Abstract
Objective:
Clinicopathologic phenotypes of dementia with Lewy bodies (DLB) and Alzheimer disease (AD) often overlap, making discrimination difficult. We performed resting state blood oxygen level–dependent (BOLD) functional connectivity MRI (fcMRI) to determine whether there were differences between AD and DLB.
Methods:
Participants (n = 88) enrolled in a longitudinal study of memory and aging underwent 3-T fcMRI. Clinical diagnoses of probable DLB (n = 15) were made according to published criteria. Cognitively normal control participants (n = 38) were selected for the absence of cerebral amyloid burden as imaged with Pittsburgh compound B (PiB). Probable AD cases (n = 35) met published criteria and had appreciable amyloid deposits with PiB imaging. Functional images were collected using a gradient spin-echo sequence sensitive to BOLD contrast (T2* weighting). Correlation maps selected a seed region in the combined bilateral precuneus.
Results:
Participants with DLB had a functional connectivity pattern for the precuneus seed region that was distinct from AD; both the DLB and AD groups had functional connectivity patterns that differed from the cognitively normal group. In the DLB group, we found increased connectivity between the precuneus and regions in the dorsal attention network and the putamen. In contrast, we found decreased connectivity between the precuneus and other task-negative default regions and visual cortices. There was also a reversal of connectivity in the right hippocampus.
Conclusions:
Changes in functional connectivity in DLB indicate patterns of activation that are distinct from those seen in AD and may improve discrimination of DLB from AD and cognitively normal individuals. Since patterns of connectivity differ between AD and DLB groups, measurements of BOLD functional connectivity can shed further light on neuroanatomic connections that distinguish DLB from AD.
Dementia with Lewy bodies (DLB) is the second most common neurodegenerative cause of dementia after Alzheimer disease (AD).1,2 In the early stages, symptoms may overlap, making it difficult to distinguish DLB from AD. In the absence of specific markers for DLB, diagnoses may be improved by utilizing neuroimaging3–5 such as MRI.6 Successful identification of imaging markers may contribute to our understanding of disease pathogenesis, improve antemortem detection, and establish thresholds for measuring progression and response to therapies.6 This approach currently is being applied in the study of AD by the Alzheimer's Disease Neuroimaging Initiative.7
Functional imaging modalities such as resting state blood oxygen level–dependent (BOLD) functional connectivity MRI (fcMRI) may also assist in discrimination. During memory tasks in AD, fcMRI studies of the default mode network (DMN)8–10 show aberrantly increased activity in the precuneus and posterior cingulate relative to cognitively intact older adults.11 BOLD connectivity studies of AD have shown decreased connectivity between posterior and anterior portions of the DMN8–11 with similar findings in cognitively intact older adults who have evidence of amyloid pathology by Pittsburgh compound B (PiB) PET.12,13
To date, BOLD fcMRI has not been studied extensively in DLB. Because the DMN has been studied in normal aging and AD and but rarely in DLB, we chose the DMN as the first site to examine with BOLD fcMRI to compare DMN connectivity in a cohort of well-characterized controls without dementia, participants with AD, and participants with DLB using the precuneus as the seed region.
METHODS
Participants.
Data were examined from 88 research participants who were enrolled in a longitudinal study of memory and aging14 at the Knight Alzheimer's Disease Research Center at Washington University in St Louis and who underwent fcMRI and PET with PiB PET.15,16 The Washington University Human Research Protection Office approved all procedures.
PiB binding potential values from the prefrontal cortex, gyrus rectus, lateral temporal cortex, and precuneus areas were averaged in each participant to calculate the mean cortical binding potential (MCBP); these regions have high PiB uptake in participants with symptomatic AD.16 Negative PiB scans are defined as a mean cortical binding potential less than 0.18, while positive scans (that is, detection of amyloid binding) are defined as a mean cortical binding potential greater than or equal to 0.18.16
Control individuals (n = 38) were selected on the basis of no dementia at their clinical assessment and having no PiB retention during amyloid imaging.16 This was done to assure that the control group did not have preclinical AD.17,18 The clinical diagnostic criteria for participants with probable AD (n = 35) were in accordance with the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association19 and these individuals had the presence of amyloid with PiB imaging.
Participants with DLB (n = 15) were recruited from a dementia specialty practice. The clinical diagnosis of probable DLB was made according to McKeith criteria1 with at least a 2-year threshold of motor to cognitive symptoms to eliminate PD dementia as a clinical diagnosis.20 As part of an imaging protocol, these individuals were enrolled in a longitudinal study and underwent identical evaluations as the cognitively normal participants and participants with AD, including clinical and cognitive evaluations, MRI, and PiB PET. Table e-1 on the Neurology® Web site at www.neurology.org depicts the characteristics of the 15 individuals with DLB. Thirteen participants with DLB completed PiB scans: 6 had positive PiB scans (mean cortical binding potential greater than or equal to 0.18) while the remaining 7 had negative scans.
Clinical evaluation.
Experienced clinicians conducted semi-structured interviews with the participant and a knowledgeable collateral source (usually a spouse or adult child). The assessment included all items of the Uniform Data Set.21 The Clinical Dementia Rating (CDR) was used to determine the presence or absence of dementia and, if present, to stage its severity.22 The CDR evaluates cognitive function in each of 6 categories (memory, orientation, judgment and problem solving, performance in community affairs, home and hobbies, and personal care) without reference to psychometric performance or results of previous evaluations. CDR 0 indicates no dementia, and CDR 0.5, 1, 2, and 3 correspond to very mild, mild, moderate, and severe dementia, respectively. Individuals with dementia with a CDR of 2 or greater were excluded as these individuals have difficulty completing psychometric assessment or undergoing imaging studies.
fcMRI.
Structural imaging was performed on a 3.0-T Siemens Allegra system. Sessions began with acquisition of a scout scan with 3 orthogonal slices, followed by a coarse 3-dimensional sagittal T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) used to automatically compute fcMRI slice tilts and offsets that optimize whole brain coverage parallel to the anterior commissure/posterior commissure plane.12,23,24 This computation (“preregistration”) standardizes the fcMRI coverage across subjects and provides highly reproducible slice positioning. High-resolution structural images were acquired using a 3-dimensional sagittal T1-weighted MPRAGE acquisition optimized for contrast-to-noise ratio and resolution12,25,26 (echo time [TE] = 16 msec, repetition time [TR] = 2,400 msec, inversion time [TI] = 1,000 msec, flip angle = 8°, 256 × 256 acquisition matrix, 1 × 1 × 1 mm voxels). The high-resolution MPRAGE was used for definitive atlas registration. High-resolution 2-D multislice oblique axial spin density/T2-weighted fast spin echo (FSE) structural images were acquired using slice tilts and positions computed by slice preregistration (TE = 455 msec, TR = 3,200 msec, 256 × 256 acquisition matrix, 1 acquisition, 1 × 1 × 1 mm voxels). The T2-weighted FSE data were used in the fcMRI atlas registration procedure. The functional images were collected in runs using a gradient spin-echo sequence (TE = 27 msec, TR = 384 msec, field of view = 256 mm, flip angle = 90°) sensitive to BOLD contrast (T2* weighting). A total of 36 contiguous, 4.0-mm-thick slices were acquired parallel to the anterior commissure/posterior commissure plane (4.0 mm approximately isotropic voxels) providing complete brain coverage. Two fcMRI runs included 164 volumes each, continuously acquired at a TR of 2.2 seconds (6 minutes each). MRI data were reconstructed into images, and then normalized across runs by 1) scaling whole-brain signal intensity to a fixed value and 2) removing the linear slope on a voxel-by-voxel basis to counteract effects of drift.24–26 The MRI data were aligned to correct for head motion using a 6-parameter rigid-body rotation and translation correction which mutually registers all frames in all runs for each subject.13,3,7 BOLD volumetric time series were concatenated and preprocessed, including temporal filtering, retaining frequencies up to 0.1 Hz. Between-subjects analyses were conducted after transformation of the data to a common atlas space and then the images were blurred with an 8-mm full width at half maximum Gaussian filter. The transforms were combined by matrix multiplication so that reslicing of data in conformity with the atlas involved only one interpolation.
Default mode network.
The DMN was first characterized in fluorodeoxyglucose (FDG) PET studies as a collection of brain regions that are most active at rest and demonstrates consistent decreases in activity associated with tasks and goal-directed behaviors.27–29 The regions of DMN are defined functionally by their coordinated behavior and commonly have the greatest activity at rest and decreased activity during the performance of goal-directed tasks.28,29 The DMN is important in self-referential activities, including evaluating salience of internal and external cues, remembering the past, and planning the future.12,24,29,30 One key region of interest, the posterior cingulate cortex and adjacent precuneus, appear to be tonically active regions that may continuously gather information relative to external and internal stimuli.27–29 When successful task performance demands focused attention, activity of the DMN is curtailed. There also appears to be a selective vulnerability of the posterior cingulate and precuneus in AD.31
Statistical analysis.
Following preprocessing, correlation maps were obtained by selecting a “seed” region in the combined bilateral precuneus (±7, −60, 21) and examining the connectivity patterns between the region of interest and other cortical regions. The precuneus seed was selected as representative of the DMN23,27 and was the same as used in previous studies of AD and in participants with amyloid deposition but not dementia.12 An image map of correlations was created using Pearson product-moment correlation and the voxel-by-voxel BOLD time course.12,23–26 Group difference significance maps were created by combining results across subjects using a random effects analysis of the Fisher z transformed correlation maps.12 Based upon the group difference map, target regions with a minimum voxel size ≥18 and a statistical threshold of p < 0.01, uncorrected for multiple comparisons, were selected. The BOLD time course from each selected target region was extracted and used as the dependent variable in an analysis of variance model, where group membership (i.e., DLB vs PiB− control, or DLB vs AD) and gender served as independent variables to examine the group differences in functional connectivity between the precuneus and target regions after taking into account the gender effect (table 1).
Table 1.
Sample characteristicsa
Abbreviations: AD = Alzheimer disease; CDR = Clinical Dementia Rating; DLB = dementia with Lewy bodies; MMSE = Mini-Mental State Examination; PiB = Pittsburgh compound B.
Values are mean (SD). For a more detailed description of the DLB cases, see table e-1.
RESULTS
Sample characteristics.
Table 1 depicts the characteristics of the study participants. There were no differences in age or education among the study participants; however, there were a higher proportion of men in the DLB group, consistent with previous reports.32 The gender effect was taken into account when the group comparison of functional connectivity was conducted. Mini-Mental State Examination scores were lower in the AD and DLB groups compared with the cognitively normal group; however, the percentage of CDR 0.5 and CDR 1 cases did not differ between AD and DLB. Consensus criteria symptoms (table e-1) in DLB included extrapyramidal symptoms (100%), cognitive fluctuations (80%), recurrent visual hallucinations (20%), and features suggestive of REM sleep disorder (47%). Six participants with DLB were taking dopaminergic agents and all participants with DLB and participants with AD were on cholinesterase inhibitors. Controls and patients with AD did not exhibit any core features of DLB.
Resting BOLD fcMRI of the default network.
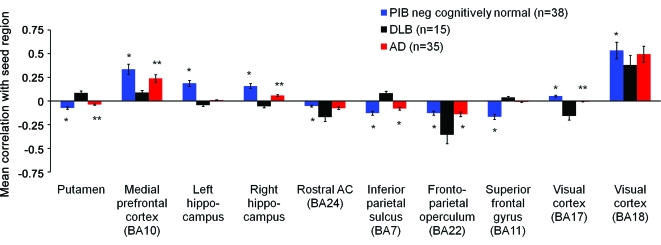
Figure e-1 shows the group mean images of the basic functional connectivity pattern by seeding the precuneus/posterior cingulate cortex in controls, cases with DLB, and cases with AD. Significant differences were found between DLB and age-matched (PiB−) cognitively normal elderly in the functional connectivity of the precuneus seed region (±7, −60, 21) (table 2). The resulting significance map was projected onto a standard brain surface (figure 1). Mean correlations between 10 cortical and subcortical regions and the precuneus seed region are shown in figure 2. DLB cases demonstrated correlations between precuneus and putamen, medial prefrontal, superior frontal gyrus, inferior parietal, and associative visual cortex. Anticorrelations were found between the precuneus seed and the rostral anterior cingulate, left and right hippocampus, frontoparietal operculum, and primary visual cortex.
Table 2.
Comparison of functional connectivity among controls, subjects with AD, and subjects with DLB
Abbreviations: AD = Alzheimer disease; BA = Brodmann area; DLB = dementia with Lewy bodies; PiB = Pittsburgh compound B.
Adjusted for gender.
Figure 1. Functional connectivity differences among dementia with Lewy bodies (DLB), control, and Alzheimer disease (AD) using the precuneus seed.
All images are shown in sagittal section and identify statistically significant regional differences in functional connectivity of the precuneus between (A) DLB and cognitively normal and (B) DLB and AD. The top images for each panel display the left medial (a) and lateral (b) surfaces, while the second row images display the right medial (c) and lateral (d) sagittal surfaces. The regions identified were in visual cortex, hippocampus, rostral anterior cingulate (AC), frontoparietal operculum, medial prefrontal cortex (PFC), inferior parietal, superior frontal gyrus, and putamen. (A) Red indicates that DLB has increased positive correlation connectivity compared with Pittsburgh compound B (PiB)–negative cognitively normal controls (inferior parietal sulcus, superior frontal gyrus, and putamen). Blue indicates that compared with controls, DLB between-regions functional connectivity has decreased positive correlation connectivity, which can be further described as decreased positive correlation connectivity (medial PFC and secondary visual cortex), or increased anticorrelation connectivity (rostral anterior cingulated, and frontoparietal operculum), or connectivity direction was changed from positive correlations to anticorrelations (left and right hippocampus, and primary visual cortex). (B) Red indicates that DLB has increased positive correlation connectivity compared with AD cases (inferior parietal sulcus, and putamen). The blue color indicates that compared with AD cases, DLB has decreased positive correlation connectivity, which can be further described as decreased positive correlation connectivity (medial PFC), increased anticorrelation connectivity (frontoparietal operculum, and visual cortex), or connectivity direction was changed from positive correlation to anticorrelation (right hippocampus). BA = Brodmann area.
Figure 2. Correlation and anticorrelation relationships in dementia with Lewy bodies (DLB), Pittsburgh compound B (PiB)+ Alzheimer disease (AD), and PiB− controls.
The graph compares regional correlation magnitudes for PiB− cognitively normal (blue), DLB (black), and PiB+ AD (red) study participants. Bars above the horizontal axis represent correlations while bars below the horizontal axis present anticorrelations. The regions identified as differing in resting state functional connectivity with the precuneus were putamen, medial prefrontal, L and R hippocampus (hip), rostral anterior cingulate (AC), inferior parietal, frontoparietal operculum, superior frontal gyrus, and primary (Brodmann area [BA] 17) and secondary (BA 18) visual cortex. *p < 0.01, **p < 0.05. Actual p values are shown in table 2.
DLB vs PiB− control connectivity.
Relative to cognitively normal participants, cases with DLB had decreased positive connectivity between the precuneus and medial prefrontal cortex, and secondary visual cortices. Correlations between the precuneus and primary visual cortex and left and right hippocampus had a changed connectivity direction from positive correlations in PiB− participants to anticorrelations in DLB. Examining anticorrelations, there was increased anticorrelation between precuneus and rostral anterior cingulate and frontoparietal operculum in DLB compared with PiB− participants, whereas the normal anticorrelation between precuneus and superior frontal gyrus, putamen, and inferior parietal sulcus in PiB− participants was positive in DLB (figures 1A and 2, table 2).
DLB vs AD connectivity.
We next examined whether differences in functional connectivity existed between DLB and AD. Compared with AD cases, DLB cases had decreased positive connectivity between the precuneus and medial prefrontal cortex, whereas the anticorrelation in connectivity in AD was increased in DLB between the frontoparietal operculum and visual cortex. In addition, the anticorrelations between the precuneus and putamen and inferior parietal regions seen in AD had a changed connectivity direction to positive correlations in DLB. In contrast, the weakly positive correlation between the precuneus and right hippocampus in AD had a changed connectivity direction to anti-correlation in DLB (figures 1B and 2, table 2).
DISCUSSION
We found that participants with DLB differed on a group level in functional connectivity within the DMN using a precuneus seed from controls without dementia and that the pattern of change in connectivity was distinct from AD. Increased connectivity was seen in the putamen and inferior parietal cortex (Brodmann area [BA] 7). Decreased connectivity was seen in the medial prefrontal cortex (BA 10), the frontoparietal operculum, and the primary visual cortex (BA 17), while a reversal of connectivity was seen in the right hippocampus.
Previous studies of BOLD fcMRI comparing subjects with AD with cognitively normal persons have demonstrated decreased precuneus resting state functional connectivity with hippocampus, parahippocampus, anterior cingulate, dorsal anterior cingulate, gyrus rectus, and superior precuneus, implicating disruptions of DMN integrity in AD corresponding to areas of amyloid deposition.12,16,30 Alterations in resting state functional connectivity between the posterior and anterior portions of the DMN have been described in AD.8–12 In the present study, however, alterations in connectivity between anterior and posterior DMN were not found for DLB. Instead, findings suggest increased connectivity between the precuneus and the dorsal attention network region (inferior parietal cortex, BA 7) and decreased connectivity between precuneus and the frontoparietal executive control networks (medial prefrontal and operculum regions, BA 10).23 The significance of these differences is not fully understood but suggests that patterns of connectivity may provide distinct fcMRI findings across different dementia etiologies.
During the performance of attention-demanding cognitive tasks, 2 opposing responses come into play.26–29 During tasks of attention, working memory, and executive function, increased activity is seen in prefrontal, anterior cingulate, and inferior parietal regions reflecting attention and executive control networks.33,34 Simultaneously, the DMN, including posterior cingulate, medial and lateral parietal cortex, and medial prefrontal cortex, exhibit decreased activity.23 As the attentional demand of a task increases, activity in task-positive regions increases in adults without dementia, while activity in task-negative regions such as the DMN decreases.23 In AD, in regions that fall within the DMN, task-based increases in activity30 were found to correspond with areas involved in amyloid deposition as measured with PiB imaging.12,16 Another critically important region for cognitive control, the anterior cingulate, demonstrated significant reductions in connectivity in PiB+ vs PiB− participants.12 In contrast, the connectivity between the precuneus and visual cortex was significantly higher in PiB+ cognitively normal individuals and participants with AD than in DLB.12 Although to date connectivity in DLB has not been well-studied, our findings suggest important changes in connectivity between the DMN and other control networks in this disorder.
Biomarkers have the potential to enhance aspects of both therapeutic trials and clinical practice.35 As future treatments increasingly target the protein chemistry underlying the different dementias, it becomes increasingly important to distinguish between the dementias during life. Imaging modalities can be used to identify attractive biomarkers and are perceived as less invasive than lumbar puncture. MRI-based measures of cerebral volume can provide a surrogate for neuronal loss and several techniques have been applied to elucidate disease processes, aid diagnosis, and enable monitoring of progression in a variety of parkinsonian disorders.36
Other investigators have used FDG PET imaging to characterize patterns of hypometabolism in AD and DLB. DLB has similar patterns of hypometabolism to AD but may also involve primary and associative visual cortices.37 However, occipital hypometabolism is only modestly effective in distinguishing DLB from AD.37 123I-FP-CIT SPECT has been used to label nigrostriatal terminal density of dopamine transporters and improve detection of probable DLB.38 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy has also been shown to discriminate DLB from AD3,39 but is not available in many countries. PET and SPECT tracers have disadvantages of having poorer spatial resolution and require injection of radiotracers. BOLD fcMRI offers the advantage of being collected during the same session as the structural MRI, permitting higher-resolution structure–function relationships.
This study has limitations. The DLB group is small (n = 15); however, all participants were well-characterized. The small size of the sample may limit our ability to detect important differences and will need to be followed by larger studies and in this study we did not correct for multiple comparisons. The analyses were done at a group level; the discriminative utility at the individual level is not addressed. Therefore, these findings cannot be used in their present state as a diagnostic marker. The sensitivity of BOLD fcMRI for presymptomatic disease cannot be addressed since impaired groups had clear clinical features of AD and DLB, and the normal controls were prescreened to assure they did not have preclinical disease. The AD and DLB cases were based on clinical diagnoses; however, we have previously established that each of the CDR stages of DLB and AD corresponds to autopsy confirmation.40 Finally, PiB imaging suggests that approximately half of the DLB sample has concurrent AD; however, the sample size is insufficient to test whether DMN differences exist between PiB+ and PiB− DLB.
The changes in functional connectivity in DLB described here suggest distinct patterns of activity that may assist in discrimination of DLB from AD and cognitively normal participants. In studies of individuals with symptomatic AD and cognitively intact controls, the use of BOLD fcMRI may identify “preclinical” disease in individuals without apparent cognitive abnormalities.12 Since patterns of connectivity differ between AD and DLB groups, measurements of BOLD functional connectivity potentially can shed further light on neuroanatomic connections that distinguish DLB from AD. Using alterations in DMN functional connectivity identified with BOLD fcMRI, we may be able to improve our understanding of the pathophysiology of different dementia etiologies such as AD and DLB.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the research participants from the Washington University Knight Alzheimer's Disease Research Center who contributed data and the staff of the Center's Clinical Core for the clinical assessments.
Supplemental data at www.neurology.org
- AD
- Alzheimer disease
- BA
- Brodmann area
- BOLD
- blood oxygen level–dependent
- CDR
- Clinical Dementia Rating
- DLB
- dementia with Lewy bodies
- DMN
- default mode network
- fcMRI
- functional connectivity MRI
- FDG
- fluorodeoxyglucose
- FSE
- fast spin echo
- MCBP
- mean cortical binding potential
- MPRAGE
- magnetization-prepared rapid gradient echo
- PiB
- Pittsburgh compound B
- TE
- echo time
- TI
- inversion time
- TR
- repetition time
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Z. Yan and Dr. Sheline.
DISCLOSURE
Dr. Galvin serves on a scientific advisory board for the American Federation for Aging Research and on the Board of Directors and the Scientific Advisory Council for the Lewy Body Dementia Association; serves on the editorial boards of Alzheimer's Disease and Associated Disorders and Acta Neuropathologica; serves on speakers' bureaus for Pfizer Inc, Eisai Inc., Novartis, and Forest Laboratories, Inc.; has served as a consultant for Novartis, Forest Laboratories, Inc., Pfizer Inc, Eisai Inc., and Medivation, Inc.; has received license fee payments for AD8 dementia screening test (copyrighted): license agreements between Washington University and Pfizer Inc, Eisai Inc., and Novartis; and receives research support from Novartis, Eli Lilly and Company, Elan Corporation, Wyeth, Bristol-Myers Squibb, the NIH/NIA, and the Alzheimer Association. Dr. Price serves as Associate Editor for the Journal of Comparative Neurology and on the editorial board of Brain Structure and Function and receives research support from the NIH (NIMH/NIA). Z. Yan reports no disclosures. Dr. Morris serves on scientific advisory boards for AstraZeneca, Bristol-Myers Squibb, Genentech, Inc., Merck Serono, Novartis, Pfizer Inc, Schering-Plough Corp., Eli Lilly and Company, Wyeth, and Elan Corporation; serves on the editorial advisory board of Alzheimer's Disease and Associated Disorders; receives royalties from publishing Mild Cognitive Impairment and Early Alzheimer's Disease (John Wiley and Sons, 2008), Dementia (Clinical Publishing, 2007), Handbook of Dementing Illnesses, 2nd edition (Taylor & Francis, 2006), and for an editorial in Lancet Neurology (Elsevier, 2008); and receives research support from Elan Corporation, Wyeth, Eli Lilly and Company, Novartis, Pfizer Inc, Avid Radiopharmaceuticals, the NIH, and the Dana Foundation. Dr. Sheline has received funding for travel and speaker honoraria from Eli Lilly and Company, for which she serves on a scientific board, as a consultant, and on the speakers' bureau; and has received research support from the NIH.
REFERENCES
- 1. McKeith IG, Dickson DW, Lowe J, et al. , Consortium on DLB Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872 [DOI] [PubMed] [Google Scholar]
- 2. Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer disease. Expert Rev Neurother 2007;7:1499–1516 [DOI] [PubMed] [Google Scholar]
- 3. Tateno M, Kobayashi S, Saito T. Imaging improves diagnosis of dementia with Lewy bodies. Psychiatry Investig 2009;6:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wild EJ, Fox NC. Serial volumetric MRI in Parkinsonian disorders. Mov Disord 2009;24(suppl 2):S691–S698 [DOI] [PubMed] [Google Scholar]
- 5. von Gunten A, Meuli R. Delineating dementia with Lewy bodies: can magnetic resonance imaging help? Front Neurol Neurosci 2009;24:126–134 [DOI] [PubMed] [Google Scholar]
- 6. Watson R, Blamire AM, O'Brien JT. Magnetic resonance imaging in Lewy body dementias. Dement Geriatr Cogn Disord 2009;28:493–506 [DOI] [PubMed] [Google Scholar]
- 7. Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 2004;101:4637–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Comput Biol 2008;4:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang HY, Wang SJ, Xing J, et al. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behav Brain Res 2009;197:103–108 [DOI] [PubMed] [Google Scholar]
- 11. Lustig C, Snyder AZ, Bhakta M, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA 2003;100:14504–14509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 2010;67:584–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hedden T, Van Dijk KR, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci 2009;29:12686–12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 1998;55:326–335 [DOI] [PubMed] [Google Scholar]
- 15. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound B. Ann Neurol 2004;55:306–319 [DOI] [PubMed] [Google Scholar]
- 16. Mintun MA, Larossa GN, Sheline YI, et al. [11C]PiB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452 [DOI] [PubMed] [Google Scholar]
- 17. Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009;30:1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol 2005;62:758–765 [DOI] [PubMed] [Google Scholar]
- 19. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 20. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707 [DOI] [PubMed] [Google Scholar]
- 21. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–216 [DOI] [PubMed] [Google Scholar]
- 22. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 23. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA 2009;106:1942–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vincent JL, Snyder AZ, Fox MD, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 2006;96:3517–3531 [DOI] [PubMed] [Google Scholar]
- 26. Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 2006;103:10046–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage 2007;37:1083–1090 [DOI] [PubMed] [Google Scholar]
- 28. Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 2009;101:3270–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 2008;100:3328–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol 1997;42:85–94 [DOI] [PubMed] [Google Scholar]
- 32. Williams M, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies versus Alzheimer's disease. Neurology 2006;67:1935–1942 [DOI] [PubMed] [Google Scholar]
- 33. Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 2000;12:1–47 [DOI] [PubMed] [Google Scholar]
- 34. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002;3:201–215 [DOI] [PubMed] [Google Scholar]
- 35. Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature 2009;461:916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Köllensperger M, Wenning GK. Assessing disease progression with MRI in atypical parkinsonian disorders. Mov Disord 2009;24(suppl 2):S699–S702 [DOI] [PubMed] [Google Scholar]
- 37. Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with Lewy bodies and those with mild Alzheimer's disease. J Nucl Med 2007;48:704–711 [DOI] [PubMed] [Google Scholar]
- 38. O'Brien JT, McKeith IG, Walker Z, et al. , DLB Study Group Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry 2009;194:34–39 [DOI] [PubMed] [Google Scholar]
- 39. Kobayashi S, Tateno M, Morii H, Utsumi K, Saito T. Decreased cardiac MIBG uptake, its correlation with clinical symptoms in dementia with Lewy bodies. Psychiatry Res 2009;174:76–80 [DOI] [PubMed] [Google Scholar]
- 40. Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology 2005;65:1232–1238 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.