Abstract
The mode by which Helicobacter pylori, the causative agent of most gastric ulcers, is transmitted remains undetermined. Epidemiological evidence suggests these organisms are waterborne; however, H. pylori has rarely been grown from potential water sources. This may be due to the ability of this organism to rapidly enter the viable but nonculturable (VBNC) state. Our investigation examines the entrance of H. pylori into this state in laboratory cultures and a natural freshwater environment as well as the relationship between morphology and culturability. To this end, membrane diffusion chambers were utilized to expose the cells to the natural fluctuations of a freshwater stream. In both the laboratory and environment, samples were assayed for culturability using plate counts and stained using a LIVE/DEAD BacLight assay for viability and morphological determinations. Additionally, water samples were collected, six environmental parameters were measured, and resuscitation conditions were examined. H. pylori was observed to lose culturability in the laboratory and stream, although viability was maintained. While the results of our study agree with those of previous studies which suggested that there is a transition in morphology from rods to cocci as culturability is lost, the morphological distribution of cells did not change as culturability was lost in the environment. The majority of cells in the VBNC state in the laboratory are cocci; however, all morphological forms were present in the environment. The results of these studies suggest that H. pylori persists in laboratory cultures and the environment in the VBNC state and that cells in this state represent a public health hazard.
Infection by the gram-negative microaerophilic rod Helicobacter pylori is associated with the development of chronic human gastritis, peptic ulcers, and gastric adenocarcinoma (2, 5, 26). It has been estimated that more that half of the world's population is infected with this organism (18). Despite such a high incidence of infection, the bacterium's reservoir and mode of transmission remain undetermined. Molecular methods have detected the presence of H. pylori DNA in river water, well water, and wastewater as well as in surface and shallow groundwater, suggesting that this organism is waterborne and might be transmitted by the fecal-oral route (12, 14, 22). However, only a single study has been published which claims that isolation of H. pylori directly from environmental sources had been performed and that the isolation occurred only following immunomagnetic separation of the cells from raw sewage (20).
It has been postulated that the inability to culture H. pylori from the environment is due to its entrance into the viable but nonculturable (VBNC) state. Cells that have entered this state are no longer culturable on routine bacteriological media, although they remain viable (25). Entrance of the bacterium into the VBNC state is induced by a variety of adverse conditions, such as temperature downshift or nutrient depletion (25). The entrance of H. pylori into the VBNC state was first suggested during laboratory studies by Shahamat et al. (29) in which cells were observed to become nonculturable in freshwater microcosms. Evidence of entry into the VBNC state was further supported through autoradiographical detection of metabolic activity in nonculturable cells (30).
Many bacterial species have been observed to alter their morphology as they enter the VBNC state. Vibrio vulnificus cells have been shown to transition from curved rods to cocci as they enter the VBNC state (24). Catrenich and Makin (6) as well as Benïssia et al. (3) observed H. pylori to undergo a similar morphological conversion as cells aged in a broth, with cells transitioning from spiral rods to “O” or “U” shapes and then to cocci. Simultaneously, culturability was observed to decrease. However, it is possible that the coccoid form of H. pylori is the VBNC morphology. This is supported by the finding that H. pylori cultures containing 90% coccoid cells and 10% spiral cells exhibited only a 1.8-fold decrease in respiratory activity compared to cultures containing 95% spiral cells and 5% cocci (9).
If the coccoid form of this bacterium is in the VBNC state, it may be capable of establishing infection in a host, as suggested by both Cellini et al. (7) and She et al. (31). Furthermore, mRNAs for VacA and UreA have been detected by reverse transcription-PCR in nonculturable H. pylori cells (23). These data indicate that VBNC forms of H. pylori might be infectious; therefore, the role of the VBNC state in this organism has important implications in epidemiology and disease prevention. We investigated the ability of H. pylori to enter the VBNC state in both the laboratory and a natural freshwater environment. In the latter case, environmental parameters which may influence the loss of culturability were also examined.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
For routine culture, H. pylori (ATCC 43504) in vented tissue culture flasks was grown in brucella broth (Becton Dickinson and Co., Cockeysville, Md.) containing 5% fetal calf serum (Sigma Chemical Co., St. Louis, Mo.), 10 mg of vancomycin/liter, 5 mg of trimethoprim/liter, and 2,500 IU of polymyxin B/liter. Cells were maintained on brucella blood agar containing 5% sheep erythrocytes (Carolina Biological Supply Company, Burlington, N.C.) and the same antibiotics. In all cases, cells were incubated at 37°C under conditions of 100% humidity and a 5% CO2 atmosphere. For laboratory study, H. pylori cells were grown for 18 h in broth and diluted 1:100 in fresh liquid medium. Samples were taken at intervals, and the optical density at 550 nm, culturability, viability, and morphological distribution were determined.
Membrane diffusion chambers.
Water was collected from a stream located in a small nature preserve on the University of North Carolina at Charlotte campus and filtered through a 0.2-μm-pore-size filter (Corning Costar Corp., Cambridge, Mass.) and subsequently autoclaved. Cultures were diluted into this sterile creek water such that the final concentration was ca. 105 to 106 CFU/ml. Aliquots (25 ml) were used to fill 30-ml sterile membrane diffusion chambers (21) that were equipped with 76-mm-diameter, 0.2-μm-pore-size polycarbonate filters (Osmonics, Inc., Livermore, Calif.). Chambers were suspended from a flotation device and anchored in the stream at a depth of ca. 0.3 m. Sampling was performed by removing one or two chambers at each time point and examining the cells within. In no case did the time from sample removal until assay exceed 30 min.
Culturability of cells.
Cell suspensions from the membrane diffusion chambers were serially diluted in sterile creek water and plated onto brucella blood agar. Cells were considered to be nonculturable when <10 CFU/ml were detected. Detected colonies were confirmed to be H. pylori by inoculation to Christensen urea agar slants (Food and Drug Administration bacteriological analytical manual; AOAC International, Gaithersburg, Md., 1995) and examined for urease production.
Viability and morphological determination.
Viability and morphology were determined by staining cells with a LIVE/DEAD BacLight bacterial viability kit (Molecular Probes, Eugene, Oreg.) according to the manufacturer's directions. Briefly, this kit employs SYTO 9 and propidium iodide to differentiate between cells with intact (live organisms) and compromised (dead organisms) membranes. BacLight-stained samples were filtered onto 0.2-μm-pore-size black polycarbonate filters (Osmonics, Inc.) in dim light. Cells were viewed using an epifluorescence microscope (Olympus model BX51) with the appropriate filter cube, and a minimum of 30 fields or 300 cells were counted in all cases. Images were captured using a SpotCAM camera and associated software (Diagnostic Instruments, Inc., Sterling Heights, Mich.).
Monitoring of environmental parameters.
The environmental parameters of the stream were measured every day of each study and included dissolved oxygen, pH, temperature, turbidity, and ammonia and phosphate levels (DR/850 portable datalogging colorimeter instrument manual; Hach Company, 1997). These were measured according to the manufacturer's instructions.
Resuscitation conditions.
VBNC cells removed from environmental chambers were plated on blood agar overlaid with 200 U of catalase (L. Sides, M. F. Hite, and J. D. Oliver, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. Q-129, 1999) or were heat shocked (A. R. Gupte and S. W. Joseph, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. Q-117, 2001) and plated on brucella blood agar.
Statistical analysis.
Linear regression was performed using Prism 2.01 software (GraphPad Software Inc., San Diego, Calif.) to determine statistically significant changes in the morphological distribution within individual sampling periods. Environmental parameter data were plotted and log transformed to achieve normality. A Spearman's coefficient rank correlation (32) was performed to determine whether any environmental parameters correlated with the variation in the length of time that cells were culturable in the environment. This was followed by a sequential Bonferroni adjustment (27). Additionally, a standardized multiple regression analysis (32) was performed to determine whether any environmental parameters accounted for variations in culturability of cells in the environment. Statistical tests of environmental parameters were performed using 8.2 software (SAS Institute, Inc., Cary, N.C.).
RESULTS AND DISCUSSION
Although culturability has been the focus of many investigations of H. pylori, none have examined culturability in a potential natural reservoir, such as a freshwater environment. Furthermore, no investigations have been reported that differentiate between culturability and viability in a population. Using a LIVE/DEAD BacLight viability assay, culturability and viability were examined in cells incubated in both the laboratory and a natural freshwater stream. Although the use of multiple viability assays is preferable (see, e.g., reference 19), other assays we investigated, such as the 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) assay of Rodriguez et al. (28) and the substrate responsiveness assay of Kogure et al. (16), did not perform well when applied to H. pylori. However, Boulos et al. (4) reported BacLight viable counts to be comparable to those of CTC assays for bacteria present in drinking water. However, viable counts determined using the BacLight stain in chlorine-stressed cells were reported to be higher than those seen with CTC assays. The authors do acknowledge that the CTC-formazan granules were very small in stressed cells, suggesting that this accounts for the differences in the stains. Furthermore, reverse transcription-PCR has become an accepted method of determining cell viability, as the half-life of bacterial mRNA is on the order of minutes (10). Our studies of H. pylori in the environment suggest that in addition to giving positive results in BacLight assays, VBNC cells continue to transcribe several genes, including those known to be virulence determinants (unpublished data).
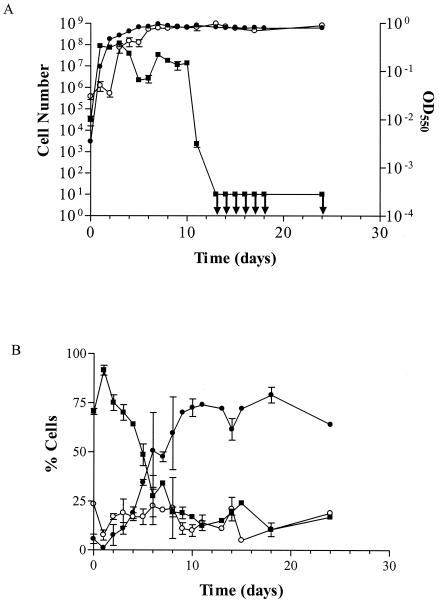
In our laboratory studies, the culturability of the cells was found to decline to <10 CFU/ml after ca. 10 days, although a large population of viable cells continued to be present (Fig. 1A). This indicates that in liquid culture in the laboratory, H. pylori enters the VBNC state as the cells age. These results showing loss of culturability and morphological conversions are consistent with data published by Benïssia et al. (3) and Catrenich and Makin (6) for H. pylori and by Alonso et al. (1) for Campylobacter coli cells. However, ours is the first investigation to show that viability is maintained in H. pylori despite this loss of culturability. In addition to maintaining viability, the cells also maintained a constant optical density (Fig. 1A), indicating that cell lysis did not occur.
FIG. 1.
(A) The viability (in cells per milliliter) (•), optical density (○), and culturability (in CFU per milliliter) (▪) of cells aged in laboratory culture. Error bars represent the standard errors of the means (SEM) of duplicate samples; ↓ indicates culturability below the limit of detection. (B) The percentages of rods, U-O forms, and cocci as cells age in laboratory culture. Error bars represent the SEM of duplicate samples. ▪, rods; ○, U-O forms; •, cocci.
The morphology of the cells as culturability was lost was also examined. Again, the findings were consistent with those of previously published studies (3, 6) in that as culturability was lost in the laboratory, the percentage of rods declined and the percentage of cocci increased (Fig. 1B). These data suggest that as culturability declines, H. pylori cells transition from culturable rods into nonculturable cocci, possibly passing through the O-U form as an intermediate stage. Because viability is maintained, this suggests that cells undergo a morphological conversion from rods to cocci as H. pylori enters the VBNC state. This change in morphology has also been observed in both the laboratory and environment for other bacterial species entering the VBNC state (25). However, when the percentage of rods was small compared to that of cocci, the culturability remained at ca. 107 for approximately 6 days. This large culturable coccus population suggests that cocci can be both culturable and nonculturable. It is also important that ca. 33% of the cocci observed in the nonculturable population in our various studies were shown to give positive results in BacLight assays. While it has been suggested by some investigators that the cocci are a death form of H. pylori (17), our studies demonstrate the viability of nonculturable cocci.
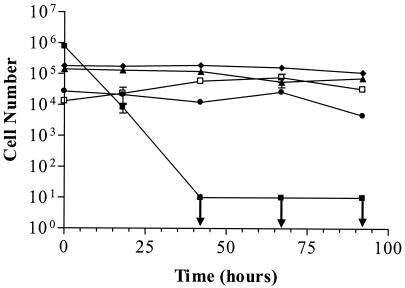
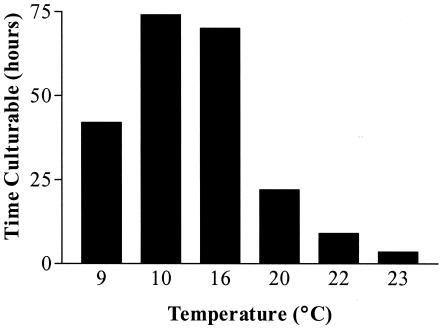
Studies conducted in a laboratory are valuable but cannot reproduce the environment bacterial cells may be exposed to in nature. As epidemiological evidence supports the fecal-oral route for the transmission of H. pylori (12-14, 22), the natural reservoir for this organism could be a freshwater body. Using membrane diffusion chambers, H. pylori cells were exposed to a natural stream environment while being monitored for culturability, viability, and morphological changes. In these environmental waters, the culturability of H. pylori cells decreased over time, reaching nonculturability in from less than 6 to ca. 70 h. Despite the loss of culturable cells in all environmental conditions, a large number of cells remained viable, as indicated by the results of the BacLight assay. For example, cells suspended in 9°C creek water lost culturability by 42 h and yet approximately 1.6 × 106 cells/ml remained viable (Fig. 2). This suggests that in similarity to the results observed in our laboratory studies, H. pylori enters the VBNC state in a natural environment. It is this rapid entrance into the VBNC state that may account for the general inability to isolate H. pylori cells from environmental sources. Lu et al. (20) recently reported the isolation of H. pylori cells from raw municipal wastewater taken from November to December in Mexico, employing immunomagnetic separation prior to culture. Their success may have been due to the high H. pylori prevalence rate in their study area (74%), the likelihood that the cells were introduced into the wastewater only shortly before the isolation was made, and the low temperature during that season. Our results (Fig. 3) suggest that H. pylori is able to remain culturable in natural waters for 2 to 3 days when the waters are at a low temperature.
FIG. 2.
The total numbers of viable cells (♦), viable rods (▴), viable O-U forms (•), and viable cocci (□) (in cells per milliliter) and the total number of culturable cells (▪) (in CFU/ml) in the environment at 9°C. Error bars represent the SEM of duplicate samples; ↓ indicates culturability below the limit of detection.
FIG. 3.
The times required for cells to lose culturability at various water temperatures.
While a considerable portion of the cell population gave BacLight-negative results in all experimental studies, ca. 10% (105 to 106) of cells remained viable. Surprisingly, linear regression analysis indicated that as culturability was lost there was no statistically significant change at any sampling period in the percentage of viable rods, O-U forms, or cocci. Figure 2 is representative of all of the environmental study results and shows the constancy of this number for each morphotype. These findings are unlike our laboratory data and those of previously published investigations and suggest that as they enter the VBNC state in the environment, there is no morphological conversion observed in H. pylori cells. Therefore, in the environment all morphological forms of this bacterium may be present in the VBNC state. Judged on the basis of investigations of the morphological conversion of V. vulnificus in the VBNC state (25), it would be expected that as H. pylori enters the VBNC state the predominant morphology would be that of cocci. However, it was found that in a population of nonculturable cells, large populations of viable rods and O-U forms in addition to cocci were present (Fig. 2). This finding is consistent with our laboratory data, which indicate that cells may exist in all morphologies in the culturable and nonculturable states.
A link between temperature and culturability was suggested by Soltesz et al. (33), as H. pylori clinical isolates and type strains were found to survive longer during transport when held at temperatures less than 15°C. The existence of such a temperature-culturability relationship was further suggested when H. pylori cells were observed to remain culturable longer in nutrient media incubated at 4°C compared to the results seen at 25, 40, and 42°C (15). It has also been found that 99% of cells maintained respiratory activity for at least 250 days at 4°C and that all respiratory activity was lost after 24 h at 37°C (11). When H. pylori cells in our studies were placed into a natural environment over a 1-year period, the length of time that cells remained culturable changed (Fig. 3). These seasonal studies involved water temperatures ranging from 9 to 23°C. In general, cells remained culturable longer in cooler (<20°C) waters than in warmer (>20°) waters. Throughout each sampling period, dissolved oxygen, pH, temperature, turbidity, and ammonia and phosphate levels were measured. Table 1 summarizes the average measurements from each study.
TABLE 1.
Average values of the environmental parameters measured during each study
| Month | Results
|
|||||
|---|---|---|---|---|---|---|
| Water temp (°C) | Ammonia (mg/liter) | Dissolved oxygen (mg/liter) | pH | Phosphate (mg/liter) | Turbidity (FAUa) | |
| November | 10 | 0.04 | 2.4 | 6.6 | 0.51 | 44 |
| February | 9 | 0.13 | 8.7 | 7.4 | 0.21 | 82 |
| April | 16 | 0.16 | 5.1 | 6.6 | 0.22 | 14 |
| August | 22 | 0.61 | 3.6 | 6.8 | 0.11 | 114 |
| August | 23 | 0.10 | 2.9 | 6.9 | 0.19 | 4.7 |
| September | 20 | 0.38 | 3.8 | 7.0 | 0.20 | 79 |
FAU, forazin attenuation units.
To determine whether there was a correlation between the number of hours H. pylori cells were culturable in the environment and the environmental parameters measured, a Spearman's coefficient of rank correlation was used. It was found that there was a significant correlation between both temperature (rs = −0.82857; P < 0.05) and phosphate (rs = 0.94286; P < 0.05) levels and culturability in the environment. When a sequential Bonferroni adjustment was applied, however, it was found that there was no statistically significant correlation between these environmental parameters and culturability. Also, multiple regression analysis was performed to determine whether any single environmental parameter accounted for the variation in culturability. It was found that no parameters met a statistically significant level of contribution to the culturability of H. pylori in the environment. Because our studies were conducted in a small, natural freshwater stream, there were many variables present which could not be accounted for or controlled, including the wash-off of construction debris into the stream during two sampling periods as well as a sewage leak during another sampling period. Although the possibility of a relationship between temperature and/or phosphate levels and culturability is suggested in our studies, a larger-scale investigation may be required to prove such a conclusion.
Several bacterial species that enter the VBNC state have been shown to resuscitate and become culturable following the modification of routine culture conditions. V. vulnificus cells resuscitate through a temperature upshift (25), while Campylobacter jejuni requires passage through embryonated eggs (8). Currently, no resuscitation techniques have been identified for H. pylori. Cells which had entered the VBNC state in the environment were exposed to a variety of resuscitation conditions and examined for growth. No growth was observed under any condition, and attempts to resuscitate this organism continue in our laboratory.
This is the first investigation to examine the viability of H. pylori in both the laboratory and a natural environment as culturability is lost. Our data suggest that H. pylori is able to enter the VBNC state as cells age in the laboratory or are exposed to a natural, freshwater environment. Cells underwent a transition from culturable rods to predominately nonculturable cocci as they entered the VBNC state in the laboratory; however, no such morphological conversion was observed in the natural environment. Thus, H. pylori appears to exist in all morphological forms in the environment. Furthermore, our studies suggest that exposure to the environment can induce this organism to enter the VBNC state and to persist in the environment until it enters a suitable host. For this reason, it is important to consider this survival state for H. pylori in natural environments.
Acknowledgments
This work was supported by the Environmental Protection Agency (R-82905701-0) and Sigma Xi Grants in Aid of Research. We acknowledge the University of North Carolina at Charlotte Graduate School for supporting publication costs.
We thank Harry Mobley for donation of the strain employed in this investigation as well as Courtney Pfeffer and Lee Lewis for assistance with statistical analysis.
REFERENCES
- 1.Alonso, J. L., S. Mascellaro, Y. Moreno, M. A. Ferrús, and J. Hernández. 2002. Double-staining method for differentiation of morphological changes and membrane integrity of Campylobacter coli cells. Appl. Environ. Microbiol. 68:5151-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asaka, M., A. R. Sepulveda, T. Sugiyama, and D. Y. Graham. 2001. Gastric cancer, p. 481-498. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. American Society for Microbiology, Washington, D.C.
- 3.Benïssia, M., P. Babin, N. Quellard, L. Pezennec, Y. Cenatiempo, and J. L. Fauchére. 1996. Changes in Helicobacter pylori ultrastructure and antigens during conversion from bacillary to the coccoid form. Infect. Immun. 64:2331-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulos, L., M. Prévost, B. Barbeau, J. Coallier, and R. Desjardins. 1999. LIVE/DEAD BacLight: application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 37:77-86. [DOI] [PubMed] [Google Scholar]
- 5.Buck, G. E. 1990. Campylobacter pylori and gastroduodenal disease. Clin. Microbiol. Rev. 3:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catrenich, C. E., and K. M. Makin. 1991. Characterization of the morphologic conversion of Helicobacter pylori from bacillary to coccoid forms. Scand. J. Gastroenterol. 181:58-64. [PubMed] [Google Scholar]
- 7.Cellini, L., N. Allcocati, D. Angelucci, T. Iezzi, E. Di Campli, L. Marzio, and B. Dainelli. 1994. Coccoid Helicobacter pylori not culturable in vitro reverts in mice. Microbiol. Immunol. 38:843-850. [DOI] [PubMed] [Google Scholar]
- 8.Chaveerach, P., A. A. H. M. ter Huurne, L. J. A. Lipman, and F. van Knapen. 2003. Survival and resuscitation of ten strains of Campylobacter jejuni and Campylobacter coli under acid conditions. Appl. Environ. Microbiol. 69:711-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole, S. P., D. Cirillo, M. F. Kagnoff, D. G. Guiney, and L. Eckmann. 1997. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect. Immun. 65:843-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway, T., and G. K. Schoolnik. 2003. Microarray expression profiling: capturing a genome-wide portrait of the transcriptome. Mol. Microbiol. 47:879-889. [DOI] [PubMed] [Google Scholar]
- 11.Gribbon, L. T., and M. R. Barer. 1995. Oxidative metabolism in nonculturable Helicobacter pylori and Vibrio vulnificus cells studied by substrate-enhanced tetrazolium reduction and digital image processing. Appl. Environ. Microbiol. 61:3379-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegarty, J. P., M. T. Dowd, and K. H. Baker. 1999. Occurrence of Helicobacter pylori in surface water in the United States. J. Appl. Microbiol. 87:697-701. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins, R. J., P. A. Vial, C. Ferreccio, J. Ovalle, P. Prado, V. Sotomayor, R. G. Russell, S. S. Wasserman, and J. Morris, Jr. 1993. Seroprevalence of Helicobacter pylori in Chile: vegetables may serve as one route of transmission. J. Infect. Dis. 163:222-226. [DOI] [PubMed] [Google Scholar]
- 14.Hulten, K., S. W. Han, H. Enroth, P. D. Klein, A. R. Opekun, R. H. Gilman, D. G. Evans, L. Engstrand, D. Y. Graham, and F. A. El-Zaatari. 1996. Helicobacter pylori in the drinking water in Peru. Gastroenterology 110:1031-1035. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, X., and M. P. Doyle. 1998. Effect of environmental and substrate factors on survival and growth of Helicobacter pylori. J. Food Prot. 61:929-933. [DOI] [PubMed] [Google Scholar]
- 16.Kogure, K., U. Simida, and N. Taga. 1979. A tentative direct microscope method for counting live marine bacteria. Can. J. Microbiol. 25:415-420. [DOI] [PubMed] [Google Scholar]
- 17.Kusters, J. G., M. M. Gerrits, J. A. G. Van Strijp, and M. J. E. Vandenbroucke-Grauls. 1997. Coccoid forms of Helicobacter pylori are the morphological manifestation of cell death. Infect. Immun. 65:3672-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert, J. R., S. K. Lin, and J. Aranda-Michel. 1995. Helicobacter pylori. Scand. J. Gastroenterol. 30(Suppl. 208):33-46. [DOI] [PubMed] [Google Scholar]
- 19.Lisle, J. T., B. H. Pyle, and G. A. McFeters. 1999. The use of multiple indices of physiological activity to access viability in chlorine disinfected Escherichia coli O157:H7. Lett. Appl. Microbiol. 29:42-47. [DOI] [PubMed] [Google Scholar]
- 20.Lu, Y., T. E. Redlinger, R. Avitia, A. Galindo, and K. Goodman. 2002. Isolation and genotyping of Helicobacter pylori from untreated municipal wastewater. Appl. Environ. Microbiol. 68:1436-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFeters, G. A., and D. G. Stuart. 1972. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane filter chambers. Appl. Microbiol. 24:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno, Y., M. A. Ferrus, J. L. Alonso, A. Jimenez, and J. Hernandez. 2003. Use of fluorescent in situ hybridization to evidence the presence of Helicobacter pylori in water. Water Res. 37:2251-2256. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson, H.-O., J. Blom, W. A. Al-Soud, Å. Ljungh, L. P. Andersen, and T. Wadström. 2002. Effect of cold starvation, acid stress, and nutrients on metabolic activity of Helicobacter pylori. Appl. Environ. Microbiol. 68:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson, L., J. D. Oliver, and S. Kjelleberg. 1991. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J. Bacteriol. 173:5024-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver, J. D. 2002. Public health significance of viable but nonculturable bacteria, p. 277-300. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. American Society for Microbiology, Washington, D.C.
- 26.Peterson, W. I. 1991. Helicobacter pylori and peptic ulcer disease. N. Engl. J. Med. 324:1043-1048. [DOI] [PubMed] [Google Scholar]
- 27.Rice, W. R. 1989. Analyzing tables of statistical tests. Evolution 43:223-225. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez, G. G., D. Phipps, K. Ishiguro, and H. F. Ridgway. 1992. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. App. Environ. Microbiol. 58:1801-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahamat, M., C. Paszko-Kolva, H. Yamamoto, and R. Colwell. 1989. Ecological studies of Campylobacter pylori. Klin. Wochenschr. 67(Suppl. XVII):62-63. [Google Scholar]
- 30.Shahamat, M., U. Mai, C. P. Paszko-Kolva, M. Kessel, and R. R. Colwell. 1993. Use of autoradiography to assess viability of Helicobacter pylori in water. Appl. Environ. Microbiol. 59:1231-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.She, F.-F., J.-Y. Lin, J.-Y. Liu, C. Huang, and D.-H. Su. 2003. Virulence of water-induced coccoid Helicobacter pylori and its experimental infection in mice. World J. Gasteroenterol. 9:516-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokal, R. R., and F. J. Rohlf. 1995. Biometry: the principles and practice of statistics in biological research, 3rd ed. W. H. Freeman and Company, New York, N.Y.
- 33.Soltesz, V., B. Zeeberg, and T. Wadstrom. 1992. Optimal survival of Helicobacter pylori under various transport conditions. J. Clin. Microbiol. 30:1453-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]