000223206.64144.68. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg AB, Dean G, Kang MS, et al. Manual versus electric vacuum aspiration for early first-trimester abortion: a controlled study of complication rates. Obstet Gynecol. 2004;103:101–107. doi: 10.1097/01.AOG.0000109147.23082.25. [DOI] [PubMed] [Google Scholar]
- 25.Acharya G, Morgan H, Paramanantham L, Fernando R. A randomized controlled trial comparing surgical termination of pregnancy with and without continuous ultrasound guidance. Eur J Obstet Gynecol Reprod Biol. 2004;114:69–74. doi: 10.1016/j.ejogrb.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Lindell G, Flam F. Management of uterine perforations in connection with legal abortions. Acta Obstet Gynecol Scand. 1995;74:373–375. doi: 10.3109/00016349509024431. [DOI] [PubMed] [Google Scholar]
- 27.Sawaya GF, Grady D, Kerlikowske K, Grimes DA. Antibiotics at the time of induced abortion: the case for universal prophylaxis based on a meta-analysis. Obstet Gynecol. 1996;87:884–890. [PubMed] [Google Scholar]
- 28.ACOG Committee on Practice Bulletins-Gynecology ACOG practice bulletin no. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180–1189. doi: 10.1097/AOG.0b013e3181a6d011. [DOI] [PubMed] [Google Scholar]
- 29.Prieto JA, Eriksen NL, Blanco JD. A randomized trial of prophylactic doxycycline for curettage in incomplete abortion. Obstet Gynecol. 1995;85:692–696. doi: 10.1016/0029-7844(95)00035-p. [DOI] [PubMed] [Google Scholar]
- 30.Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JP. Expectant, medical, or surgical management of first-trimester miscarriage: a metaanalysis. Obstet Gynecol. 2005;105:1104–1113. doi: 10.1097/01.AOG.0000158857.44046.a4. [DOI] [PubMed] [Google Scholar]
- 31.Moon KS, Richter KS, Levy MJ, Widra EA. Does dilation and curettage versus expectant management for spontaneous abortion in patients undergoing in vitro fertilization affect subsequent endometrial development? Fertil Steril. 2009;92:1776–1779. doi: 10.1016/j.fertnstert.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 32.Creinin MD. Change in serum beta-human chorionic gonadotropin after abortion with methotrexate and misoprostol. Am J Obstet Gynecol. 1996;174:776–778. doi: 10.1016/s0002-9378(96)70463-5. [DOI] [PubMed] [Google Scholar]
- 33.Marrs RP, Kletzky OA, Howard WF, Mishell DR., Jr Disappearance of human chorionic gonadotropin and resumption of ovulation following abortion. Am J Obstet Gynecol. 1979;135:731–736. doi: 10.1016/0002-9378(79)90383-1. [DOI] [PubMed] [Google Scholar]
- 34.Perriera LK, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143–149. doi: 10.1016/j.contraception.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 35.DaVanzo J, Hale L, Razzaque A, Rahman M. Effects of interpregnancy interval and outcome of the preceding pregnancy on pregnancy outcomes in Matlab, Bangladesh. BJOG. 2007;114:1079–1087. doi: 10.1111/j.1471-0528.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madden T, Westhoff C. Rates of follow-up and repeat pregnancy in the 12 months after first-trimester induced abortion. Obstet Gynecol. 2009;113:663–668. doi: 10.1097/AOG.0b013e318195dd1e. [DOI] [PubMed] [Google Scholar]
- 37.Reeves MF, Smith KJ, Creinin MD. Contraceptive effectiveness of immediate compared with delayed insertion of intrauterine devices after abortion: a decision analysis. Obstet Gynecol. 2007;109:1286–1294. doi: 10.1097/01.AOG.0000265336.14160.cc. [DOI] [PubMed] [Google Scholar]