Abstract
Protozoan pathogens are a highly diverse group of unicellular organisms, several of which are significant human pathogens. One group of protozoan pathogens includes obligate intracellular parasites such as agents of malaria, leishmaniasis, babesiosis, and toxoplasmosis. The other group includes extracellular pathogens such as agents of giardiasis and amebiasis. An unfortunate unifying theme for most human protozoan pathogens is that highly effective treatments for them are generally lacking. We will review targeting protozoan mitogen-activated protein kinases (MAPKs) as a novel drug discovery approach towards developing better therapies, focusing on Plasmodia, Leishmania, and Toxoplasma, about which the most is known.
1. General Properties of MAPKs
Virtually all eukaryotic organisms possess MAPKs, signal transduction molecules that regulate cell functions such as tissue morphogenesis, cytoskeletal rearrangements, proliferation, differentiation, survival, immune responses, and adaptation/stress-response [1–3]. Encephalitozoon cuniculi is the only example to date of a eukaryote apparently lacking any MAPKs [4]. The MAPK superfamily, which evolved 1.0 to 1.5 billion years ago [5], comprises proline-directed serine/threonine kinases that are classified based on the primary amino acid sequence within the catalytic domains and must possess a [TS]XX[LIVM]XT[RK] [WY]YRXPEX[LIVM] signature sequence at its core [6–8]. The phosphorylation lip (solid underline beneath the sequence above) is required for MAPK activation by upstream regulators and is contiguous with the proline-directed (P+1) peptide binding pocket (double underline beneath the sequence above), conferring substrate specificity, and is capable of being singly [(pT)XX)] or dually [(pT)X(pY)] phosphorylated in response to particular extracellular stimuli [9]. In addition, MAPKs possess 11 subdomains [5, 10] with numerous highly conserved residues required for ATP binding, phosphotransferase activity, and substrate specificity [7].
MAPKs are often controlled by highly evolutionarily conserved regulatory cascades involving sequential phosphorylation by three component modules consisting of MAPK kinase kinases (MKKKs, Ste11-like kinases) and MAPK kinases (MKKs, Ste7-like kinases), terminating in the phosphorylation of specific MAPKs [11]. Many MAPK cascades have recently been expanded to include a fourth tier involving proteins aptly termed MKKKKs (Ste20-like kinases) [12] that can either serve in a noncatalytic capacity as a scaffold to promote pathway assembly (and MKKK auto-activation) or can phosphorylate specific MKKKs [13]. Once activated, MAPKs phosphorylate a wide variety of proteins including MAPK-activated protein kinases and transcription factors, ultimately resulting in changes in gene expression [14, 15]. MAPK signaling can also have additional epigenetic effects by affecting histone modification [16].
MAPKs are grouped into subfamilies on the basis of amino acid sequence similarity, mechanism of activation, and the type of MAPK cascade to which they belong. Cyclin-dependent kinases share very high amino acid sequence identity with MAPKs [17] but generally lack a phosphorylation lip. Differences in the precise amino acid composition of the phosphorylation lip have historically been used to classify MAPKs, as outlined below. Our phylogenetic studies [18] have established, however, that homology between many short strings of amino acids found in MAPKs is of equal or greater importance when classifying MAPKs within different subfamilies.
Four conventional MAPK subfamilies exist, which are also described as “typical”, that is, capable of dual phosphorylation [8]. These conventional MAPK groups include the extracellular signal-regulated kinases (e.g., mammalian ERK1 and ERK2, possessing a TEY motif at the phosphorylation lip, [19]), c-Jun-activated kinases (e.g., mammalian JNK1, JNK2, and JNK3 (TPY motif) [20]), p38 stress-response MAPKs (e.g., mammalian p38α, p38β, p38γ, and p38δ (TGY motif), [21]), and mammalian ERK5 (big MAPK-1, BMK-1 (TEY motif) [22, 23]). ERK5 is unusual because it possesses a long carboxy-terminal extension consisting of a transactivation domain and a nuclear localization signal facilitating translocation into the nucleus upon MAPK activation [8]. Multiple isoforms of MAPKs often exist within individual cells, which can either be activated by different MKKs or can themselves phosphorylate alternate downstream substrates [24]. Additional phylogenetically distinct MAPK subfamilies are defined by categorizing distantly related MAPKs including those from plants (TEY motif), yeasts (T[EN]Y motif), and protozoans (TXY motif, where X is often D or E, but many exceptions exist) [5, 18].
Several atypical MAPK subfamilies also exist, largely representing MAPKs that can only be monophosphorylated within their activation loops. Mammalian ERK3 [25] and ERK4 [8], possessing an SEG motif in the phosphorylation lip and an RXPR motif in the substrate binding pocket, are representative members of one major subfamily of atypical MAPKs, while Nemo-like kinases (NLKs, with a T[HQ]E motif) comprise a second major atypical MAPK subfamily [26]. Greater sequence diversity exists in the phosphorylation lip of atypical protozoan MAPKs (most commonly TGH or TSH motifs) compared to metazoan MAPKs, but members of this subfamily otherwise closely resemble typical MAPKs.
Monophosphorylated human ERK2 has 10- to 100-fold less kinase activity than dually phosphorylated ERK2 [27], illustrating that dual phosphorylation (as is the case for typical MAPKs) achieves greater signal amplification and range of responses than can be achieved by monophosphorylation (as is the case for atypical MAPKs). In addition, different upstream activators can preferentially phosphorylate the threonine or tyrosine within the activation loop of typical MAPKs, allowing signals from two different origins to elicit a response [28]. Typical MAPKs are also subject to a tertiary level of control through the expression of phosphatases specific for either phosphothreonine or phosphotyrosine in the activation loop [29].
Human ERK8 (homologous to rat ERK7) represents a prototypical member of a large atypical MAPK subfamily [30]. Although these large atypical MAPKs contain a TEY motif capable of dual phosphorylation, activation of mammalian ERK8 (or ERK7) is not under the control of any known MKK family member. Instead, they are activated by autophosphorylation of their activation loops in response to conformational changes in their carboxy-terminal extensions [31]—a highly unusual feature for mammalian MAPKs. Their carboxy-terminal extensions possess a nuclear localization signal that is only exposed in the activated state, thereby facilitating MAPK translocation to the nucleus, which in turn regulates cell proliferation [32].
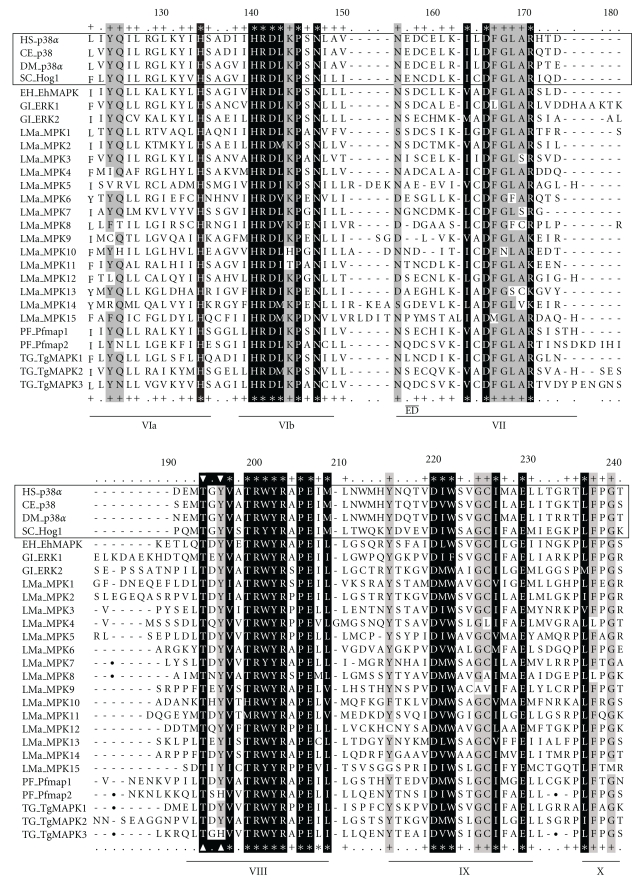
We performed ClustalW alignment [33] comparing the amino acid sequences of representative metazoan (Homo sapiens [21, 34], Drosophila melanogaster [35], Caenorhabditis elegans [36]) and yeast (Saccharomyces cerevisiae [6]) p38 MAPKs to unique protozoan MAPKs described in this review (Figure 1). Human p38α was selected as a prototypical MAPK for comparison for three principal reasons. First, a plethora of p38 MAPK inhibitor drugs currently exists [37, 38]. Second, the binding specificity of the pyridinylimidazole p38 MAPK inhibitor SB203580 to the ATP binding pocket of human p38α is well understood [39, 40]. Third, we have shown that p38 MAPK inhibitors effectively inhibit the in vitro replication of protozoan parasites such as Plasmodium falciparum (Brumlik et al., submitted), L. donovani (Brumlik et al., unpublished observations), and T. gondii [41]. We have further demonstrated that the pyridinylimidazole p38 MAPK inhibitor RWJ67657 protects mice from lethal challenge with T. gondii [42]. Figure 1 demonstrates that while the overall structure of MAPKs is highly conserved even between distantly related eukaryotes, unique features exist that could lead to the design of MAPK inhibitors specific for protozoan parasites.
Figure 1.
ClustalW alignment of representative MAPKs of diverse evolutionary origin, with each of the 11 subdomains indicated (Roman numerals). Conserved acidic residues within the ED site (subdomain VII) and common docking (CD) domain, which immediately follows subdomain XI, have been underlined. The first four sequences represent p38 MAPKs of metazoan and yeast origin and are boxed as reference sequences to which other protozoan MAPKs can be compared. Invariant MAPK residues (within allowed substitution groups) are highlighted in black and denoted by an asterisk. Highly conserved residues (>80% conservation) are highlighted in grey and denoted by a plus sign. In the absence of grey shading, plus signs indicate residues conserved in the majority of aligned sequences. Allowed substitution groups include acidic/amide (DE, DN, EQ), aliphatic (LIVM), aromatic (FYW), basic (KR), and hydroxyl/polar residues (STG). The positions of insertion sequences removed prior to ClustalW alignment are indicated by filled circles. White triangles denote the position of the TX[XY] phosphorylation lip. Two letter abbreviations precede the name of each MAPK sequence, indicating the genus and species of origin for each MAPK. CE: Caenorhabditis elegans; DM: Drosophila melanogaster; EH: Entamoeba histolytica; GI: Giardia intestinalis; HS: Homo sapiens; LMa: Leishmania major; PF: Plasmodium falciparum; SC: Saccharomyces cerevisiae; TG: Toxoplasma gondii. Accession numbers of all aligned sequences are listed in Tables 1 and 2.
2. Phylum Apicomplexa
Apicomplexa is a large, diverse phylum comprising over 5000 species, of which seven are known human pathogens (in the genera Babesia, Cryptosporidium, Cyclospora, Isospora, Plasmodium, Sarcocystis, and Toxoplasma). There are no reports of functional studies of MAPKs from Babesia, Cryptosporidium, Cyclospora, Isospora, or Sarcocystis to our knowledge. This section will thus focus on Plasmodium and Toxoplasma.
2.1. Genus Plasmodium
The genus Plasmodium contains four significant human pathogens, all agents of malaria: P. falciparum, P. vivax, P. ovale, and P. malariae. P. falciparum, which causes the most severe form of malaria, possesses only two MAPKs. Its Pfmap-1 represents a typical MAPK that is predominantly expressed in gametocytes [43] while Pfmap-2 represents an atypical MAPK (Table 1, Figure 1), which instead possesses a TSH phosphorylation lip [44]. PfPK7, which bears extremely limited homology to mammalian MKK3 and MKK6 that activate host p38 MAPK, does not appear to be a true MKK homologue. Furthermore, PfPK7 is unable to phosphorylate either recombinant Pfmap-1 or Pfmap-2 in vitro [45], suggesting that it does not represent a long-sought-after member of an MAPK cascade in Plasmodium. Moreover, no P. falciparum MKK genes have been identified, suggesting that P. falciparum MAPK signaling does not utilize typical MAPK cascades [46]. P. falciparum Pfmap-2 is instead activated by Pfnek-1, a never-in-mitosis/Aspergillus- (NIMA-) related kinase [47]. Since homology amongst MKKs and MKKKs is much lower than that for members of the MAPK superfamily, it is conceivable that genes encoding these proteins exist but have simply not been annotated as such in the P. falciparum genome.
Table 1.
Non-Trypanosomatid mitogen-activated protein kinases discussed in this review.
| Organism | MAPK | Accession no. | Phosphorylation lip | Classification | Function | References |
|---|---|---|---|---|---|---|
| Caenorhabditis elegans | p38 | AAB00664 | TGY | Typical | Stress-response | [36] |
| Drosophila melanogaster | p38α | AF035547 | TGY | Typical | Stress-response | [35] |
| Entamoeba histolytica | EhMAPK | AY460178 | TDY | Typical | ? | |
| Giardia intestinalis | ERK1 | AY149274 | TEY | Typical | Encystation | [49] |
| ERK2 | AY149275 | TDY | Typical | Encystation | [49] | |
| Homo sapiens | p38α | Q16539 | TGY | Typical | Stress-response | [21, 34] |
| Plasmodium falciparum | Pfmap-1 | Q94656 | TDY | Typical | ? | |
| Pfmap-2 | Q25917 | TSH | Atypical | Essential for differentiation | [48] | |
| Saccharomyces cerevisiae | Hog1 | AAA34680 | TGY | Typical | Stress-response | [6] |
| Toxoplasma gondii | TgMAPK1 (BARKY) |
AY684849 | TDY | Typical | Proliferation†, differentiation†, virulence† | |
| TgMAPK2 | DQ115400 | TDY | Typical | ? | ||
| TgMAPK3 | XP_0022369585 | TGH | Atypical | ? |
†Brumlik et al., submitted.
Pfmap-1 is neither required for schizogony nor gametocytogenesis in human erythrocytes cultured in vitro, nor for gametogenesis and/or sporogony in the mosquito vector [48]. However, Pfmap-2 protein levels are elevated in pfmap-1 knockout parasites, suggesting that Pfmap-1 fulfills an important function necessitating compensatory adaptation in parasites lacking this enzyme. Pfmap-2 is essential for the completion of the P. falciparum asexual cycle [48]. Functional characterizations of MAPKs from P. vivax, P. ovale, and P. malaria, the other Plasmodium species causing malaria, have yet to be reported to our knowledge.
2.2. Genus Toxoplasma
T. gondii, the sole member of the genus Toxoplasma, can cause significant morbidity or mortality in hosts with compromised cellular immunity. Like P. falciparum, T. gondii appears to be another protozoan parasite that lacks typical MAPK activation cascades. Preliminary examination of the T. gondii genome suggests that it encodes four MAPKs. However, the TGME49_021550 locus (situated on chromosome II) lacks coding sequences corresponding to several essential MAPK motifs (including an incomplete MAPK signature sequence), thereby disqualifying it as a functional MAPK gene. Of the remaining three MAPK genes (Table 1, Figure 1), we have cloned and sequenced both the genes encoding tgMAPK1, situated on chromosome XI [50], and tgMAPK2 (chromosome VIII; [18]). We have also sequenced the third MAPK gene, tgMAPK3 (chromosome Ib).
TgMAPK1 is a critical virulence determinant during acute T. gondii infection (Brumlik et al., submitted). By expressing it in Hog1-deficient yeast lacking its own stress-response MAPK, we restored yeast ability to grow under osmotic stress [50], providing evidence for this MAPK's role as a stress-response MAPK. Since TgMAPK1 expression affects tachyzoite/bradyzoite stage differentiation (manuscript in preparation), we renamed it “BARKY” (bradyzoite antigen regulator, kinase Y).
BARKY is a typical MAPK based on conventional criteria [50] although it possesses three insertion sequences. Using mass spectroscopy, we confirmed the presence of a 34 amino acid insert situated between the GXGXXGXV motif (subdomain I) and the invariant lysine residue within the VAXK motif of subdomain II, a region responsible for anchoring the nontransferable α- and β-phosphates of ATP during catalysis. BARKY is also predicted to encode a 93 amino acid insert situated between the DFGLAR motif that interacts with the Mg++ bound to ATP and the phosphorylation lip, which links the proline-directed peptide binding pocket in an extended conformation following phosphorylation of its activation loop (subdomains VII and VIII, resp.). Finally, using mass spectroscopy, we identified a 20 amino acid insert between subdomains IX and X.
Phylogenetic analysis demonstrates that T. gondii BARKY most closely resembles Cryptosporidium hominis MAPK (with 52% amino acid sequence identity across all 11 of the MAPK subdomains), with a corresponding homologue in C. parvum. No other closely related MAPK homologues were identified either within or outside the phylum Apicomplexa at the time of publication [18].
Alternative splicing within exons 3-4 and exons 7-8 of the BARKY gene results in multiple BARKY isoforms, producing protein variants that could differentially respond to upstream signals or have altered substrate specificity. In support, we have detected 50, 58, and ~130 kDa proteins in T. gondii tachyzoite cell-free extracts by Western blotting. We have also employed mass spectroscopy to detect peptide fragments that confirm the existence of the full length (130 kDa) BARKY protein in tachyzoites grown in vitro. We cannot exclude the possibility that the smaller forms of the protein result from proteolytic degradation, but reverse transcriptase-polymerase chain reaction has demonstrated the presence of BARKY transcripts with a stop codon-situated 84 nucleotides into exon 7, as well as an alternative BARKY splice variant encoding exon 8 that adds a 766 amino acid extension to the carboxy-terminus (Brumlik et al., unpublished observations). These features are reminiscent of extensions identified in many Leishmania MAPKs [51].
There are no reported functional data for T. gondii TgMAPK2 but it is expressed in T. gondii tachyzoites at the expected molecular weight of 73 kDa (Brumlik et al., unpublished observations). Phylogenetic analysis places this MAPK in a group of closely related Apicomplexan MAPKs which includes Cryptosporidium hominis MAPK1, P. falciparum Pfmap-1, and Theileria annulata MAPK (all sharing roughly 70% amino acid sequence identity across all 11 of the MAPK subdomains). TgMAPK2 shares significant amino acid sequence identity with MAPKs from non-Apicomplexan protozoans including L. mexicana LmxMPK2 (62%) and Trypanosoma brucei TbMAPK2 (62%), each possessing a typical TDY phosphorylation lip. The deduced amino acid sequence of TgMAPK2 shares 55% identity with human ERK8 across all 11 MAPK subdomains, demonstrating the remarkable evolutionary conservation of this MAPK subfamily member. In addition, T. gondii TgMAPK2 possesses multiple copies of a VSSSHHG repeat in its carboxy-terminal extension, the exact number of repeats being strain-dependent [18]. While the role of this repeat remains unknown, it is striking that P. falciparum Pfmap-1 possesses an analogous series of imperfect KKYVD[GSE][GSL]N repeats in its carboxy-terminal extension [43]. Short amino acid repeats often facilitate oligomerization or serve as contact points for protein-protein interactions. Interestingly, TgMAPK2 is also predicted to possess a nuclear localization signal within its carboxy-terminal extension.
T. gondii TgMAPK3 is predicted to be an atypical 63 kDa MAPK with a TGH phosphorylation lip. It shares significant amino acid sequence identity with several Apicomplexan MAPKs such as Cryptosporidium hominis MAPK2 (67%), P. falciparum Pfmap-2 (58%), and Theileria annulata MAPK2 (50%), with low amino acid sequence identity to non-Apicomplexan MAPKs [18].
3. Phylum Sarcomastigophora
3.1. Trypanosomatid MAPKs
Trypanosomatids (members of the family Trypanosomatidae) are a diverse group of protozoan parasites of which two genera are human pathogens: Trypanosoma and Leishmania.
3.1.1. Genus Leishmania
Several different Leishmania species cause human disease of varying clinical presentation and severity, of which L. major generally causes the most serious illnesses. Genome sequencing has identified 15 putative complete MAPK genes in L. major (Table 2), the alignments of which are shown in Figure 1. Two partial L. major MAPK genes have also been identified (LmjF03.0210 and LmjF13.07800) [52] but have been excluded from further consideration because they lack the coding region for the complete MAPK signature sequence. All 15 L. major MAPK homologues have also been identified in L. mexicana (Table 2), L. infantum, and L. brasiliensis [52].
Table 2.
Mitogen-activated protein kinases and their corresponding homologues in Trypanosomatids.
| L. major (LMa) MAPK | LMa aaccn. | L mexicana (LMx) MAPK | LMx aaccn. | T. brucei (TB) MAPK | TB aaccn. | T. cruzi (TC) MAPK | TC aaccn. #1 | TC aaccn. #2 | Phosphorylation lip LMa/LMx/TB/ TC#1/TC#2 |
Function | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LmaMPK1 | Q4Q0B0 | LmxMPK1 | Z95887 | KFR1 | Q26802 | — | Q4CSB9 | — | TDY/TDY/ TEY/TGY |
Essential for intracellular parasite survival of bloodstream stage (LMx, TB), IFN-γ-induced proliferation of bloodstream stage (TB) | [52–55] |
| LmaMPK2 | Q4Q204 | LmxMPK2 | AJ293280 | — | Q38B88 | — | Q4CZ09 | Q4CR01 | TDY/TDY/TDY/ TDY/TDY |
Essential for intracellular parasite survival of bloodstream stage (LMx, TB) | [52, 53] |
| LmaMPK3 | Q4QHG6 | LmxMPK3 | AJ293281 | — | Q580X5 | TcMPK3 | Q4D0A7 | Q4CKS6 | TDY/TDY/TDY/ TDY/TDY |
Flagellar length (LMx) | [56, 57] |
| LmaMPK4 | Q4QD66 | LmxMPK4 | AJ293282 | TbMAPK2 | Q38B88 | — | Q4D3Y2 | — | TQY/TQY/ TDY/TEY |
Stage-specific induction of phosphotransferase activity(LMx) | [52, 58, 59] |
| LmaMPK5 | Q4Q701 | LmxMPK5 | AJ293283 | TbMAPK5 | Q586Y9 | — | Q4DHF7 | Q4DCP6 | TDY/TDY/TDY/ TDY/TDY |
Differentiation (TB) | [60] |
| LmaMPK6 | Q4Q4U7 | LmxMPK6 | AJ293284 | TbECK1 | Q381A7 | — | Q4DD40 | — | TDY/TDY/ TEY/TDY |
Proliferation; stage-specificinduction of phosphotransferase activity (TB) | [61] |
| LmaMPK7 | Q4QFZ0 | LmxMPK7 | AJ293285 | — | — | — | — | — | TDY/TDY | Proliferation; stage-specificinduction of phosphotransferase activity (LMa) | [59, 62] |
| LmaMPK8 | Q4Q8L2 | LmxMPK8 | AJ293286 | — | — | — | — | — | TNY/TNY | ? | |
| LmaMPK9 | Q4QDK3 | LmxMPK9 | AJ293287 | — | Q387N8 | — | Q4DYK0 | Q4DD15 | TEY/TEY/TEY/ TEY/TEY |
Flagellar length (LMx) | [57, 63] |
| LmaMPK10 | Q4QHJ8 | LmxMPK10 | DQ308411 | — | Q580Z7 | — | Q4D4Q4 | Q4CU32 | THY/THY/THY/ THY/THY |
Stage-specific induction of phosphotransferase activity (LMa, LMx) | [59, 62] |
| LmaMPK11 | Q4Q449 | LmxMPK11 | DQ026027 | — | Q389D8 | — | Q4CZQ7 | Q4DC97 | TDY/TDY/TDY/ TDY/TDY |
? | |
| LmaMPK12 | Q4Q7S2 | LmxMPK12 | DQ026026 | TbMAPK4 | Q585N3 | — | Q4DHP2 | — | TQY/TQY/TSY/THY | ? | |
| LmaMPK13 (LF4) | Q4FVX2 | LmxMPK13 (LF4) | DQ812905 | MOK | Q38E60 | — | Q4E4I5 | Q4DWW0 | TEY/TEY/TEY/ TEY/TEY |
Flagellar length (LMx) | [57] |
| LmaMPK14 | Q4FYW2 | LmxMPK14 | DQ812906 | — | Q57WV2 | — | Q4D0S5 | Q4D7J6 | TDY/TDY/TDY/ TDY/TDY |
Flagellar length (LMx) | [57] |
| LmaMPK15 | Q4Q3Y0 | LmxMPK15 | DQ812907 | — | Q389P3 | — | Q4DKI1 | — | TIY/TIY/TFY/TFY | ? |
aaccn.; accession no.
Each of the 15 unique Leishmania MAPKs (Figure 1) is a typical MAPK by the classical definition (i.e., the activation loop is comprised of a TXY motif). The majority of these MAPKs possess carboxy-terminal extensions (Figure 1), some of them over 1000 amino acids long (as for LmaMPK8). This region may be analogous to the corresponding region of human ERK5 or ERK8, each of which possesses a C-terminal transactivation domain and nuclear localization signal [22, 23]. LmaMPK6, 7, and 8 are predicted to contain nuclear localization signals within their carboxy-terminal extensions, making them even more closely resemble human ERK5 and ERK8, as well as T. gondii TgMAPK2.
Deletion analysis of the genes encoding L. mexicana LmxMPK1 and LmxMPK2 demonstrates that both are essential for amastigote (bloodstream stage) survival [52, 53]. L. mexicana LmxMPK4 is essential to both promastigote (sandfly stage) and amastigote forms [58] and is phosphorylated on T190 and Y192 of its phosphorylation lip by the MKK LmxMKK5 [64]. Overexpression of L. major LmaMPK4, 7, or 10 (homologues of LmxMPK4, 7, and 10, resp.) causes stage-specific induction of phosphotransferase activity. Moreover, LmaMPK7 activation specifically regulates parasite growth [62]. In each case, kinase activity was low or absent in cell-free extracts from promastigotes but significantly increased after exposure to pH 5.5 and 34°C., which simulates the stress encountered by the parasite in the acidified phagolysosome upon invasion of macrophages [59]. L. mexicana LmxPK4 is an MKK that controls parasite differentiation [65] and thus represents a potential upstream activator of at least one of the MAPKs affecting stage differentiation.
Several L. mexicana MAPKs regulate flagellar length, many of which possess carboxy-terminal extensions [66]. Deletion mutants for LmxMPK3 had shortened flagella and overexpression of LmxMPK3 in the deletion background complemented this defect [56, 57]. Deletion mutants for LmxMPK9, LmxMPK13, or LmxMPK14 generated promastigotes with elongated flagella, an effect that could be reversed by overexpressing these MAPKs in null mutants [57, 63]. LmxMPK13 is the homologue of LF4 from the protozoan microalga Chlamydomonas reinhardtii, which also regulates flagellar length [67]. L. mexicana LmxMKK is the MAPKK responsible for regulating flagellar length [68] and activates LmxMPK3 [56] and perhaps affects other MAPKs regulating flagellar length.
Analysis of the L. mexicana genome has identified two additional putative MKK genes in addition to L. mexicana lmxPK4, lmxMKK, and lmxMKK5 for which functions have yet to be determined. L. mexicana also putatively encodes 23 MKKKs and a single MKKKK [51], the functions of which remain unknown.
3.1.2. Genus Trypanosoma
Subspecies of T. brucei cause African sleeping sickness, whereas T. cruzi causes New World trypanosomiasis (Chagas disease). Genomic sequencing has identified 13 MAPK genes in T. brucei, each of which has at least one, but often two virtually identical copies of MAPK homologues in T. cruzi (with each copy having greater than 99% amino acid sequence identity to the other (Table 2)) [51, 52]. Homologous T. brucei or T. cruzi MAPK domains are ~90% identical to each other and each has a single corresponding homologue in Leishmania spp. (sharing over 80% amino acid sequence identity across the 11 MAPK subdomains). LmxMPK7 and LmxMPK8 are the only two Leishmania MAPKs that lack homologues in either T. brucei or T. cruzi. Thus we exclusively used the L. major MAPK sequences (LmaMPK1-15) for ClustalW alignment, reducing redundant examples of highly homologous MAPKs in the analysis.
Although all Trypanosoma MAPKs possess a classical TXY motif, a feature also conserved in all Leishmania MAPK homologues (Table 2), the central amino acid in the TXY motif varies between MAPK homologues from different Trypanosomatid species (Table 2) and thus is not as evolutionarily constrained as in mammalian MAPKs.
T. brucei/cruzi MPK10 (accession nos. Q580Z7/Q4D4Q4) and MPK11 (accession nos. Q389D8/Q4CZQ7) have not yet been officially named (see Table 2). Regardless, these MAPKs (and their Leishmania MAPK homologues) are exceptional in possessing a MAPK signature sequence that deviates with respect to the precise position of the threonine in the proline-directed (P+1) peptide binding pocket (see Figure 1, center of subdomain VIII, residues 197-207). This likely alters the precise spatial orientation of the proline-directed peptide binding pocket relative to the phosphorylation lip, perhaps placing these MAPKs in a separate subfamily.
KFR1 (KSS1- and FUS3-related kinase 1), the T. brucei homologue of L. mexicana LmxMPK1, mediates interferon-γ-induced amastigote proliferation and phosphorylates serine residues on host histone H1, myelin basic protein, and β-casein [54, 55]. T. brucei TbECK1, which is the trypanosome homologue of L. mexicana LmxMPK6, possesses a carboxy-terminal extension that regulates kinase activity in all life cycle stages. Expression of a truncated TbECK1 protein lacking large parts of this extension caused T. brucei to grow slowly with abnormal morphology [61]. T. brucei procyclic forms lacking TbMAPK5, the homologue of L. mexicana LmxMPK5, likewise showed impaired differentiation into the bloodstream form [60]. TbMAPK2, the T. brucei homologue of LmxMPK4, regulates cell cycle progression from the procyclic (tsetse fly midgut) form to the bloodstream form [69]. TbMAPK5 controls T. brucei differentiation [60]. No functional studies of T. cruzi MAPKs have been published to date to our knowledge.
Phylogenetic analysis of T. brucei and T. cruzi suggests a single gene orthologous to the five putative MKK genes in L. major [51]. Only about one-third of the putative L. major MKKK genes have phylogenetic branching patterns consistent with the existence of orthologous genes in T. brucei and T. cruzi [51]. In most cases, L. major and T. brucei MKKK genes appear to be paralogues, having arisen from gene duplication events [51], suggesting significant evolutionary divergence in the circuitry of signaling cascades in Trypanosomatids. Three unique MKKKK genes have been identified in T. cruzi and two in T. brucei [51]. Functions have yet to be ascribed to any of these putative upstream MAPK activators.
3.2. Other Sarcomastigophora
Two MAPKs have been identified and characterized in the protozoan intestinal parasite Giardia lamblia, ERK1 and ERK2 (Table 1, Figure 1), each of which plays distinct roles in encystation [49]. In addition, one MAPK gene has been identified in the Trichomonas vaginalis genome [70]. However, functional studies have yet to be performed on MAPKs from either parasite.
3.3. Subphylum Sarcodina (the Amoebae)
This subphylum of amoebas contains three human pathogenic genera: Entamoeba, Naegleria, and Acanthamoeba. The E. histolytica EhMAPK gene encodes a putative MAPK with significant homology to human ERK8 [71]. We are not aware of any further MAPK analyses in this genus or of any reports of MAPK genes or function in Naegleria or Acanthamoeba.
4. Protozoan MAPKs as Therapeutic Targets
MAPKs direct many functions critical to pathogen homeostasis and survival, including proliferation [62], differentiation [52, 53], regulation of cytoskeletal features such as the biosynthesis of flagella [56, 57, 63], and stress-responses [50]. Because protozoan MAPKs share many common structural features and are vastly more closely related to each other than to human MAPKs [18, 72], it should be possible to design drugs specifically or preferentially targeting protozoan MAPKs. For example, Leishmania mexicana LmxMPK1 and LmxMPK2 are essential MAPKs required for differentiation [52, 53], with corresponding homologues in other Leishmania species and in T. brucei and T. cruzi [51], but bearing scant resemblance to human MAPKs, making them excellent candidates for drug development [72]. Specifically targeting these MAPKs could have far reaching therapeutic potential since one drug could be used to treat a broad range of Trypanosomatid infections based on the high degree of homology between Trypanosomatid MAPKs [51].
P. falciparum Pfmap-2 is likewise an excellent druggable target as this MAPK is essential for the parasite to complete asexual replication in infected human erythrocytes [48] and it is highly dissimilar to human MAPKs. Although we have yet to determine which of the T. gondii MAPKs are essential to parasite survival, reducing BARKY expression dramatically impairs parasite virulence (Brumlik et al., submitted), making BARKY a useful target for MAPK inhibitor drugs.
Agents interfering with the function of MAPKs that affect stage differentiation, such as T. gondii BARKY, or affect parasite growth, such as L. major LmaMPK7 or T. brucei TbECK1, likely would be useful antiparasitic agents. T. brucei KFR1 is an interesting MAPK target, as it regulates effects of the host immune response (interferon-γ-induced amastigote proliferation) and could be considered in combination with an immune strategy. L. mexicana LmxMPK1 is homologous to KFR1 and could mediate similar effects, being a useful drug discovery target in this respect. Agents impairing the function of MAPKs controlling flagellar development or function, such as LmxMPK3, LmxMPK9, LmxMPK13, or LmxMPK14, could inhibit parasite dissemination and might be useful alone, or in combination with parasiticidal agents.
Our work with T. gondii BARKY demonstrates multiple MAPK splice variants that can occur naturally in parasites. A better understanding of the function of these splice variants could help develop agents specifically targeting variants relevant to disease pathogenesis. Likewise, our genomic analyses, and those of others, have demonstrated unusual repeat motifs in several protozoan parasite MAPKs (including in T. gondii and Plasmodium species) encoding large numbers of potential phosphorylation sites. An understanding of the functional significance of these motifs could help develop useful antiparasitic agents. Given the relatively unique nature of the phosphorylation site repeat motifs, these sites possibly could lead to highly parasite-specific drugs.
Protozoan MAPKs need not subserve critical functions to be useful drug discovery targets. For example, L. mexicana LmxMPK6 affects parasite morphology (which has indirect consequences on its growth rate following infection) and has homologues in related disease-causing Trypanosomatids. Drugs impairing LmxMPK6 function could be used in conjugation with existing anti-Leishmania therapies to boost their efficacy and could have broad-spectrum effects.
Upstream components of the MAPK cascades such as the MKKs or MKKKs in pathogenic protozoan parasites are also potentially useful drug discovery targets. For example, the L. mexicana MKK, LmxPK4, controls parasite differentiation and thus is an excellent candidate. Because protozoan MKKs and MKKKs are even more distantly related to mammalian counterparts than MAPKs, a further potential advantage to this approach is that drugs inhibiting parasite MKK function could be less likely to have undesirable side-effects compared to drugs targeting specific MAPKs.
A potential disadvantage to targeting upstream MAPK regulators relates to our incomplete understanding of how they function. For example, MAPKs such as human p38α are capable of MKK-independent activation and can undergo autophosphorylation in the presence of transforming growth factor-β-activated protein kinase 1 [73]. In this case, it would not be possible to block p38α activation by targeting the conventional upstream MKKK and MKK components of the p38 MAPK cascade.
Many protozoan MAPKs possess vestiges of the common docking (CD) domain and ED site (Figure 1)—surface-exposed acidic residues in human p38α MAPK that facilitate binding to upstream and downstream MAPK partners [74, 75]. D313, D315, and D316 comprise the CD domain in human p38α MAPK. This region acts in concert with the ED161 site to bind to short strings of 2–5 basic amino acids situated on proteins with which p38α interacts [74]. Protozoan MAPKs lacking a conserved CD domain (e.g., Leishmania major LMaMPK9 and 15) and/or ED site (e.g., Leishmania major LMaMPK3, 7, 9, 11, 15, and T. gondii TgMAPK1) are prime candidates for drug development since these domains have diverged considerably from their corresponding mammalian counterparts.
In addition, the highly variable carboxy-terminal extensions, which are present in over half the protozoan MAPKs shown in Figure 1, are excellent targets for drug development owing to their unique structures. Drugs targeted to these extensions would have a low probability of affecting mammalian MAPKs.
SB203580 is a pyridinylimidazole competitive ATP inhibitor affecting human p38 MAPK phosphotransferase activity through hydrogen bonding between its pyridine ring nitrogen and the MAPK backbone amide of M 109 in the THLM109 motif (subdomain V; Figure 1) [39]. A second critical hydrogen bond occurs between a nitrogen atom on the imidazole ring and the invariant lysine in the VAXK 53 motif (subdomain II). Finally, the fluorophenyl ring of SB203580 interacts with the hydrophobic environment created by T106 and H107[39].
Because SB203580 is much smaller than ATP (as are all pyridinylimidazole p38 MAPK inhibitors), it does not fully occupy this region, leaving two large hydrophobic pockets on either side of the pyridine ring [39]. By designing novel pyridinylimidazoles or structurally related pharmacophores that properly fill the ATP binding pocket of pertinent protozoan MAPKs, it could be possible to develop novel antiparasitic agents that are more potent and specific than existing drugs. Such drugs will be less likely to have unintended consequences on host p38 MAPK, which is a potential drawback of several existing p38 MAPK inhibitors.
Recent molecular modeling studies using competitive ATP inhibitors against LCRK3 in L. donovani, a cyclin-dependent kinase that is a distant relative of the MAPK superfamily, indicate that such compounds could have significant inhibitory activity against L. donovani LCRK3 [76]. Our work has shown that the human p38 MAPK inhibitors RWJ67657, RWJ68198, and SB203580 reduced the replication of L. donovani promastigotes in axenic culture. Moreover, SB203580 effectively inhibited the replication of the bloodstream stage cultured ex vivo (Brumlik et al., unpublished observations).
X-ray crystallographic studies of human p38α MAPK complexed with ATP have demonstrated that the THLM109 motif in the center of subdomain V (Figure 1) forms two critical hydrogen bonds with the adenosine moiety [77]. Based on our ClustalW alignment, many other amino acids can evidently serve this same purpose in other MAPKs (Figure 1; subdomain V), although the binding affinity of ATP (and competitive ATP inhibitor drugs) could be affected by such differences. Structural studies have further shown that the invariant GXGXXGXV38 motif in subdomain I coordinates the nontransferable α- and β-phosphates of ATP, while catalytic transfer of the γ-phosphate is mediated by hydrogen bonding between an essential lysine in the VAXK 53 motif (subdomain II), the RE68 motif in subdomain III, and the underlined residues in the HRD 168XK 170PXN 173 motif (subdomain VIb) [78]. Thus, to design novel competitive ATP inhibitors against protozoan MAPKs, one must not only account for the invariant residues comprising the ATP binding site in all MAPKs but also pay particular attention to the permissible structural changes in subdomain V of protozoan MAPKs that specifically affect the binding of competitive ATP inhibitors.
We have shown that SB203580 [50] and another pyridinylimidazole human p38 MAPK inhibitor, RWJ67657, significantly inhibit BARKY autophosphorylation (Brumlik et al., unpublished observations). These agents reduced T. gondii proliferation in vitro [41] and treated otherwise fatal T. gondii infection in mice [42]. We further assessed the efficacy of two human p38 MAPK inhibitors to treat parasitic infections and showed that RWJ67657 and the pyrrolobenzimidazole RWJ68198 effectively blocked the replication of P. falciparum cultured in human erythrocytes ex vivo. Drug treatment resulted in trophozoites that were markedly diminished in size (Brumlik et al., submitted).
We demonstrated that RWJ67657 protected mice from otherwise fatal infection with the protozoan Encephalitozoon cuniculi [42] although it encodes no known MAPKs. Inhibition of host p38 MAPK could improve the host immune response to E. cuniculi, as has been demonstrated for T. gondii [79], or RWJ67657 could have therapeutic off-target effects in either the host or parasite. Better understandings of the mechanism of action in this case will further help drug development.
A large number of p38 MAPK inhibitors have recently progressed into phase I and II clinical trials, thus providing basic inhibitor pharmacophores that can be modified to target critical protozoan MAPKs specifically while at the same time having less host toxicity (a problem with many agents in the pyridinylimidazole class).
5. Conclusions
MAPKs play essential roles in virtually all eukaryotes. Thus, inhibiting protozoan MAPK functions represents a scientifically sound approach to developing novel classes of antiprotozoan agents. As protozoan MAPKs are only distantly related to mammalian MAPKs and have distinct active sites, it is reasonable to expect that selective agents can be developed to target pathogen proteins with minimal collateral effects on human counterparts.
Although only a very modest body of work on the structure and function of protozoan MAPKs currently exists, the available evidence already suggests the general utility of inhibiting protozoan parasite MAPK function as a treatment strategy. Several specific MAPK candidates have also already emerged from such work. As interest in MAPKs increases, the rate of important discoveries and their preclinical and clinical translation will also increase.
Specific roles for MAPKs cannot be predicted based solely on sequence similarity to protein homologues. For example, P. falciparum MAPK Pfmap-2 is essential for the completion of asexual replication in human erythrocytes [48] and yet its closest homologue in P. berghei, Pbmap-2 (with 93% amino acid sequence identity within its catalytic domains to Pfmap-2 [80]), is dispensable for both asexual replication and gametocyte formation in the mouse erythrocyte. Pbmap-2 instead plays a critical role in exflagellation in the mosquito midgut [81]. Thus, while the structure of the MAPKs themselves remains highly evolutionarily constrained even among closely related Plasmodium species, the circuitry of the various signal transduction pathways themselves has undergone significant divergent evolution. Therefore it is critical to establish specific roles of particular MAPKs prior to drug development.
Once the function of a MAPK from a pathogenic protozoan parasite has been established, one can exploit the phylogenetic differences between MAPKs of protozoan and metazoan origin to design specific MAPK inhibitors. Refining the structure of human MAPK inhibitor pharmacophores already in existence should speed development of new MAPK-inhibiting antiprotozoan drugs. We also expect to see additional new classes of drugs developed, which will be aided by additional structure/function studies. Targeting upstream MAPK regulators is an approach that also bears investigation, but which will likely lag owing to significant current knowledge gaps in understanding these regulators.
A considerable challenge is to persevere with such research given the relatively scant resources available for such work, in spite of the fact that over one-half billion people in many of the poorest parts of the world are infected by pathogenic protozoan parasites [82].
References
- 1.Bagrodia S, Cerione RA. PAK to the future. Trends in Cell Biology. 1999;9(9):350–355. doi: 10.1016/s0962-8924(99)01618-9. [DOI] [PubMed] [Google Scholar]
- 2.Pearson G, Robinson F, Gibson TB, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 4.Miranda-Saavedra D, Stark MJR, Packer JC, Vivares CP, Doerig C, Barton GJ. The complement of protein kinases of the microsporidium Encephalitozoon cuniculi in relation to those of Saccharomyces cerevisiae and Schizosaccharomyces pombe. BMC Genomics. 2007;8, article 309 doi: 10.1186/1471-2164-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kültz D, Burg M. Evolution of osmotic stress signaling via MAP kinase cascades. Journal of Experimental Biology. 1998;201(22):3015–3021. doi: 10.1242/jeb.201.22.3015. [DOI] [PubMed] [Google Scholar]
- 6.Kültz D. Phylogenetic and functional classification of mitogen- and stress- activated protein kinases. Journal of Molecular Evolution. 1998;46(5):571–588. doi: 10.1007/pl00006338. [DOI] [PubMed] [Google Scholar]
- 7.Hanks SK. Genomic analysis of the eukaryotic protein kinase superfamily: a perspective. Genome Biology. 2003;4(5, article 111) doi: 10.1186/gb-2003-4-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochimica et Biophysica Acta. 2007;1773(8):1376–1387. doi: 10.1016/j.bbamcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90(5):859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 10.Hanks SK, Quinn AM. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods in Enzymology. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- 11.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiological Reviews. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 12.Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends in Cell Biology. 2001;11(5):220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 13.Avruch J. MAP kinase pathways: the first twenty years. Biochimica et Biophysica Acta. 2007;1773(8):1150–1160. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seger R, Krebs EG. The MAPK signaling cascade. FASEB Journal. 1995;9(9):726–735. [PubMed] [Google Scholar]
- 15.Edbauer D, Cheng D, Batterton MN, et al. Identification and characterization of neuronal mitogen-activated protein kinase substrates using a specific phosphomotif antibody. Molecular & Cellular Proteomics. 2009;8(4):681–695. doi: 10.1074/mcp.M800233-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 17.Miyata Y, Nishida E. Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochemical and Biophysical Research Communications. 1999;266(2):291–295. doi: 10.1006/bbrc.1999.1705. [DOI] [PubMed] [Google Scholar]
- 18.Lacey MR, Brumlik MJ, Yenni RE, Burow ME, Curiel TJ. Toxoplasma gondii expresses two mitogen-activated protein kinase genes that represent distinct protozoan subfamilies. Journal of Molecular Evolution. 2007;64(1):4–14. doi: 10.1007/s00239-005-0197-x. [DOI] [PubMed] [Google Scholar]
- 19.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24(1):21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 20.Kyriakis JM, Banerjee P, Nikolakaki E, et al. The stress-activated protein kinase subfamily of c-jun kinases. Nature. 1994;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 21.Whitmarsh AJ. A central role for p38 MAPK in the early transcriptional response to stress. BMC Biology. 2010;8, article 47 doi: 10.1186/1741-7007-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou G, Zhao Qin Bao , Dixon JE. Components of a new human protein kinase signal transduction pathway. Journal of Biological Chemistry. 1995;270(21):12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 23.Lee JD, Ulevitch RJ, Han J. Primary structure of BMK1: a new mammalian MAP kinase. Biochemical and Biophysical Research Communications. 1995;213(2):715–724. doi: 10.1006/bbrc.1995.2189. [DOI] [PubMed] [Google Scholar]
- 24.Enslen H, Brancho DM, Davis RJ. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. The EMBO Journal. 2000;19(6):1301–1311. doi: 10.1093/emboj/19.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng M, Boulton TG, Cobb MH. ERK3 is a constitutively nuclear protein kinase. Journal of Biological Chemistry. 1996;271(15):8951–8958. doi: 10.1074/jbc.271.15.8951. [DOI] [PubMed] [Google Scholar]
- 26.Yamada M, Ohnishi J, Ohkawara B, et al. NARF, an Nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF) Journal of Biological Chemistry. 2006;281(30):20749–20760. doi: 10.1074/jbc.M602089200. [DOI] [PubMed] [Google Scholar]
- 27.Zhou BO, Zhang ZY. The activity of the extracellular signal-regulated kinase 2 is regulated by differential phosphorylation in the activation loop. Journal of Biological Chemistry. 2002;277(16):13889–13899. doi: 10.1074/jbc.M200377200. [DOI] [PubMed] [Google Scholar]
- 28.Zanke BW, Rubie EA, Winnett E, et al. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. Journal of Biological Chemistry. 1996;271(47):29876–29881. doi: 10.1074/jbc.271.47.29876. [DOI] [PubMed] [Google Scholar]
- 29.Swain PS, Siggia ED. The role of proofreading in signal transduction specificity. Biophysical Journal. 2002;82(6):2928–2933. doi: 10.1016/S0006-3495(02)75633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe MK, Saelzler MP, Rafael Espinosa III, et al. ERK8, a new member of the mitogen-activated protein kinase family. Journal of Biological Chemistry. 2002;277(19):16733–16743. doi: 10.1074/jbc.M112483200. [DOI] [PubMed] [Google Scholar]
- 31.Abe MK, Kahle KT, Saelzler MP, Orth K, Dixon JE, Rosner MR. ERK7 is an autoactivated member of the MAPK family. Journal of Biological Chemistry. 2001;276(24):21272–21279. doi: 10.1074/jbc.M100026200. [DOI] [PubMed] [Google Scholar]
- 32.Abe MK, Kuo WL, Hershenson MB, Rosner MR. Extracellular signal-regulated kinase 7 (ERK7), a novel ERK with a C- terminal domain that regulates its activity, its cellular localization, and cell growth. Molecular and Cellular Biology. 1999;19(2):1301–1312. doi: 10.1128/mcb.19.2.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Current Protocols in Bioinformatics. 2002;2(unit 2.3) doi: 10.1002/0471250953.bi0203s00. [DOI] [PubMed] [Google Scholar]
- 34.Sheikh-Hamad D, Di Mari J, Suki WN, Safirstein R, Watts BA, III, Rouse D. p38 Kinase activity is essential for osmotic induction of mRNAs for HSP70 and transporter for organic solute betaine in Madin-Darby canine kidney cells. Journal of Biological Chemistry. 1998;273(3):1832–1837. doi: 10.1074/jbc.273.3.1832. [DOI] [PubMed] [Google Scholar]
- 35.Han ZS, Enslen H, Hu X, et al. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Molecular and Cellular Biology. 1998;18(6):3527–3539. doi: 10.1128/mcb.18.6.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue H, Hisamoto N, Jae HA, et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes and Development. 2005;19(19):2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadsworth SA, Cavender DE, Beers SA, et al. RWJ 67657, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. Journal of Pharmacology and Experimental Therapeutics. 1999;291(2):680–687. [PubMed] [Google Scholar]
- 38.Sweitzer SM, Medicherla S, Almirez R, et al. Antinociceptive action of a p38α MAPK inhibitor, SD-282, in a diabetic neuropathy model. Pain. 2004;109(3):409–419. doi: 10.1016/j.pain.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Tong L, Pav S, White DM, et al. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nature Structural Biology. 1997;4(4):311–316. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Canagarajah BJ, Boehm JC, et al. Structural basis of inhibitor selectivity in MAP kinases. Structure. 1998;6(9):1117–1128. doi: 10.1016/s0969-2126(98)00113-0. [DOI] [PubMed] [Google Scholar]
- 41.Wei S, Marches F, Daniel B, Sonda S, Heidenreich K, Curiel T. Pyridinylimidazole p38 mitogen-activated protein kinase inhibitors block intracellular Toxoplasma gondii replication. International Journal for Parasitology. 2002;32(8):969–977. doi: 10.1016/s0020-7519(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 42.Wei S, Daniel BJ, Brumlik MJ, et al. Drugs designed to inhibit human p38 mitogen-activated protein kinase activation treat Toxoplasma gondii and Encephalitozoon cuniculi infection. Antimicrobial Agents and Chemotherapy. 2007;51(12):4324–4328. doi: 10.1128/AAC.00680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doerig CM, Parzy D, Langsley G, Horrocks P, Carter R, Doerig CD. A MAP kinase homologue from the human malaria parasite, Plasmodium falciparum . Gene. 1996;177(1-2):1–6. doi: 10.1016/0378-1119(96)00281-8. [DOI] [PubMed] [Google Scholar]
- 44.Dorin D, Alano P, Boccaccio I, et al. An atypical mitogen-activated protein kinase (MAPK) homologue expressed in gametocytes of the human malaria parasite Plasmodium falciparum. Identification of a MAPK signature. Journal of Biological Chemistry. 1999;274(42):29912–29920. doi: 10.1074/jbc.274.42.29912. [DOI] [PubMed] [Google Scholar]
- 45.Dorin D, Semblat JP, Poullet P, et al. PfPK7, an atypical MEK-related protein kinase, reflects the absence of classical three-component MAPK pathways in the human malaria parasite Plasmodium falciparum . Molecular Microbiology. 2005;55(1):184–196. doi: 10.1111/j.1365-2958.2004.04393.x. [DOI] [PubMed] [Google Scholar]
- 46.Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5(1, article 79) doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorin D, Roch KL, Sallicandro P, et al. Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum: biochemical properties and possible involvement in MAPK regulation. European Journal of Biochemistry. 2001;268(9):2600–2608. doi: 10.1046/j.1432-1327.2001.02151.x. [DOI] [PubMed] [Google Scholar]
- 48.Dorin-Semblat D, Quashie N, Halbert J, et al. Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Molecular Microbiology. 2007;65(5):1170–1180. doi: 10.1111/j.1365-2958.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 49.Ellis JG, Davila M, Chakrabarti R. Potential involvement of extracellular signal-regulated kinase 1 and 2 in encystation of a primitive eukaryote, Giardia lamblia: stage-specific activation and intracellular localization. Journal of Biological Chemistry. 2003;278(3):1936–1945. doi: 10.1074/jbc.M209274200. [DOI] [PubMed] [Google Scholar]
- 50.Brumlik MJ, Wei S, Finstad K, et al. Identification of a novel mitogen-activated protein kinase in Toxoplasma gondii. International Journal for Parasitology. 2004;34(11):1245–1254. doi: 10.1016/j.ijpara.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Parsons M, Worthey EA, Ward PN, Mottram JC. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics. 2005;6(article 127) doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiese M. Leishmania MAP kinases—familiar proteins in an unusual context. International Journal for Parasitology. 2007;37(10):1053–1062. doi: 10.1016/j.ijpara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Wiese M. A mitogen-activated protein (MAP) kinase homologue of Leishmania mexicana is essential for parasite survival in the infected host. The EMBO Journal. 1998;17(9):2619–2628. doi: 10.1093/emboj/17.9.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hua SB, Wang CC. Differential accumulation of a protein kinase homolog in Trypanosoma brucei . Journal of Cellular Biochemistry. 1994;54(1):20–31. doi: 10.1002/jcb.240540104. [DOI] [PubMed] [Google Scholar]
- 55.Hua SB, Wang CC. Interferon-γ activation of a mitogen-activated protein kinase, KFR1, in the bloodstream form of Trypanosoma brucei . Journal of Biological Chemistry. 1997;272(16):10797–10803. doi: 10.1074/jbc.272.16.10797. [DOI] [PubMed] [Google Scholar]
- 56.Erdmann M, Scholz A, Melzer IM, Schmetz C, Wiese M. Interacting protein kinases involved in the regulation of flagellar length. Molecular Biology of the Cell. 2006;17(4):2035–2045. doi: 10.1091/mbc.E05-10-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erdmann M. LmxMPK3, a mitogen-activated protein kinase involved in length control of a eukaryotic flagellum. Hamburg, Germany: University of Hamburg; 2009. Thesis dissertation. [Google Scholar]
- 58.Wang Q, Melzer IM, Kruse M, Sander-Juelch C, Wiese M. LmxMPK4, a mitogen-activated protein (MAP) kinase homologue essential for promastigotes and amastigotes of Leishmania mexicana . Kinetoplastid Biology and Disease. 2005;4, article 6 doi: 10.1186/1475-9292-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morales MA, Renaud O, Faigle W, Shorte SL, Späth GF. Over-expression of Leishmania major MAP kinases reveals stage-specific induction of phosphotransferase activity. International Journal for Parasitology. 2007;37(11):1187–1199. doi: 10.1016/j.ijpara.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Pfister DD, Burkard G, Morand S, Renggli CK, Roditi I, Vassella E. A mitogen-activated protein kinase controls differentiation of bloodstream forms of Trypanosoma brucei . Eukaryotic Cell. 2006;5(7):1126–1135. doi: 10.1128/EC.00094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellis J, Sarkar M, Hendriks E, Matthews K. A novel ERK-like, CRK-like protein kinase that modulates growth in Trypanosoma brucei via an autoregulatory C-terminal entension. Molecular Microbiology. 2004;53(5):1487–1499. doi: 10.1111/j.1365-2958.2004.04218.x. [DOI] [PubMed] [Google Scholar]
- 62.Morales MA, Pescher P, Späth GF. Leishmania major MPK7 protein kinase activity inhibits intracellular growth of the pathogenic amastigote stage. Eukaryotic Cell. 2010;9(1):22–30. doi: 10.1128/EC.00196-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bengs F, Scholz A, Kuhn D, Wiese M. LmxMPK9, a mitogen-activated protein kinase homologue affects flagellar length in Leishmania mexicana . Molecular Microbiology. 2005;55(5):1606–1615. doi: 10.1111/j.1365-2958.2005.04498.x. [DOI] [PubMed] [Google Scholar]
- 64.John von Freyend S, Rosenqvist H, Fink A, et al. LmxMPK4, an essential mitogen-activated protein kinase of Leishmania mexicana is phosphorylated and activated by the STE7-like protein kinase LmxMKK5. International Journal for Parasitology. 2010;40(8):969–978. doi: 10.1016/j.ijpara.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Kuhn D, Wiese M. LmxPK4, a mitogen-activated protein kinase kinase homologue of Leishmania mexicana with a potential role in parasite differentiation. Molecular Microbiology. 2005;56(5):1169–1182. doi: 10.1111/j.1365-2958.2005.04614.x. [DOI] [PubMed] [Google Scholar]
- 66.Rotureau B, Morales MA, Bastin P, Späth GF. The flagellum-mitogen-activated protein kinase connection in Trypanosomatids: a key sensory role in parasite signalling and development? Cellular Microbiology. 2009;11(5):710–718. doi: 10.1111/j.1462-5822.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- 67.Berman SA, Wilson NF, Haas NA, Lefebvre PA. A novel MAP kinase regulates flagellar length in Chlamydomonas . Current Biology. 2003;13(13):1145–1149. doi: 10.1016/s0960-9822(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 68.Wiese M, Kuhn D, Grünfelder CG. Protein kinase involved in flagellar-length control. Eukaryotic Cell. 2003;2(4):769–777. doi: 10.1128/EC.2.4.769-777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Müller IB, Domenicali-Pfister D, Roditi I, Vassella E. Stage-specific requirement of a mitogen-activated protein kinase by Trypanosoma brucei . Molecular Biology of the Cell. 2002;13(11):3787–3799. doi: 10.1091/mbc.E02-02-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlton JM, Hirt RP, Silva JC, et al. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis . Science. 2007;315(5809):207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ray D, Dutta S, Banerjee S, Banerjee R, Raha S. Identification, structure, and phylogenetic relationships of a mitogen-activated protein kinase homologue from the parasitic protist Entamoeba histolytica . Gene. 2005;346:41–50. doi: 10.1016/j.gene.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 72.Naula C, Parsons M, Mottram JC. Protein kinases as drug targets in trypanosomes and Leishmania. Biochimica et Biophysica Acta. 2005;1754(1-2):151–159. doi: 10.1016/j.bbapap.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge B, Gram H, Di Padova F, et al. MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α . Science. 2002;295(5558):1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- 74.Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nature Cell Biology. 2000;2(2):110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 75.Tanoue T, Maeda R, Adachi M, Nishida E. Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. The EMBO Journal. 2001;20(3):466–479. doi: 10.1093/emboj/20.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Awale M, Kumar V, Saravanan P, Mohan CG. Homology modeling and atomic level binding study of Leishmania MAPK with inhibitors. Journal of Molecular Modeling. 2010;16(3):475–488. doi: 10.1007/s00894-009-0565-3. [DOI] [PubMed] [Google Scholar]
- 77.Wilson KP, Fitzgibbon MJ, Caron PR, et al. Crystal structure of p38 mitogen-activated protein kinase. Journal of Biological Chemistry. 1996;271(44):27696–27700. doi: 10.1074/jbc.271.44.27696. [DOI] [PubMed] [Google Scholar]
- 78.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109(3):275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 79.Kim L, Del Rio L, Butcher BA, et al. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. Journal of Immunology. 2005;174(7):4178–4184. doi: 10.4049/jimmunol.174.7.4178. [DOI] [PubMed] [Google Scholar]
- 80.Rangarajan R, Bei AK, Jethwaney D, et al. A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei. EMBO Reports. 2005;6(5):464–469. doi: 10.1038/sj.embor.7400404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Molecular Microbiology. 2005;58(5):1253–1263. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- 82.Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nature Chemical Biology. 2006;2(12):701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]