Abstract
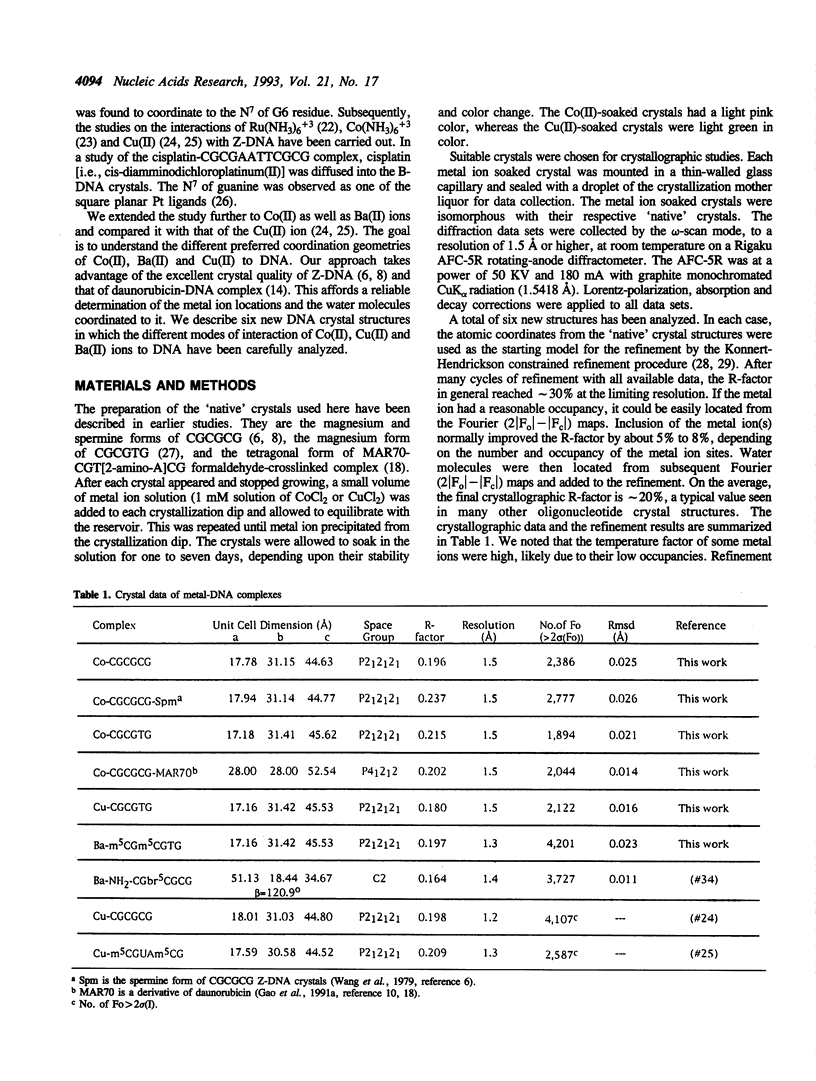
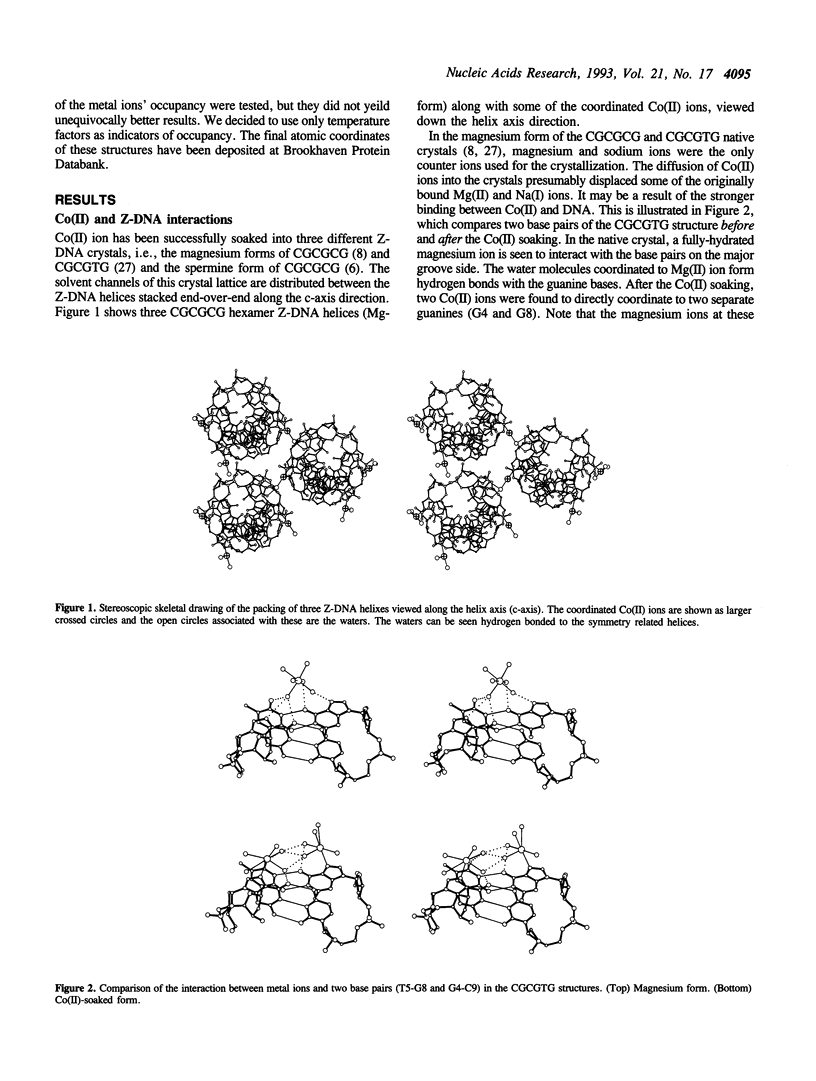
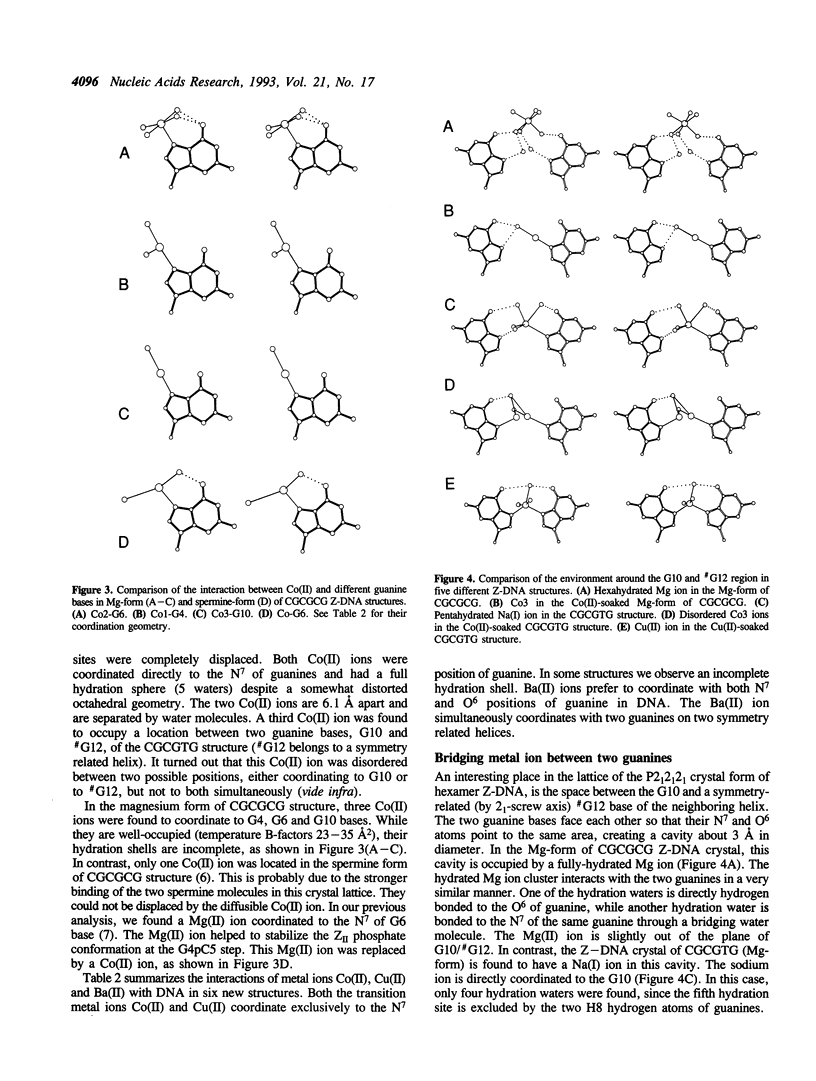
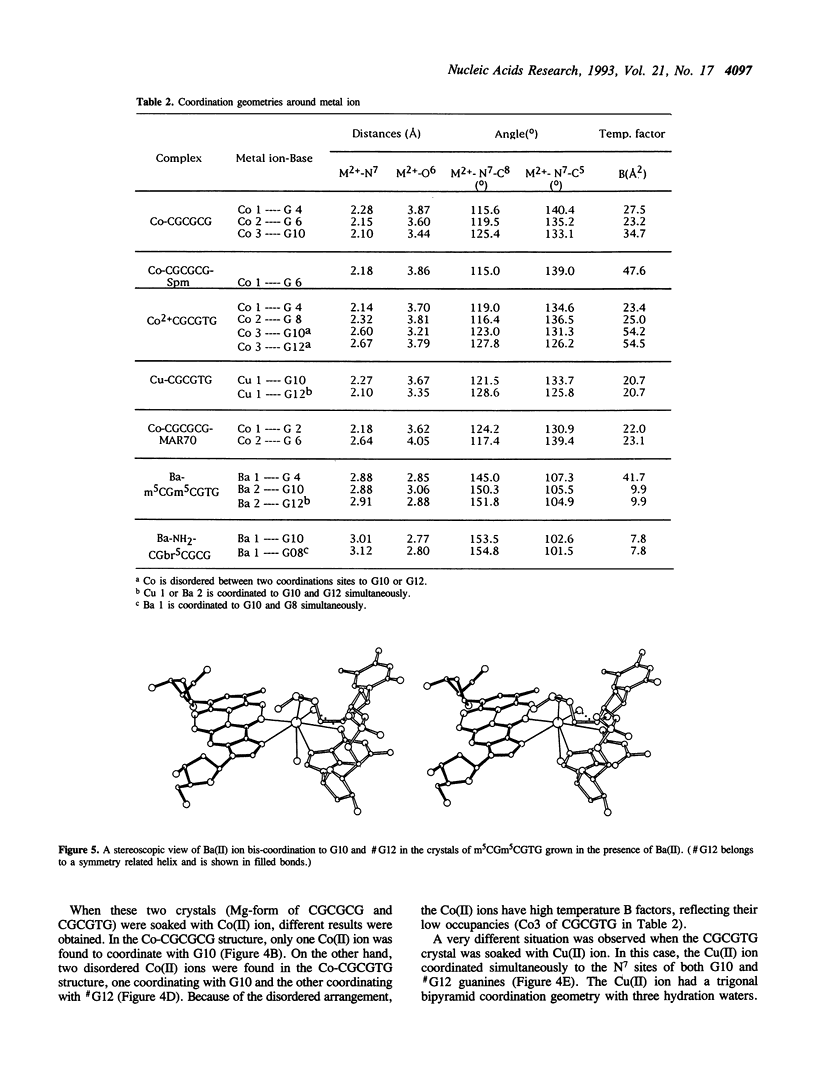
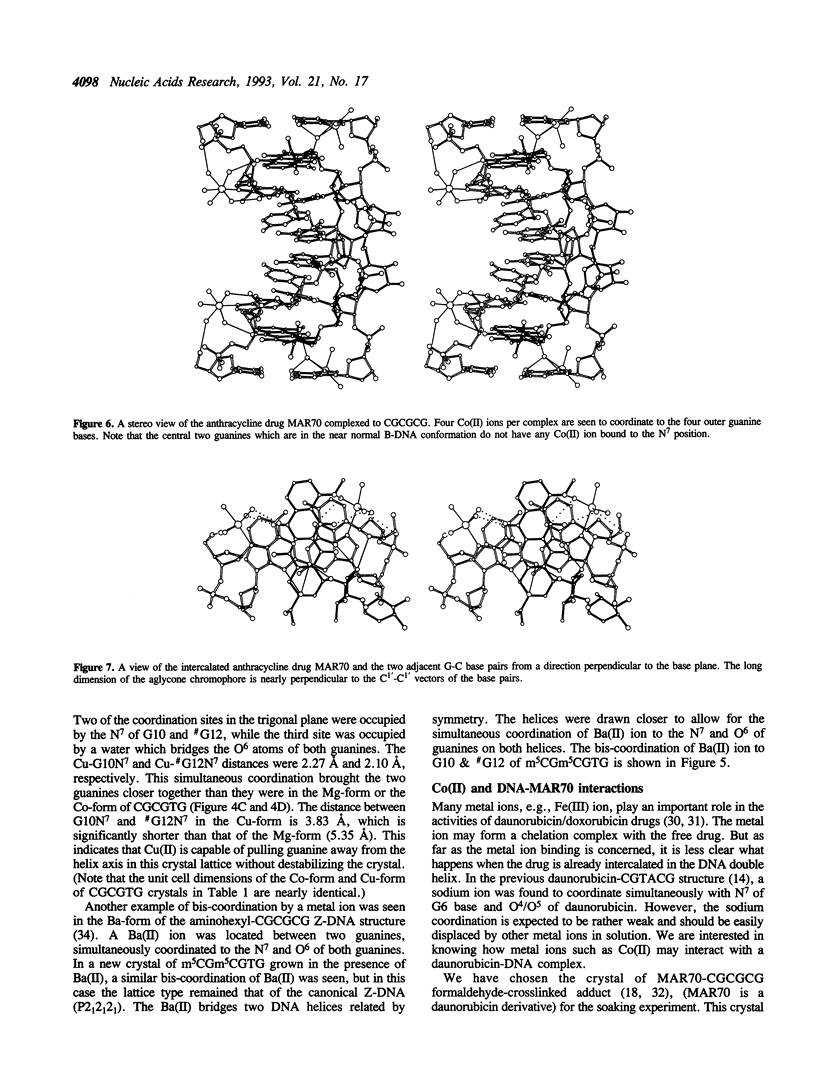
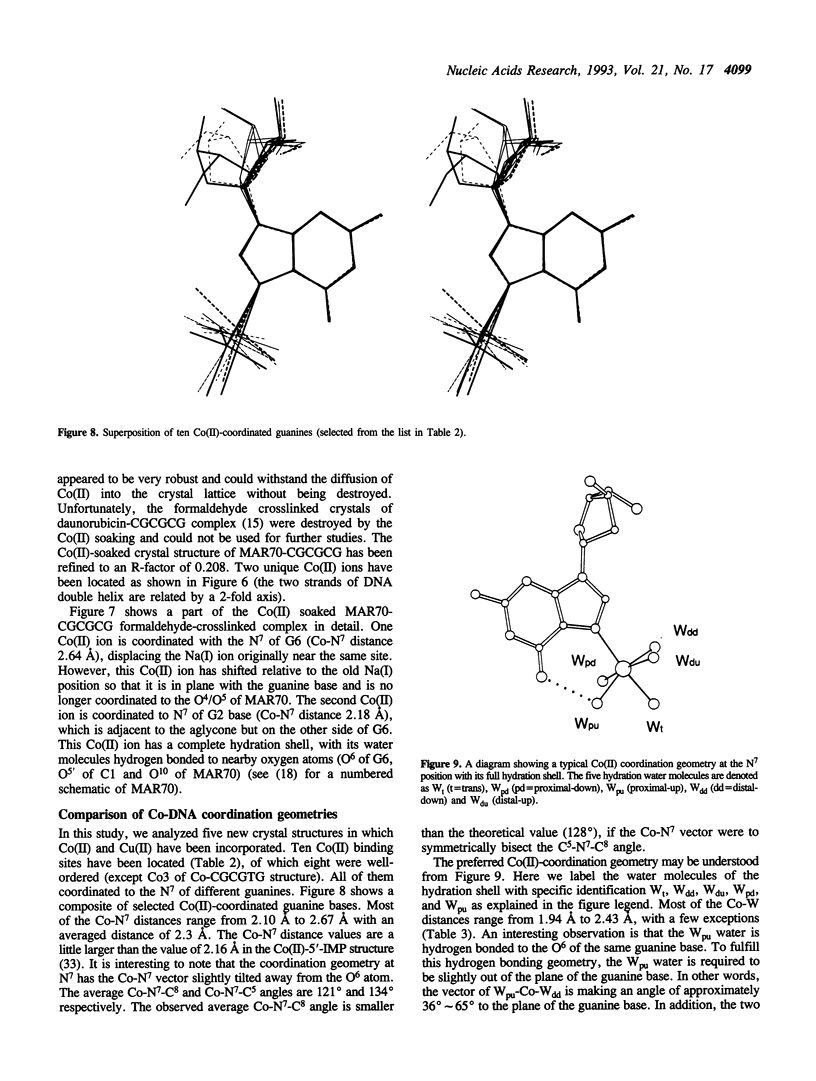
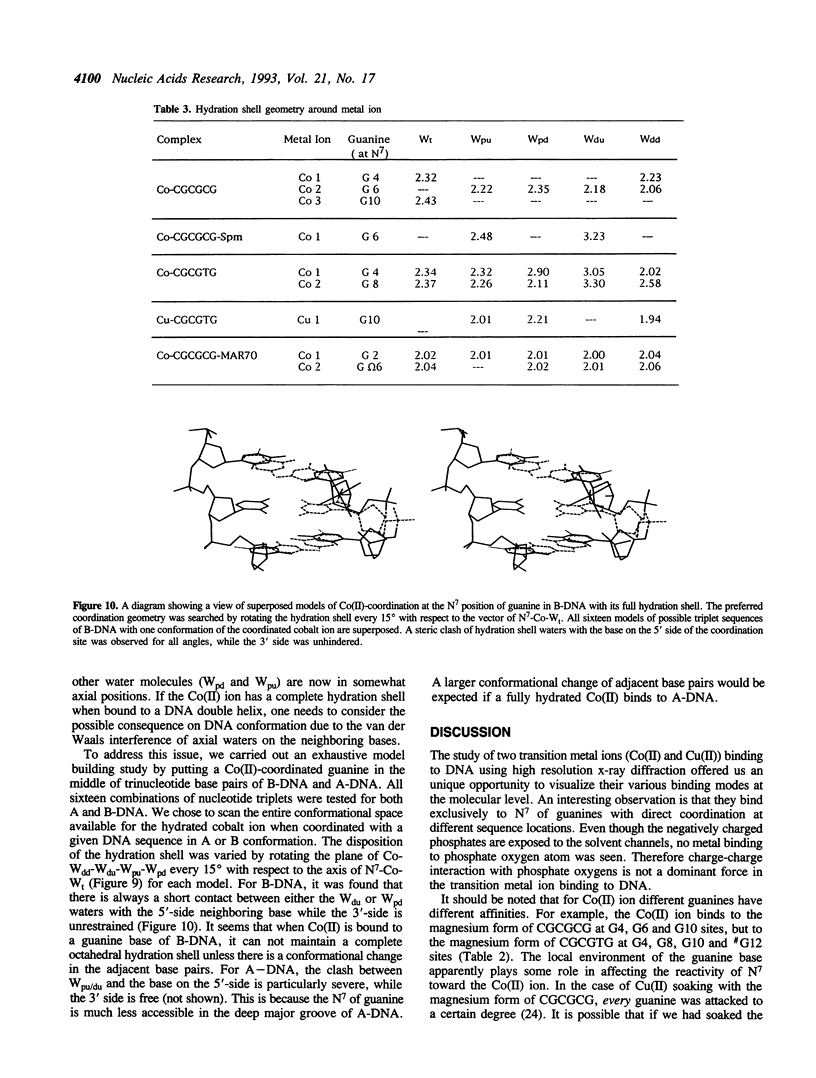
Metal ion coordination to nucleic acids is not only required for charge neutralization, it is also essential for the biological function of nucleic acids. The structural impact of different metal ion coordinations of DNA helices is an open question. We carried out X-ray diffraction analyses of the interactions of the two transition metal ions Co(II) and Cu(II) and an alkaline earth metal ion Ba(II), with DNA of different conformations. In crystals, Co(II) ion binds exclusively at the N7 position of guanine bases by direct coordination. The coordination geometry around Co(II) is octahedral, although some sites have an incomplete hydration shell. The averaged Co-N7 bond distance is 2.3 A. The averaged Co-N7-C8 angle is 121 degrees, significantly smaller than the value of 128 degrees if the Co-N7 vector were to bisect the C5-N7-C8 bond angle. Model building of Co(II) binding to guanine N7 in B-DNA indicates that the coordinated waters in the axial positions would have a van der Waals clash with the neighboring base on the 5' side. In contrast, the major groove of A-DNA does not have enough room to accommodate the entire hydration shell. This suggests that Co(II) binding to either B-DNA or A-DNA may induce significant conformational changes. The Z-DNA structure of Cu(II)-soaked CGCGTG crystal revealed that the Cu(II) ion is bis-coordinated to N7 position of G10 and #G12 (# denotes a symmetry-related position) bases with a trigonal bipyramid geometry, suggesting a possible N7-Cu-N7 crosslinking mechanism. A similar bis-coordination to two guanines has also been seen in the interaction of Cu(II) in m5CGUAm5CG Z-DNA crystal and of Ba(II) with two other Z-DNA crystals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behlen L. S., Sampson J. R., DiRenzo A. B., Uhlenbeck O. C. Lead-catalyzed cleavage of yeast tRNAPhe mutants. Biochemistry. 1990 Mar 13;29(10):2515–2523. doi: 10.1021/bi00462a013. [DOI] [PubMed] [Google Scholar]
- Eliot H., Gianni L., Myers C. Oxidative destruction of DNA by the adriamycin-iron complex. Biochemistry. 1984 Feb 28;23(5):928–936. doi: 10.1021/bi00300a021. [DOI] [PubMed] [Google Scholar]
- Frederick C. A., Coll M., van der Marel G. A., van Boom J. H., Wang A. H. Molecular structure of cyclic deoxydiadenylic acid at atomic resolution. Biochemistry. 1988 Nov 1;27(22):8350–8361. doi: 10.1021/bi00422a010. [DOI] [PubMed] [Google Scholar]
- Gao Y. G., Liaw Y. C., Li Y. K., van der Marel G. A., van Boom J. H., Wang A. H. Facile formation of a crosslinked adduct between DNA and the daunorubicin derivative MAR70 mediated by formaldehyde: molecular structure of the MAR70-d(CGTnACG) covalent adduct. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4845–4849. doi: 10.1073/pnas.88.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. G., van der Marel G. A., van Boom J. H., Wang A. H. Molecular structure of a DNA decamber containing an anticancer nucleoside arabinosylcytosine: conformational perturbation by arabinosylcytosine in B-DNA. Biochemistry. 1991 Oct 15;30(41):9922–9931. doi: 10.1021/bi00105a016. [DOI] [PubMed] [Google Scholar]
- Geierstanger B. H., Kagawa T. F., Chen S. L., Quigley G. J., Ho P. S. Base-specific binding of copper(II) to Z-DNA. The 1.3-A single crystal structure of d(m5CGUAm5CG) in the presence of CuCl2. J Biol Chem. 1991 Oct 25;266(30):20185–20191. doi: 10.2210/pdb1d40/pdb. [DOI] [PubMed] [Google Scholar]
- Gessner R. V., Frederick C. A., Quigley G. J., Rich A., Wang A. H. The molecular structure of the left-handed Z-DNA double helix at 1.0-A atomic resolution. Geometry, conformation, and ionic interactions of d(CGCGCG). J Biol Chem. 1989 May 15;264(14):7921–7935. doi: 10.2210/pdb1dcg/pdb. [DOI] [PubMed] [Google Scholar]
- Gessner R. V., Quigley G. J., Wang A. H., van der Marel G. A., van Boom J. H., Rich A. Structural basis for stabilization of Z-DNA by cobalt hexaammine and magnesium cations. Biochemistry. 1985 Jan 15;24(2):237–240. doi: 10.1021/bi00323a001. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Hahn M. C-C-A-G-G-C-m5C-T-G-G. Helical fine structure, hydration, and comparison with C-C-A-G-G-C-C-T-G-G. J Biol Chem. 1992 Apr 15;267(11):7332–7341. [PubMed] [Google Scholar]
- Ho P. S., Frederick C. A., Quigley G. J., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. G.T wobble base-pairing in Z-DNA at 1.0 A atomic resolution: the crystal structure of d(CGCGTG). EMBO J. 1985 Dec 16;4(13A):3617–3623. doi: 10.1002/j.1460-2075.1985.tb04125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. S., Frederick C. A., Saal D., Wang A. H., Rich A. The interactions of ruthenium hexaammine with Z-DNA: crystal structure of a Ru(NH3)6+3 salt of d(CGCGCG) at 1.2 A resolution. J Biomol Struct Dyn. 1987 Feb;4(4):521–534. doi: 10.1080/07391102.1987.10507657. [DOI] [PubMed] [Google Scholar]
- Holbrook S. R., Sussman J. L., Warrant R. W., Church G. M., Kim S. H. RNA-ligant interactions. (I) Magnesium binding sites in yeast tRNAPhe. Nucleic Acids Res. 1977 Aug;4(8):2811–2820. doi: 10.1093/nar/4.8.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Rhodes D., Brown R. S., Klug A. A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J Mol Biol. 1977 Apr 15;111(3):315–328. doi: 10.1016/s0022-2836(77)80054-5. [DOI] [PubMed] [Google Scholar]
- Jean Y. C., Gao Y. G., Wang A. H. Z-DNA structure of a modified DNA hexamer at 1.4-A resolution: aminohexyl-5'-d(pCpGp[br5C]pGpCpG). Biochemistry. 1993 Jan 12;32(1):381–388. doi: 10.1021/bi00052a047. [DOI] [PubMed] [Google Scholar]
- Jia X., Marzilli L. G. Zinc ion-DNA polymer interactions. Biopolymers. 1991 Jan;31(1):23–44. doi: 10.1002/bip.360310104. [DOI] [PubMed] [Google Scholar]
- Kagawa T. F., Geierstanger B. H., Wang A. H., Ho P. S. Covalent modification of guanine bases in double-stranded DNA. The 1.2-A Z-DNA structure of d(CGCGCG) in the presence of CuCl2. J Biol Chem. 1991 Oct 25;266(30):20175–20184. doi: 10.2210/pdb1d39/pdb. [DOI] [PubMed] [Google Scholar]
- Liaw Y. C., Gao Y. G., Robinson H., Sheldrick G. M., Sliedregt L. A., van der Marel G. A., van Boom J. H., Wang A. H. Cyclic diguanylic acid behaves as a host molecule for planar intercalators. FEBS Lett. 1990 May 21;264(2):223–227. doi: 10.1016/0014-5793(90)80253-f. [DOI] [PubMed] [Google Scholar]
- Muindi J. R., Sinha B. K., Gianni L., Myers C. E. Hydroxyl radical production and DNA damage induced by anthracycline-iron complex. FEBS Lett. 1984 Jul 9;172(2):226–230. doi: 10.1016/0014-5793(84)81130-8. [DOI] [PubMed] [Google Scholar]
- Piccirilli J. A., Vyle J. S., Caruthers M. H., Cech T. R. Metal ion catalysis in the Tetrahymena ribozyme reaction. Nature. 1993 Jan 7;361(6407):85–88. doi: 10.1038/361085a0. [DOI] [PubMed] [Google Scholar]
- Privé G. G., Heinemann U., Chandrasegaran S., Kan L. S., Kopka M. L., Dickerson R. E. Helix geometry, hydration, and G.A mismatch in a B-DNA decamer. Science. 1987 Oct 23;238(4826):498–504. doi: 10.1126/science.3310237. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan V., Sundaralingam M. The crystal structures of metal complexes of nucleic acids and their constituents. CRC Crit Rev Biochem. 1979;6(3):245–336. doi: 10.3109/10409237909102565. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Gao Y. G., Liaw Y. C., Li Y. K. Formaldehyde cross-links daunorubicin and DNA efficiently: HPLC and X-ray diffraction studies. Biochemistry. 1991 Apr 23;30(16):3812–3815. doi: 10.1021/bi00230a002. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Rich A. Interactions between an anthracycline antibiotic and DNA: molecular structure of daunomycin complexed to d(CpGpTpApCpG) at 1.2-A resolution. Biochemistry. 1987 Feb 24;26(4):1152–1163. doi: 10.1021/bi00378a025. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Westhof E., Dumas P., Moras D. Crystallographic refinement of yeast aspartic acid transfer RNA. J Mol Biol. 1985 Jul 5;184(1):119–145. doi: 10.1016/0022-2836(85)90048-8. [DOI] [PubMed] [Google Scholar]
- Wing R. M., Pjura P., Drew H. R., Dickerson R. E. The primary mode of binding of cisplatin to a B-DNA dodecamer: C-G-C-G-A-A-T-T-C-G-C-G. EMBO J. 1984 May;3(5):1201–1206. doi: 10.1002/j.1460-2075.1984.tb01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi K., Privé G. G., Dickerson R. E. Analysis of local helix geometry in three B-DNA decamers and eight dodecamers. J Mol Biol. 1991 Jan 5;217(1):201–214. doi: 10.1016/0022-2836(91)90620-l. [DOI] [PubMed] [Google Scholar]


