Abstract
Seborrheic dermatitis is a common chronic-recurrent inflammatory disorder that most commonly affects adults; however, a more transient infantile form also occurs. The definitive cause of seborrheic dermatitis is unknown. However, proliferation of Malassezia species has been described as a contributing factor. The adult form of seborrheic dermatitis affects up to approximately five percent of the general population. The disorder commonly affects the scalp, face, and periauricular region, with the central chest, axillae, and genital region also involved in some cases. Pruritus is not always present and is relatively common, especially with scalp disease. A variety of treatments are available including topical corticosteroids, topical antifungal agents, topical calcineurin inhibitors, and more recently, a nonsteroidal “device ”cream. This article reviews the practical topical management of seborrheic dermatitis in the United States, focusing on the adult population.
Seborrheic dermatitis (SD) is a chronic inflammatory skin disorder characterized in immunocompetent adult patients by periods of exacerbation and remission. The reported prevalence of SD in the overall adult population ranges from 1 to 5 percent, and the disorder can affect any ethnicity.1, 2 There is a transient infantile form of SD that presents and resolves within the first 3 to 4 months of life; however, some cases may be more persistent with recurrences over several months. Infantile SD may be limited to scalp involvement (“cradle cap”) or may be more diffuse. The adult form of SD, which is far more common than infantile SD and appears to affect men more than women, may present first around puberty, correlating with the increase in cutaneous lipids resulting from androgen-driven sebaceous gland development and sebum secretion.3 The course of adult SD in affected individuals is variable throughout adulthood with some noting only occasional periods of exacerbation and others experiencing greater chronicity with more frequent recurrences. The common presence of SD after the age of 50 years has been noted.3, 4
In patients with acquired immunodeficiency syndrome (AIDS), the incidence of SD increases markedly, affecting from 30 to 80 percent of individuals. This increase in prevalence of SD in people with AIDS likely correlates with T cell lymphopenia, affecting counts of CD4+ cells involved in immune surveillance.3,5–7 In an animal model of SD, researchers were able to simulate SD seen in AIDS patients, showing apparent correlation with lowered CD4+ T lymphocyte counts and fungal proliferation.7
Clinical Presentation of Adult Seborrheic Dermatitis
Adult SD presents most often on the face and/or scalp as ill-defined erythematous patches associated with fine (pityriasiform) scaling, involving one are more sites of predilection. These commonly affected sites include scalp, anterior hairline (Figure 1), eyebrows, glabella region of the forehead, nasal alar creases, melolabial folds (Figure 2), ears (including the external canals, anterior auricular region, retroauricular region), central chest (sternum area), and genital region. Pruritus is not a mandatory feature of adult SD, but is often present, especially with scalp involvement. It has long been debated whether or not dandruff (pityriasis sicca), which is defined as fine scalp scaling without the visible presence of inflammation, is part of the spectrum of adult SD, with more recent evidence suggesting that in most cases dandruff is a mild form of SD.3, 4 The scalp scaling associated with SD and dandruff is often bothersome as flakes, which are shed from the scalp and are often visibly apparent on darker clothing (Figure 3). In some cases, SD may produce thicker more confluent areas of involvement, sometimes with oval, discoid plaques (medallion lesions) (Figure 4).
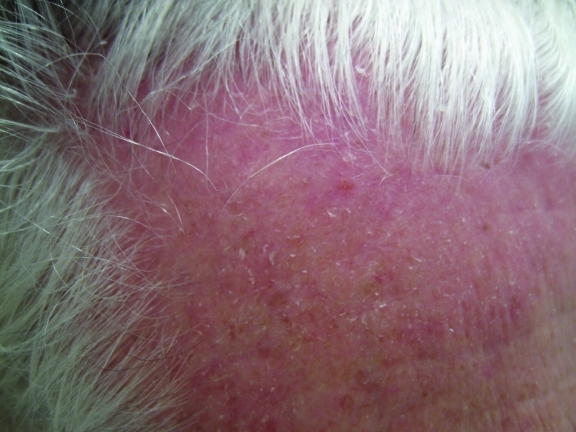
Figure 1.
Seborrheic dermatitis of the anterior hairline characterized by pink erythema and fine scaling in a 68-year-old Caucasian man
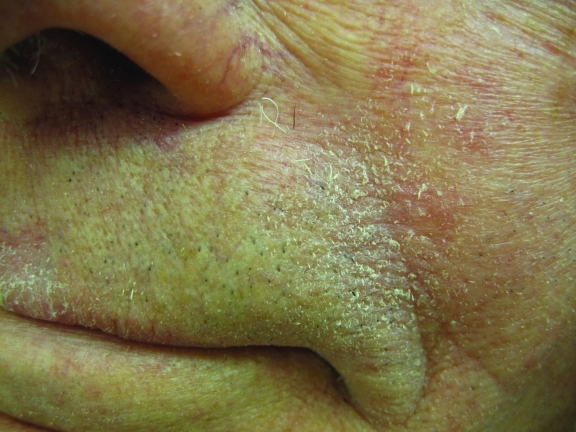
Figure 2.
Seborrheic dermatitis of the melolabial fold characterized by pink erythema and fine scaling in a 68-year-old Caucasian man
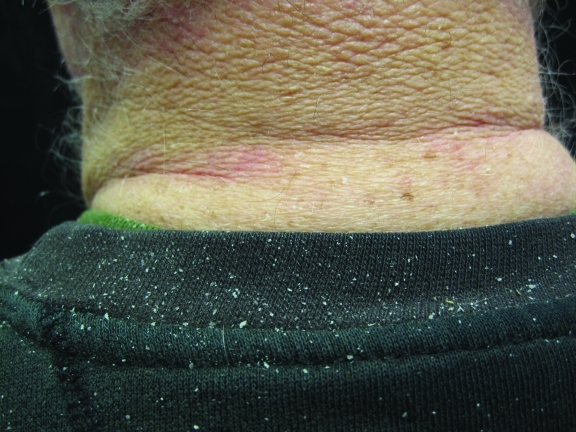
Figure 3.
Severe scalp scaling (dandruff) associated with continued shedding of scalp flakes, which fall on dark clothing and are readily visible
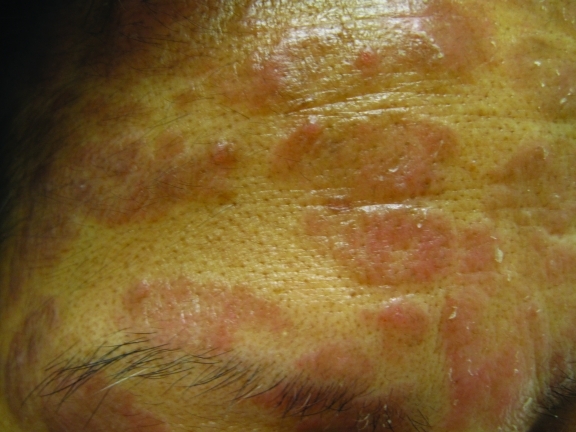
Figure 4.
Discoid, waxy, fine, scaly plaques of “medallion” seborrheic dermatitis on the face of a 50-year-old Filipino woman
Etiology of Adult Seborrheic Dermatitis
Although the etiology of adult SD is not definitely known, there are three principal factors that appear to play a role: sebaceous gland secretion, alteration in colonization and metabolism of cutaneous microflora (Malassezia spp), and individual susceptibility and host response.8
The role of Malassezia spp in the pathogenesis of adult SD remains controversial. However, the correlation between use of ketoconazole shampoo, reduction in Malassezia spp, and clinical improvement of scalp AD has caused researchers to suspect that these commensal yeasts play an important role.3 Although proliferation of Malassezia spp has been associated with exacerbation of SD, some reports have refuted this association.3,9,10 As Malassezia spp are not only present on the skin surface, but also within layers of the stratum corneum, variations in technique in obtaining specimen and quantifying the organism likely explain the differences in findings among available studies.3 The Malassezia spp that have been most commonly associated with SD are M. globosa and M. restricta, both of which are commensal yeasts that require an exogenous source of lipids.1, 3
It has been suggested that M. globosa and M. restricta are capable of degrading lipids in sebum with production of free fatty acids and triglycerides, followed by consumption of certain saturated fatty acids. The remaining modified unsaturated short-chain fatty acids are more capable of penetrating skin and inducing inflammation.8 Another explanation suggests altered host immune function or response in those with SD. The observation in SD of increase in natural killer cells (NK1+) and CD16+ cells, increase in inflammatory interleukins, and activation of complement in lesional skin as compared to nonlesional skin and in skin of healthy controls suggests an augmented inflammatory response in individuals with SD.11 The marked severity of SD in AIDS patients and the observation that SD lesions worsen in correlation with progressive worsening of AIDS also suggest that SD is associated with immune alteration and varied host response, the details of which warrant further investigation.3
Management of Adult Seborrheic Dermatitis
The main goals of therapy for SD are to clear the visible signs of the disease and to ameliorate the associated symptoms, especially pruritus. As SD is associated with intermittent periods of relapse over several years, some preventative regimens have been identified.8
Thorough reviews of the literature demonstrate that no standard scales have been identified for assessing the severity of SD, with the major outcome measures being symptom improvement, recurrence rate (short term), changes in colony counts of Malassezia spp, patient satisfaction, and cosmetic acceptability of the therapy.8 Despite the presence of several clinical trials evaluating many treatments for SD, systematic review of available data states that few randomized, controlled trials have “met the criteria for clinical evidence.”3, 8 Nevertheless, there are multiple studies that demonstrate efficacious therapies for SD involving both the scalp and glabrous skin sites.3, 8
Multiple topical therapies, primarily shampoo and cream formulations, have been used to treat SD of the scalp and/or glabrous skin.3, 8 One extensive review of topical treatment options for SD, involving scalp or other sites, included several studies inclusive of multiple agents (number of studies, N=number of actively treated subjects): selenium sulfide 2.5% shampoo (1, N=95), propylene glycol solution 35 to 50% (1, N=37), hydrocortisone 1% cream (3, N>58), ketoconazole 2% shampoo (3, N=181), ketoconazole 2% cream (4, N=89), miconazole 2% cream (1, N=22), bifonazole 1% shampoo (2, N=59), ciclopirox 1% cream (1, N=57), ciclopirox 1.5% shampoo (1, N=102), and lithium succinate 8%/zinc sulfate 0.05% ointment (1, N=82).3 Other topical agents used to treat SD have included terbinafine 1% cream/solution, metro-nidazole 1% gel, and azelaic acid 15% gel, although data are limited.3, 8 A more recently published “evidence-based” review of SD treatment has evaluated these same agents with inclusion of additional studies and agents, such as the topical calcineurin inhibitors.8 The following discusses the practical application for the clinician, with focus on the major agents used for SD that have been well studied and assessed in thorough reviews. Practical applications are based on the available published data, thorough literature reviews, and clinical experience.
Shampoos for Scalp Seborrheic Dermatitis
Ketoconazole 2% shampoo (Nizoral, McNeil Consumer Healthcare, a Division of McNeil-PPC, Inc.). Ketoconazole is an imidazole antifungal agent with activity against Malassezia spp and possibly a mild direct antiinflammatory effect.3 A total of seven double-blind, randomized, vehicle-controlled trials were identified using ketoconazole shampoo or cream in an “evidence-based” review, with the largest trial (N=575) demonstrating excellent clinical results in 88 percent of subjects.8 Importantly, ketoconazole 2% shampoo, available by prescription in the United States, has been shown to be more effective than ketoconazole 1% shampoo (P<0.001), available over the counter in the United States, more effective than selenium sulfide 2.5% shampoo (P=0.0026) for SD, and better tolerated than selenium sulfide 2.5% shampoo.12, 13 The usual recommended frequency with ketoconazole 2% shampoo is twice weekly over a usual duration of four weeks.3,8,12,13 Intermittent use of ketoconazole 2% shampoo, such as once weekly, has been shown to be effective in preventing relapse of SD.12, 14 The favorable safety profile of ketoconazole 2% shampoo is supported by studies demonstrating negligible percutaneous absorption and low potential for irritancy or contact sensitivity.8
Ciclopirox 1% shampoo (Loprox, Medicis, Scottsdale, Arizona). Ciclopirox exhibits antifungal activity against Malassezia spp as well as other superficial fungi.3 An evidence-based review evaluated five double-blind, randomized, vehicle-controlled trials of ciclopirox, including dose-response evaluation.8 Twice-weekly use appears to be optimal over a usual duration of at least four weeks.3,8,15 As with ketoconazole 2% shampoo, the safety and tolerability of ciclopirox 1% shampoo is highly favorable.3,8,16
Other shampoos. Efficacy for scalp SD has been demonstrated in some studies with selenium sulfide 2.5% shampoo (Selsun, Ross Products Division, Abbott Laboratories, Columbus, Ohio) and zinc pyrithione 1% and 2% shampoo (Head & Shoulders, Procter & Gamble), with some studies demonstrating that the former is the more effective of the two agents.17 Ketoconazole 2% shampoo appears to have a greater prophylactic effect against relapse than selenium sulfide.12,12,17 Salicyclic acid shampoos may be used for adjunctive benefit to reduce scaling; however, their efficacy has not been well studied for adult scalp SD. Tar shampoos, also not well studied for adult scalp SD, can stain blond, white, or gray hair a greenish or brown color.17 Additionally, selenium sulfide shampoo may cause residual odor, discolor hair, or create a sense of hair being more oily.17
Practical application. Ketoconazole 2% shampoo or ciclopirox 1% shampoo may be effective as monotherapy in patients with mild-to-moderate scalp SD when used twice weekly over at least four weeks duration. More frequent use is not likely to afford additional benefit in most patients. In more severe cases, the additional use of a topical corticosteroid (TCS) at bedtime, either using a solution, foam, or spray, over the first 1 to 2 weeks, is often helpful in expediting resolution of signs and symptoms, with the antifungal shampoo treatment continued over at least four weeks of use. If frequent relapse is problematic, either antifungal shampoo may be used long term once or twice a week.
Nonsteroidal “Leave on” Formulations for Scalp Seborrheic Dermatitis
Ketoconazole 2% foam (Extina, Stiefel/Glaxo SmithKline). An alcohol-based “leave on” foam formulation of ketoconazole 2% (Extina) is also available for scalp SD. However, especially in more refractory cases, quicker and greater benefit is more likely with use of a mid-to-high-potency TCS over a duration of 1 to 2 weeks.
Nonsteroidal “Leave on” Formulations for Non-Scalp Seborrheic Dermatitis
Although several formulations have been evaluated for non-scalp SD, the predominant nonsteroidal topical agents that have been used in the United States are ketoconazole 2% cream and ciclopirox 1% cream.3, 8 Topical calcineurin inhibitor therapy (e.g., pimecrolimus) has also been evaluated and is sometimes used, especially in patients with frequent relapse.8 More recently, a nonsteroidal “device” cream has been approved in the United States and has been shown to be effective and safe.18
Antifungal agents. Ketoconazole 2% cream (Nizoral, Kuric, JSJ Pharmaceuticals)/2% gel (Xolegel, Aqua Pharmaceuticals, LLC, West Chester, Pennsylvania). Application of ketoconazole 2% cream twice daily over four weeks has been shown to improve SD, including for glabrous skin of the face and chest; however, the onset response is often slower than with a TCS.3, 8 Other formulations of ketoconazole 2%, such as a gel or foam, may provide an advantage in some patients, such as for body sites with hair present (e.g., central chest of male individuals, groin, axillae).
Ciclopirox 1% cream (Loprox). In a double-blind, vehicle-controlled trial of subjects with facial SD (N=129), ciclopirox 1% cream twice daily was significantly superior to vehicle cream after 28 days (P<0.01), followed by successful maintenance control over the next 28 days with once-daily application.19
Other antifungals. Data are also available with the use of topical miconazole 2% cream (Micatin, WellSpring Pharmaceutical Corporation, Sarasota, Florida; Monistat, McNeil-PPC), terbinafine 1% solution (Lamisil, Novartis Pharmaceuticals Corporation, East Hanover, New Jersey), and terbinafine 1% cream (Lamisil). Data on these other agents are limited.3, 8
Topical calcineurin inhibitors. The topical calcineurin inhibitors (TCIs), pimecrolimus 1% cream (Elidel, Novartis Pharmaceuticals Corporation) and tacrolimus 0.03% and 0.1% ointment (Protopic, Astellas Pharma US, Inc., Deerfield, Illinois), are approved in the United States for the treatment of active lesions of atopic dermatitis in patients over two years of age. These agents decrease cutaneous inflammation in atopic dermatitis by inhibiting T lymphocyte cytokine production.20 Due to their anti-inflammatory effects, and the absence of side effects that are associated with more prolonged application of TCS agents, especially on the face, TCIs have been studied for the treatment of facial SD.20 Likely due to the greater cosmetic acceptability of a cream versus an ointment, especially on facial skin, the majority of studies in SD have been completed with pimecrolimus 1% cream.8,21–27 The frequency of application for pimecrolimus 1% cream is twice daily.
Pimecrolimus 1% cream. In a double-blind, randomized, vehicle-controlled, four-week study of primarily adult male patients (mean age 59.6 years) with moderate-to-severe facial SD, pimecrolimus 1% cream (n=47) was compared with vehicle cream (n=49).21 The study results suggested that pimecrolimus 1% cream is effective for facial SD, proving superiority to vehicle as early as two weeks (P=0.0062) with good tolerability in both study arms. Several other reports, including open-phase trials and case report series, all completed over durations of 4 to 16 weeks, have demonstrated that pimecrolimus 1% cream is efficacious for SD, primarily of the facial region.22–27 Reports demonstrating positive therapeutic outcomes have also included patients not fully responsive to TCS, in Korean patients with facial SD (N=20), and in African Americans with SD associated with hypopigmentation (N=5).22–27 Some authors have noted that marked improvement occurs within the first two weeks of use.21, 27 Overall, pimecrolimus 1% cream is well tolerated. However, rosaceiform dermatitis and rosacea-like demodicidosis have been reported with its use, including for facial SD.28, 29 In addition, as SD is an off-label use for TCIs, it is important to recognize that both TCIs have in their US Food and Drug Administration (FDA)-approved labeling a “black box warning” related to potential concerns regarding malignancy association based on animal data. It appears that based on current knowledge, there is a lack of scientific evidence correlating TCI use and increased risk of malignancy.30
Other nonsteroidal topical formulations. Nonsteroidal topical device cream (Promiseb, Promius Pharma, LLC, Bridgewater, New Jersey). A water-based, fragrance-free, nonsteroidal topical device (NSTD) cream, approved in the United States by the FDA as a “medical device,” is indicated “to manage and relieve the signs and symptoms of seborrhea and SD, such as itching, erythema, scaling, and pain.”18 Application is 2 to 3 times a day to areas affected by SD. As a topical “medical device,” this formulation is not able to claim any individual active ingredient(s). However, some ingredients that may contribute to improvement in SD after application of this NSTD cream include the biocide piroctone olamine, multiple antioxidants (e.g., telmesteine, tocopheryl acetate, ascorbyl tetraiso-palmitate), multiple emollients and skin conditioning agents (e.g., ethylhexyl palmitate, bisabolol, shea butter, Vitis vinifera), and alglycera, which contains allantoin and glycyrrhetinic acid, the latter reported to exhibit anti-inflammatory activity.18 Two studies evaluated the antifungal activity of the NSTD cream, one completed in an animal model and another on the chest region in human subjects.31, 32 In the guinea pig model, once-daily topical application for three days of either the NSTD cream or ciclopirox cream reduced M furfur counts to below the limit of quantification.31 Evaluation of colony-forming units (CFUs) of Malassezia spp using tape stripping after twice-daily application of NSTD cream for seven days to one side of the chest was compared to results from the untreated side in healthy human volunteers (N=10).32 At the end of the study, the percentage reduction in CFUs of Malassezia spp was 94 percent on the treated side versus 49 percent on the untreated side (P=0.03). These studies suggest that the NSTD cream may be effective for SD, at least partially due to reduction in Malassezia spp, with this antifungal effect likely related to the presence of piroctone olamine.
A randomized, investigator-blinded, parallel-group, multicenter, pilot study was completed to compare the safety and efficacy of the NSTD cream (n=38) twice daily versus desonide cream 0.05% (n=39) twice daily for mild-to-moderate facial SD in adults, with a treatment duration of at least 14 days and a maximum of up to 28 days.33 Both treatments showed marked improvement in signs and symptoms of facial SD after 14 days and after 28 days of treatment. The percentage of subjects achieving “clear” or “almost clear” based on Investigator Global Asessment (IGA) over the course of the study was 92 percent in the desonide study arm and 85 percent in the NSTD cream arm. Although the number of patients rated as “clear” by IGA at Day 14 was greater in the desonide-treated group (39%) than in the NSTD cream-treated group (20%), 71.4 percent of the subjects who were clear at Day 14 in the NSTD cream arm remained clear at Day 28 as compared to 14.3 percent in the desonide arm (P=0.0173).33 Although the number of patients studied was small, this pilot study demonstrated that response may be faster in onset with the TCS within the first two weeks, but with more prolonged application the efficacy of both therapies was comparable. In addition, once clearing occurs, relapse may be more common and occur sooner with the TCS, although larger studies with longer duration of follow up are needed to further address timing and rate of relapse with different therapeutic agents.
Other nonsteroidal formulations. Both topical metronidazole (0.75% gel, 1% gel) and azelaic 15% gel have been shown to be effective in some patients with facial SD and may serve as monotherapy in patients with both papulopustular rosacea and mild-to-moderate facial SD.8,34–36 Lithium succinate 8%/zinc sulfate 0.05% ointment has also been shown to be effective for SD.3, 8
Practical application. For the treatment of mild-to-moderate, adult, non-scalp SD, especially on facial skin, many patients respond favorably to twice-daily application of a variety of nonsteroidal therapies, including ketoconazole 2% cream, ciclopirox 1% cream, pimecrolimus 1% cream, or NSTD cream. If associated symptoms are mild, many patients will exhibit improvement in signs and symptoms of SD within 1 to 4 weeks. With the exception of pimecrolimus 1% cream, these agents may be continued long term to prevent relapse without fear of adverse sequelae. It is possible that once-daily use of either of the antifungal agents will be adequate to prevent relapse of facial SD in many patients. All four of these agents may be used intermittently to control milder flares with reasonable rapidity. However, in cases that are more brisk with moderate-to-severe involvement, and/or are associated with moderate-to-severe symptoms, a short course of a TCS used over 1 to 2 weeks once or twice day (depending on potency), and used in combination with a nonsteroidal agent, is rational. Once the SD is “cooled down” to a milder state or is cleared, which usually occurs within a few to several days, the TCS can be stopped abruptly or tapered off in frequency over the following 1 to 2 weeks, with the nonsteroidal agent continued for at least a few more weeks to prevent relapse. There is no one way to approach SD. The clinician may modulate his or her approach to facial SD management in the individual patient based on disease severity, response to treatment, and tendency for relapse.
Topical Corticosteroid Therapy for Scalp and Non-Scalp Seborrheic Dermatitis
Review of adult SD treatments revealed that there has been a relative conspicuous absence of recent published data on the use of TCS for SD, especially when compared to the plethora of studies on the use of TCS for psoriasis.3,8,37 Nevertheless, TCS are considered to be first-line or second-line agents for SD, depending upon the severity of disease.
In many cases a low to low-mid potency TCS is effective in rapidly clearing visible signs and associated symptoms. For scalp SD, many cases are associated with greater severity of pruritus than with facial SD, warranting the need for initial treatment over the first week or two with a higher potency TCS, followed by tapering in frequency over the next 1 to 2 weeks. It has been noted that relapse of SD occurs sooner and more frequently with use of TCS as compared to antifungal agents and other nonsteroidal topical therapies.3,8,33 As TCS are available in a variety of vehicle formulations, the clinician may select the potency and vehicle of the TCS based on the severity and sites of involvement. In most cases, TCS use will likely be limited to a maximum of 1 to 4 weeks, as response is usually rapid. Tapering of frequency as opposed to abrupt discontinuation, although not formally studied, may potentially reduce the risk of relapse that has been observed with TCS use for SD.
Practical application. The use of TCS for adult SD, regardless of site or severity, is to achieve the most rapid control of signs and symptoms. Therefore, a TCS, usually of low to low-mid potency may be used for 1 to 2 weeks, and sometimes less. For adult scalp SD, higher potency agents are sometimes needed especially for diffuse involvement, and or moderate to severe symptoms such as pruritus, stinging, and/or burning. It is the preference of this author to use a low to mid-potency agent for 1 to 2 weeks on the face or other glabrous skin sites in adults, with tapering to every other day over an additional 1 to 2 weeks. This anecdotally appears to reduce relapse, which, when it occurs soon after discontinuation of TCS use, is really a form of rebound. Use of a vehicle that is nonirritating, and hopefully hydrating, such as moisturizing hydrogel, a lipocream, or emollient cream, is often helpful. It is beneficial, especially in adult patients prone to frequent recurrences of facial SD, to combine the short-term TCS use with concomitant topical nonsteroidal therapy, the latter continued over a more prolonged duration to sustain therapeutic benefit and prevent relapse. For adult scalp SD, especially moderate to severe, use of a mid-to-high-potency TCS solution, foam, or spray often controls the disorder within 1 to 2 weeks, and sometimes longer. This therapy may be tapered in a manner similar to what has been described above for facial SD. Use of ketoconazole 2% shampoo or ciclopirox 1% shampoo twice weekly can be used concomitantly with a TCS for adult scalp SD, and continued after the TCS is stopped. It is important to respect that prolonged used of TCS may cause adverse reactions such as atrophy, striae, and telangiectasis.8, 37
Conclusion
This article reviews several therapies for adult SD of the scalp and non-scalp, especially the face. Practical applications based on available data are provided to assist the clinician in modulating and combining therapeutic approaches. As SD is a chronic disease, optimal results depend on our ability to combine our best evidence based on data, clinical experience, and the “art” of adjusting therapy based on the needs of the individual case.
References
- 1.Erchiga VC, Martos OJ, Cassano AV, et al. Malassezia globosa as the causative organism of pityriasis versicolor. Br J Dermatol. 2000;143:799–803. doi: 10.1046/j.1365-2133.2000.03779.x. [DOI] [PubMed] [Google Scholar]
- 2.Fritsch PO, Reider N. Other eczematous dermatoses. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. Vol. 1. New York: Mosby; 2003. pp. 215–218. [Google Scholar]
- 3.Gupta AK, Bluhm R, Cooper EA, et al. Seborrheic dermatitis. Dermatol Clin. 2003;21:401–412. doi: 10.1016/s0733-8635(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 4.Lynch PJ. Dermatologic problems of the head and neck. Otolaryngol Clin North Am. 1982;15:271–285. [PubMed] [Google Scholar]
- 5.Smith KS, Skelton HG, Yeager J, et al. Cutaneous findings in HIV-1 positive patients: a 42-month prospective study. J Am Acad Dermatol. 1994;31:746–754. doi: 10.1016/s0190-9622(94)70236-5. [DOI] [PubMed] [Google Scholar]
- 6.Schaub NA, Drewe J, Sponagel L, et al. Is there a relationship between risk groups or initial CD4 T cell counts and prevalence of seborrheic dermatitis in HIV-infected patients? Dermatology. 1999;198:126–129. doi: 10.1159/000018087. [DOI] [PubMed] [Google Scholar]
- 7.Oble DA, Collett E, Hsieh M, et al. A novel T cell receptor transgenic animal model of seborrheic dermatitis-like skin disease. J Invest Dermatol. 2005;124:151–159. doi: 10.1111/j.0022-202X.2004.23565.x. [DOI] [PubMed] [Google Scholar]
- 8.Picardo M, Cameli N. Seborrheic dermatitis. In: Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B, editors. Evidence-Based Dermatology. Second Edition. Malden, Massachusetts: Blackwell Publishing; 2008. pp. 164–170. [Google Scholar]
- 9.Gupta AK, Kohli Y, Summerbell RC, et al. Quantitative culture of Malassezia species from different body sites of individuals with and without seborrheic dermatitis. Med Mycol. 2001;11:1–9. doi: 10.1080/mmy.39.3.243.251. [DOI] [PubMed] [Google Scholar]
- 10.Ashbee HR, Ingham E, Holland KT, et al. The carriage of Malassezia furfur serovars A, B and C in patients with pityriasis versicolor, seborrheic dermatitis, and controls. Br J Dermatol. 1993;129:533–540. doi: 10.1111/j.1365-2133.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 11.Faergemann J, Bergbrant IM, Dohse M. Seborrhoeic dermatitis and Pityrosporum (Malassezia) folliculitis: characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol. 2001;144:549–556. doi: 10.1046/j.1365-2133.2001.04082.x. [DOI] [PubMed] [Google Scholar]
- 12.Pierard-Franchimont C, Pierard GE, Arrese JE, et al. Effect of ketoconazole 1% and 2% shampoos on severe dandruff and seborrheic dermatitis: clinical, squamometric, and mycologic assessments. Dermatology. 2001;202:171–176. doi: 10.1159/000051628. [DOI] [PubMed] [Google Scholar]
- 13.Danby FW, Maddin WS, Margesson LJ, et al. A randomized, double-blind, placebo-controlled trial of ketoconazole 2% shampoo versus selenium sulfide 2.5% shampoo in the treatment of moderate to severe dandruff. J Am Acad Dermatol. 1993;29:1008–1012. doi: 10.1016/0190-9622(93)70282-x. [DOI] [PubMed] [Google Scholar]
- 14.Peter RU, Richarz-Barthauer U. Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicenter, double-blind, placebo-controlled trial. Br J Dermatol. 1995;132:441–445. doi: 10.1111/j.1365-2133.1995.tb08680.x. [DOI] [PubMed] [Google Scholar]
- 15.Abeck D. Rationale of frequency of use of ciclopirox 1% shampoo in the treatment of seborrheic dermatitis: results of a double-blind, placebo-controlled study comparing the efficacy of once, twice, and three times weekly usage. Int J Dermatol. 2004;43(Suppl 1):13–16. doi: 10.1111/j.1461-1244.2004.02382.x. [DOI] [PubMed] [Google Scholar]
- 16.Lebwohl M, Plott T. Safety and efficacy of ciclopirox 1% shampoo for the treatment of seborrheic dermatitis of the scalp in the US population: results of a double-blind, vehicle-controlled trial. Int J Dermatol. 2004;43(Suppl 1):17–20. doi: 10.1111/j.1461-1244.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 17.Brodell RT, Cooper KD. Therapeutic shampoos. In: Wolverton SE, editor. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadephia: Saunders Elsevier; 2007. pp. 719–729. [Google Scholar]
- 18.Promiseb Topical Cream [package insert] NJ: Promius Pharma LLC; 2009. Bridgewater. [Google Scholar]
- 19.Dupuy P, Maurette C, Amoric JC, et al. Randomized, placebo-controlled, double-blind study on clinical efficacy of ciclopiroxolamine 1% cream in facial seborrhoeic dermatitis. Br J Dermatol. 2001;144:1033–1037. doi: 10.1046/j.1365-2133.2001.04194.x. [DOI] [PubMed] [Google Scholar]
- 20.Cook BA, Warshaw EM. Role of topical calcineurin inhibitors in the treatment of seborrheic dermatitis: a review of pathophysiology, safety, and efficacy. Am J Clin Dermatol. 2009;10:103–118. doi: 10.2165/00128071-200910020-00003. [DOI] [PubMed] [Google Scholar]
- 21.Warshaw EM, Wohlhuter RJ, Liu A, et al. Results of a randomized, double-blind, vehicle-controlled efficacy trial of pimecrolimus cream 1% for the treatment of moderate to severe facial seborrheic dermatitis. J Am Acad Dermatol. 2007;57:257–264. doi: 10.1016/j.jaad.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Kim BS, Kim SH, Kim MB, et al. Treatment of seborrheic dermatitis with pimecrolimus cream 1%: an open-label clinical study in Korean patients. J Korean Med Sci. 2007;22:868–872. doi: 10.3346/jkms.2007.22.5.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koc E, Arca E, Kose O, et al. An open, randomized, prospective, comparative study of topical pimecrolimus 1% cream and topical ketoconazole 2% cream in the treatment of seborrheic dermatitis. J Dermatol Treat. 2009;20:4–9. doi: 10.1080/09546630802286993. [DOI] [PubMed] [Google Scholar]
- 24.Ozden MG, Tekin NS, Ilter N, et al. Topical pimecrolimus 1% cream for resistant seborrheic dermatitis of the face: an open label study. Am J Clin Dermatol. 2010;11:51–54. doi: 10.2165/11311160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Cunha PR. Pimecrolimus cream 1% is effective in seborrheic dermatitis refractory to topical corticosteroids. Acta Derm Venereol. 2006;86:69–70. doi: 10.1080/00015550510040040. [DOI] [PubMed] [Google Scholar]
- 26.Cicek D, Kandi B, Bakar S, Turgut D. Pimecrolimus 1% cream, methylprednisolone aceponate 0.1% cream, and metronidaziole 0.75% gel in the treatment of seborrhoeic dermatitis: a randomized clinical study. J Dermatol Treat. 2009;1:1–6. doi: 10.3109/09546630802687349. [DOI] [PubMed] [Google Scholar]
- 27.High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083–1088. doi: 10.1016/j.jaad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Gorman CR, White SW. Rosaceiform dermatitis as a complication of treatment of facial seborrheic dermatitis with 1% pimecrolimus cream. Arch Dermatol. 2005;141:1168. doi: 10.1001/archderm.141.9.1168. [DOI] [PubMed] [Google Scholar]
- 29.Yoon TY, Kim HJ, Kim MK. Pimecrolimus-induced rosacea-like demodicidosis. Int J Dermatol. 2007;46:1103–1105. doi: 10.1111/j.1365-4632.2007.03325.x. [DOI] [PubMed] [Google Scholar]
- 30.Thaci D, Salgo R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: facts and controversies. Clin Dermatol. 2010;28:52–56. doi: 10.1016/j.clindermatol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Nalamothu V, O'Leary AL, Kandavilli S, et al. Evaluation of a nonsteroidal topical cream in a guinea pig model of Malassezia furfur infection. Clinics Dermatol. 2009;27:S3–S5. doi: 10.1016/j.clindermatol.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Kircik L. An open-label, single-center pilot study to determine the antifungal activity of a new nonsteroidal cream (Promiseb Topical Cream) after 7 days of use in healthy volunteers. Clinics Dermatol. 2009;27:S6–S9. doi: 10.1016/j.clindermatol.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Elewski B. An investigator-blinded, randomized, 4-week, parallel-group, multicenter pilot study to compare the safety and efficacy of a nonsteroidal cream (Promiseb Topical Cream) and desonide cream 0.05% in the twice-daily treatment of mild to moderate seborrheic dermatitis of the face. Clinics Dermatol. 2009;27:S10–S15. doi: 10.1016/j.clindermatol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Zip CM. Innovative use of topical metronidazole. Dermatol Clin. 2010;28:525–534. doi: 10.1016/j.det.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Bikowski J. Facial seborrheic dermatitis: a report on current status and therapeutic horizons. J Drugs Dermatol. 2009;8:125–133. [PubMed] [Google Scholar]
- 36.McFalda WL, Roebuck HL. Rational management of papulopustular rosacea with concomitant facial seborrheic dermatitis: a case report. J Clin Aesthet Dermatol. 2011;4:40–42. [PMC free article] [PubMed] [Google Scholar]
- 37.Lebwohl MG, Heymann WR, Berth-Jones J, Coulson . In: Seborrheic eczema. Treatment of Skin Disease: Comprehensive Treatment Strategies. 3rd ed. Williams J, Coulson I, editors; Philadelphia: Saunders Elsevier; 2010. pp. 694–696. [Google Scholar]