Abstract
Background/Objective:
Patients with posterior cortical atrophy (PCA) often have Alzheimer disease (AD) at autopsy, yet are cognitively and anatomically distinct from patients with clinical AD. We sought to compare the distribution of β-amyloid and glucose metabolism in PCA and AD in vivo using Pittsburgh compound B (PiB) and FDG-PET.
Methods:
Patients with PCA (n = 12, age 57.5 ± 7.4, Mini-Mental State Examination [MMSE] 22.2 ± 5.1), AD (n = 14, age 58.8 ± 9.6, MMSE 23.8 ± 6.7), and cognitively normal controls (NC, n = 30, age 73.6 ± 6.4) underwent PiB and FDG-PET. Group differences in PiB distribution volume ratios (DVR, cerebellar reference) and FDG uptake (pons-averaged) were assessed on a voxel-wise basis and by comparing binding in regions of interest (ROIs).
Results:
Compared to NC, both patients with AD and patients with PCA showed diffuse PiB uptake throughout frontal, temporoparietal, and occipital cortex (p < 0.0001). There were no regional differences in PiB binding between PCA and AD even after correcting for atrophy. FDG patterns in PCA and AD were distinct: while both groups showed hypometabolism compared to NC in temporoparietal cortex and precuneus/posterior cingulate, patients with PCA further showed hypometabolism in inferior occipitotemporal cortex compared to both NC and patients with AD (p < 0.05). Patients with AD did not show areas of relative hypometabolism compared to PCA.
Conclusions:
Fibrillar amyloid deposition in PCA is diffuse and similar to AD, while glucose hypometabolism extends more posteriorly into occipital cortex. Further studies are needed to determine the mechanisms of selective network degeneration in focal variants of AD.
Posterior cortical atrophy (PCA) is a focal neurodegenerative disorder of higher visual processing and spatial praxis with relative sparing of memory and insight.1 At autopsy, the majority of patients are found to have underlying Alzheimer disease (AD) as the causative pathology.1–3
The distribution of AD pathology in PCA is controversial, with some studies demonstrating higher numbers of amyloid plaques in primary and association visual cortex in PCA compared to typical AD4,5 and others showing no difference in the distribution of Aβ pathology.2,3 Postmortem comparisons are limited in that they study the end stages of disease, which makes relating changes seen at autopsy to in vivo disease evolution difficult.
Fibrillar Aβ pathology can be measured during life using PET with [11C]-labeled Pittsburgh compound B (PiB-PET).6 PiB binding during life correlates strongly with distribution and burden of fibrillar Aβ found at autopsy.7,8 Previous case reports have described increased occipital PiB binding in individual patients with PCA.9–11 However, occipital PiB binding is also seen in AD.6,12,13 To our knowledge, no study has directly compared PiB uptake at a group level in PCA and AD.
Based on the preponderance of postmortem data and previously demonstrated dissociations between PiB binding and clinical features of AD,14,15 we hypothesized that patients with PCA would show diffuse cortical PiB binding in a pattern indistinguishable from clinically “typical” AD, but greater glucose hypometabolism than patients with AD in occipital cortex, correlating with their selective impairment in visual processing.
METHODS
Study population.
Patients were recruited from PCA and AD cohorts followed at the University of California San Francisco Memory and Aging Center. All patients underwent a history and physical examination, a structured caregiver interview, and neuropsychological tests.16 Visuospatial function was assessed using the modified Rey-Osterrieth design, copy of intersecting pentagons, and the number location task from the Visual Object and Space Perception battery (VOSP).17 Verbal memory was assessed using the 9-item California Verbal Learning Test18 and visual memory with recall of the modified Rey-Osterrieth design. The remainder of the neuropsychological test battery has been previously described.16 Clinical diagnosis was assigned by consensus at a multidisciplinary conference.
Medical records of patients clinically diagnosed with PCA were reviewed by a neurologist (M.H.R.) blinded to PET data to ensure they met the following criteria19: 1) presentation with progressive visual or visuospatial impairment in the absence of ophthalmologic impairment; 2) evidence of complex visual disorder on examination: elements of Balint syndrome, visual agnosia, dressing apraxia, or environmental disorientation; 3) proportionately less memory loss. To meet criteria, patients were required to present with early complaints of visuospatial impairment in the absence of memory complaints. Impairments in higher visual processing on neurologic examination were required. A neuropsychological profile showing predominant visuospatial impairments with relative sparing of verbal episodic memory (in the judgment of the investigator) was also obligatory. Visual memory scores were not considered as these can be impaired in PCA due to primary visuospatial dysfunction rather than true memory loss. Structural MRI was reviewed to confirm a pattern of posterior cortical atrophy involving visual association areas. Subjects with early memory impairment, ophthalmologic disease, extrapyramidal symptoms or signs, hallucinations, cognitive fluctuations, or significant occipito-parietal MRI T2 white matter hyperintensities were excluded. Twelve eligible patients with PCA were enrolled in the study (table 1).
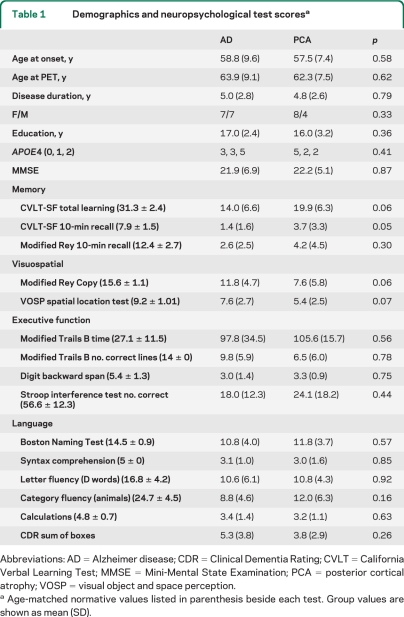
Table 1.
Demographics and neuropsychological test scoresa
Abbreviations: AD = Alzheimer disease; CDR = Clinical Dementia Rating; CVLT = California Verbal Learning Test; MMSE = Mini-Mental State Examination; PCA = posterior cortical atrophy; VOSP = visual object and space perception.
Age-matched normative values listed in parenthesis beside each test. Group values are shown as mean (SD).
A comparison group of 14 patients meeting standard clinical criteria for AD (National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association20) were selected to match the PCA cohort for age, sex, and Mini-Mental State Examination (MMSE) score (table 1). An imaging control group of 30 cognitively normal older individuals (NC) were recruited from the community (mean age = 73.6 ± 6.4, 22 female, 8 male, mean education = 17.7 ± 1.8 years).21 All participants underwent PET imaging between April 2005 and October 2009.
Standard protocol approvals, registrations, and consents.
The study was approved by the University of California Berkeley, UCSF, and Lawrence Berkeley National Laboratory institutional review boards for human research. Consent for the study was provided by the participants or their assigned surrogate decision-makers.
Image analysis.
Image acquisition and preprocessing.
All subjects underwent PET imaging with [11C] PiB and [18F] FDG at Lawrence Berkeley National Laboratory on a Siemens ECAT EXACT HR PET scanner in 3-dimensional acquisition mode. Tracer synthesis, PET acquisition, and preprocessing were performed as previously described22 (see appendix e-1 on the Neurology® Web site at www.neurology.org for details). One patient with PCA did not undergo FDG due to technical reasons.
For PiB, voxel-wise distribution volume rations were calculated using Logan graphical analysis, with the cerebellum time-activity curve used as a reference tissue input function (t = 35–90 minutes).23,24 FDG frames for each subject were summed and normalized to mean activity in the pons.25
Given the differential atrophy patterns seen in AD and PCA,26 we corrected PET data for atrophy by applying a 2-compartmental partial volume correction27 to all subjects with an MRI (10/12 PCA, 12/14 AD, and all NC) (see appendix e-1).15,27 Since the benefit of partial volume correction for PiB data are controversial, all primary analyses were performed using uncorrected PET data, while confirmatory analyses were conducted with atrophy-corrected data in the subset of patients with an MRI.
Voxel-wise group comparisons.
Individual subject PiB and FDG volumes were spatially normalized to Montreal Neurological Institute (MNI) space using SPM 5 (see appendix e-1).15 All normalized images were smoothed with a 12-mm kernel. Voxel-wise comparisons of PiB distribution volume ratio and pons-normalized FDG images were performed in an analysis of covariance model that included diagnosis (PCA, AD, NC) as the condition and education and sex as covariates. Pairwise contrasts were performed among the 3 groups. To allow broad visualization of the data, results were displayed on a template brain as T-maps thresholded at p < 0.001 uncorrected for multiple comparisons. Voxels were considered significant at p < 0.05 after family-wise error (FWE) correction for AD/PCA vs NC contrasts, and at p < 0.001 uncorrected for direct AD vs PCA contrasts.
Region of interest definition.
PET values were extracted in normalized space from regions of interest (ROIs) derived from the Automated Anatomic Labeling Atlas.28 A custom ROI was created by generating 10-mm spheres around the peak voxel detected in right lateral occipitotemporal cortex in a previous study contrasting gray matter volumes in PCA and AD (MNI coordinates x = 39, y = −85, z = −6)29 and the corresponding voxel in the left hemisphere.
To represent tracer binding in occipital cortex relative to global PiB or FDG uptake, an occipital percentage (occipital pct) was calculated: Occipital pct = (mean occipital PiB or FDG)/(mean cortical PiB or FDG).
Statistical analysis.
Group differences in continuous variables were examined using t tests or one-way analysis of variance and Tukey post hoc contrasts. Differences in dichotomous variables were measured using Pearson χ2.
RESULTS
Subject characteristics.
By design, patients with PCA and patients with AD were well-matched for age, education, and disease severity (table 1). NC were older than the patients (p < 0.0001) but were matched for sex and education. The APOE ϵ4 genotype was more common in patients with AD than controls (p = 0.02), but there was no difference in frequency between PCA and AD.
On neuropsychological testing, patients with AD performed more poorly on verbal memory tests (p < 0.05, table 1) and patients with PCA showed nonsignificant trends toward worse performance on visuospatial tasks (p = 0.06 for Rey copy, p = 0.07 for VOSP number location). No differences were found on tests of executive function or language.
All clinically diagnosed patients with PCA and patients with AD showed increased global PiB binding compared to controls on visual inspection, supporting the assumption of underlying AD pathology. None of the subjects have undergone autopsy.
PiB: Voxel-wise comparisons.
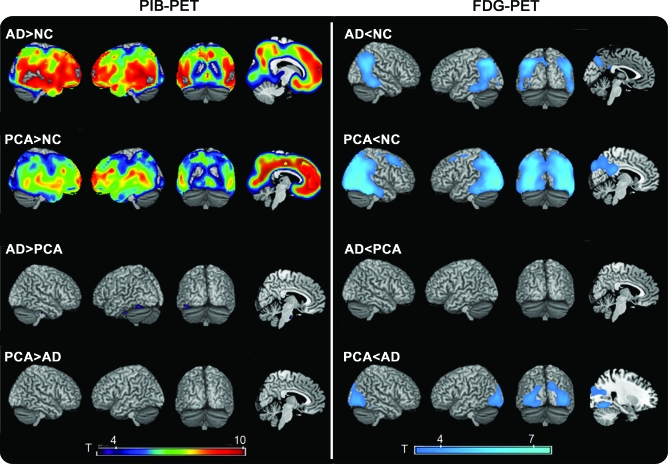
Compared to NC, both patients with AD and patients with PCA showed diffuse, symmetric PiB binding throughout cortex with sparing of primary sensorimotor and visual areas and hippocampus (p [FWE] <0.05, figure 1). There were no regions of increased PiB binding in the PCA > AD condition at a threshold of p < 0.001 uncorrected. In the AD > PCA comparison, small clusters of elevated binding were seen in pons and cerebellum at p < 0.001 uncorrected, but these were not considered significant as they were not located in regions that showed elevated PiB in AD vs NC.
Figure 1. Patterns of Pittsburgh compound B (PiB) and FDG binding in Alzheimer disease (AD) and posterior cortical atrophy (PCA) compared to normal controls (NC) and to each other.
T-score maps are rendered on the ch2 template brain. All results are presented at a threshold of p < 0.001, uncorrected for multiple comparisons.
PiB: ROI comparisons.
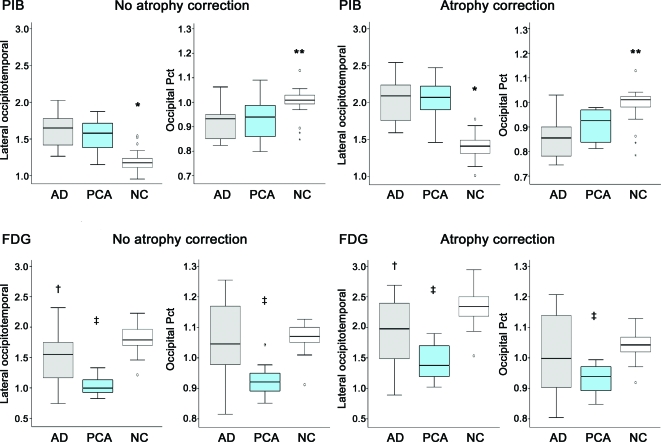
Both AD and PCA groups had significantly higher PiB uptake throughout all ROIs in comparison to NC (p < 0.001, table e-1) except in hippocampus in which a significant difference was detected only between AD and NC (p < 0.05). There were no differences in PiB uptake between AD and PCA in any ROIs or in the occipital pct (0.93 ± 0.08 in PCA, 0.91 ± 0.07 in AD, p = 0.81, figure 2). Following atrophy correction, there was a trend for higher PiB in PCA compared to AD in cuneus and lingual gyrus (p = 0.09 for both) but not in other ROIs or in occipital pct (0.91 ± 0.07 in PCA, 0.86 ± 0.09 in AD, p = 0.26).
Figure 2. Box plots representing pre-atrophy and post-atrophy Pittsburgh compound B (PiB) and FDG uptake for the lateral occipitotemporal region and occipital percentage (ratio of occipital to global cortical PiB/FDG).
*Less than Alzheimer disease (AD) and posterior cortical atrophy (PCA), p < 0.05. **Greater than AD and PCA, p < 0.05. †Less than normal controls (NC), p < 0.05. ‡Less than AD and NC, p < 0.05.
FDG: Voxel-wise comparisons.
Compared to NC, both patient groups showed hypometabolism in bilateral angular gyrus, middle and inferior temporal gyrus, and inferior parietal lobule, with hypometabolism in PCA extending posteriorly to bilateral inferior, middle, and superior occipital gyrus, precuneus, and right calcarine cortex (p [FWE] <0.05, figure 1). When directly compared to AD, patients with PCA showed relative hypometabolism in bilateral fusiform gyrus, inferior, middle, and superior occipital gyrus, left lingual gyrus, right inferior temporal cortex, bilateral cuneus, and bilateral calcarine cortex (p < 0.001 uncorrected). These differences did not survive multiple comparisons correction. There were no regions of relative hypometabolism in AD compared to PCA at p < 0.001 uncorrected.
FDG: ROI comparisons.
Compared to NC, FDG uptake was decreased bilaterally in both PCA and AD throughout lateral temporoparietal cortex, precuneus, inferior and middle occipital gyrus, and the lateral occipitotemporal ROI (p < 0.05, table e-2). Subjects with PCA alone showed hypometabolism in bilateral fusiform gyrus, cuneus, superior occipital, and left lingual gyrus (p < 0.05). These findings remained significant after atrophy correction (p < 0.05). Compared to AD, patients with PCA showed lower FDG in the lateral occipitotemporal cortex, inferior, middle, and superior occipital gyrus, and right cuneus (p < 0.05, figure 2). Following atrophy correction, there was lower metabolism in PCA relative to AD in bilateral inferior occipital cortex and the lateral occipitotemporal ROI (p < 0.05). Pre-atrophy and post-atrophy correction, the FDG occipital pct in PCA (0.92 ± 0.06 pre-atrophy correction, 0.93 ± 0.05 post-correction) was lower than in AD (1.06 ± 0.13 pre-atrophy, 1.02 ± 0.14 post-correction, p < 0.05 for both) and NC (1.07 ± 0.04 pre-atrophy, 1.04 ± 0.04 post-atrophy correction, p < 0.05 for both). There were no regions of hypometabolism in AD relative to PCA.
DISCUSSION
In this study we compared clinical features, fibrillar Aβ deposition, and regional glucose metabolism in matched patients presenting clinically with PCA and AD. As expected, patients with PCA showed greater impairment in visuospatial tasks and patients with AD showed relative deficits in episodic memory. All patients with PCA showed high PiB uptake, consistent with previous reports that the clinical syndrome of PCA is very often associated with underlying AD pathology.1–3 The pattern of cortical PiB binding in PCA was diffuse, affecting anterior and posterior cortical regions, visual and nonvisual areas, and on direct statistical comparison was indistinguishable from the pattern seen in AD. In contrast, patients with PCA showed a more posterior pattern of glucose hypometabolism with greater occipital involvement than was seen in patients with clinically “typical” AD. These findings suggest that distinct clinical features and regional hypometabolism in PCA and AD are not related to fibrillar amyloid distribution.
Previous autopsy studies have yielded conflicting results regarding the distribution of amyloid pathology in PCA, with some studies reporting 3–5 times more plaques in visual areas in patients with PCA compared to patients with typical AD,5 and others finding similar plaque counts in these regions in PCA and AD.2,3 The reasons for these discrepant findings are unclear, but may include subject factors (e.g., age, disease severity, failure to control for copathology) as well as the methods used to quantify plaques (e.g., lesion counts vs more rigorous quantification, staining techniques, discrimination between diffuse vs neuritic plaques). Our data suggest that, in the mild-to-moderate disease stage, there is no difference in the distribution of fibrillar Aβ pathology between PCA and AD. These findings are congruent with observations from patients with underlying AD who present with another focal cortical syndrome, primary progressive aphasia (PPA). Though neurodegeneration in PPA is highly asymmetric and preferentially involves the language network,30,31 the distribution of amyloid pathology (as measured by PiB or at autopsy) in AD-associated cases is symmetric and indistinguishable from that found in typical AD.14,32
Previously, our group used voxel-based morphometry (VBM) to compare atrophy patterns in patients with early age-at-onset AD, PCA, and logopenic aphasia (LPA), a PPA variant associated with underlying AD.31 We found that patients with all 3 syndromes showed overlapping atrophy in precuneus/posterior cingulate and lateral temporoparietal cortex. Additional right ventral-occipital/parietal atrophy was found in PCA and left middle/superior temporal gyrus atrophy in LPA. Similar results were reported in another study contrasting atrophy patterns in AD and PCA.31 Likewise, in this study we found comparable glucose hypometabolism in AD and PCA in temporoparietal cortex and precuneus, with extension of hypometabolism into occipitotemporal cortex in PCA. These observations suggest that precuneus/posterior cingulate and lateral temporoparietal involvement is a common feature of AD-associated syndromes, with extension of neurodegeneration into other, distinct regions in PCA and LPA. It is intriguing to speculate whether common involvement of these regions in AD is related to their proposed function as highly interconnected “cortical hubs,” which may render them susceptible to both early Aβ aggregation (due to high synaptic activity with resultant Aβ release) and Aβ-mediated neurodegeneration (due to high metabolic demand).33 The high level of connectivity may also enable the spread of disease from these regions into several cortical networks,34 including those underlying memory, language, and visual function. Our work in early-onset AD,15 PPA,14 and now PCA suggests that the respective involvement of these networks is not explained by the distribution of amyloid. Rather, it may be the relative vulnerability of networks in an individual that determines the neurodegenerative pattern and clinical phenotype of AD. In most patients the posterior “default mode network” may be most vulnerable to Aβ-mediated neurodegeneration, perhaps because of high metabolic demand,35 leading to a “typical” amnestic AD phenotype. In individuals with PPA, however, the language network may be particularly vulnerable, as suggested by the high rate of developmental language disorders in individuals who develop PPA later in life.36 To our knowledge, such premorbid risk factors have not been systematically studied in PCA, where visual networks are disproportionately affected. Future studies that estimate premorbid “reserve” in specific cognitive domains, investigate the integrity and function of the associated neural networks, and relate these findings to clinical and degenerative phenotype may help elucidate the mechanisms of selective network degeneration in focal variants of AD.
Another possible explanation for the dissociation between the patterns of PiB and FDG binding in this study is that the fibrillar Aβ deposits imaged by PiB may not be the critical pathology driving neurodegeneration. Soluble Aβ oligomers are considered to be the most neurotoxic Aβ species,37,38 yet are not bound by PiB. It is possible the patients with PCA have high concentrations of Aβ oligomers in visual areas, though it is not clear why this would not be reflected by higher concentrations of fibrillar Aβ that is thought to be in equilibrium with the soluble compartment. Finally, the degenerative pattern is likely more closely related to the distribution of neurofibrillary pathology. Higher neurofibrillary tangle (NFT) counts in visual regions in PCA compared to AD is a consistent finding across pathology studies,2,3,5 and asymmetric NFT have also been reported in PPA.32 It is unknown, however, whether in AD NFT form independently or secondary to Aβ-driven processes.
Our results differ from case reports of single subjects with PCA with posterior-predominant PiB binding10 or disproportionate occipital PiB compared to AD.9 While most subjects with PCA in our cohort showed diffuse PiB binding, individual subjects with posterior-predominant binding patterns could be identified, though conversely frontal-predominant binding was seen in one subject, and similar variability in binding patterns was also seen in the “typical” AD group. Following atrophy correction, there was a trend toward higher mean PiB values for PCA compared to AD in the cuneus and lingual gyrus (p = 0.09), leaving open the possibility that subtle increases in occipital amyloid are found in PCA and that our study was underpowered to detect them, though it is doubtful that they are of the 3- to 5-fold higher magnitude reported in some pathology studies.5 Conversely, this result may be due to overinflation of PET counts by the atrophy correction procedure, which remains controversial for PiB data. Importantly, regions that showed marked hypometabolism in PCA compared to AD showed essentially identical PiB uptake in the 2 groups even after atrophy correction (e.g., lateral occipitotemporal and inferior occipital cortex), supporting the assertion that PiB and FDG patterns are dissociated in the 2 syndromes.
Our study has limitations. The sample sizes were relatively small, potentially limiting our power to detect differences between AD and PCA, though the size of our cohorts is comparable to those found in previous studies of PCA, and had sufficient power to detect differences in FDG binding and marginal differences in clinical performance. Although initial studies suggest that in vivo PiB binding correlates highly with postmortem Aβ measures,11,8 the limitations of this technique have not yet been fully identified. It is possible that patients with PCA in our study had a secondary pathology in addition to AD, though this is less likely given the exclusion of subjects with clinical features suggestive of dementia with Lewy bodies, corticobasal degeneration, or prion disease. Since we matched the AD and PCA groups for age, our AD control group is largely composed of early age-at-onset patients (mean age at onset 58.8 years). This may have minimized both clinical and anatomic differences between AD and PCA, since patients with early-onset AD have greater visuospatial impairment and posterior cortical atrophy and relatively preserved episodic memory and medial temporal lobes compared to late-onset patients.39,40
Supplementary Material
- AD
- Alzheimer disease
- DVR
- distribution volume ratio
- FWE
- family-wise error
- LPA
- logopenic aphasia
- MMSE
- Mini-Mental State Examination
- MNI
- Montreal Neurological Institute
- NC
- normal control
- NFT
- neurofibrillary tangle(s)
- PCA
- posterior cortical atrophy
- PiB
- Pittsburgh compound B
- PPA
- primary progressive aphasia
- ROI
- region of interest
- VBM
- voxel-based morphometry
- VOSP
- Visual Object and Space Perception battery.
DISCLOSURE
Dr. Rosenbloom, A. Alkalay, N. Agarwal, and Dr. Baker report no disclosures. Dr. O'Neill receives research support from Genzyme Corporation, the US Department of Energy, the US Army Medical Research & Materiel Command, and the NIH. Dr. Janabi receives research support from the NIH. I.V. Yen, M. Growdon, J. Jang, C. Madson, and E.C. Mormino report no disclosures. Dr. Rosen serves on a scientific advisory board for Avanir Pharmaceuticals; receives publishing royalties for The Emotional Brain (Oxford University Press); and receives research support from the NIH (NIA, NINDS, DHS/ADP/ARCC) and the Larry L. Hillblom Foundation. Dr. Gorno-Tempini receives research support from the NIH (NINDS, NIA), the John Douglas French Alzheimer's Foundation, the Alzheimer's Association, the Larry L. Hillblom Foundation, the Koret Family Foundation, and the McBean Family Foundation. Dr. Weiner serves on scientific advisory boards for Bayer Schering Pharma, Eli Lilly and Company, CoMentis, Inc., Neurochem Inc, Eisai Inc., Avid Radiopharmaceuticals Inc., Aegis Therapies, Genentech, Inc., Allergan, Inc., Lippincott Williams & Wilkins, Bristol-Myers Squibb, Forest Laboratories, Inc., Pfizer Inc, McKinsey & Company, Mitsubishi Tanabe Pharma Corporation, and Novartis; has received funding for travel from Nestlé and Kenes International and to attend conferences not funded by industry; serves on the editorial board of Alzheimer's & Dementia; has received honoraria from the Rotman Research Institute and BOLT International; serves as a consultant for Elan Corporation; receives research support from Merck & Co., Radiopharmaceuticals Inc., the NIH, the Veterans Administration, and the State of California; and holds stock in Synarc and Elan Corporation. Dr. Miller serves on a scientific advisory board for the Alzheimer's Disease Clinical Study; serves as an Editor for Neurocase and as an Associate Editor of ADAD; receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge, 2009), Handbook of Neurology (Elsevier, 2009), and The Human Frontal Lobes (Guilford, 2008); serves as a consultant for Lundbeck Inc., Elan Corporation, and Allon Therapeutics, Inc.; serves on speakers' bureaus for Novartis and Pfizer Inc.; and receives research support from Novartis and the NIH/NIA and the State of California Alzheimer's Center. Dr. Jagust has served on a scientific advisory board for Genentech, Inc.; serves as Associate Editor for Frontiers in Human Neuroscience and on the editorial boards of Annals of Neurology, Brain Imaging and Behavior, and Alzheimer's Disease and Associated Disorders; receives publishing royalties for Imaging the Aging Brain (Oxford University Press, 2009); has served as a consultant for Synarc, Elan Corporation/Janssen Alzheimer Immunotherapy, Genentech, Inc., Abbott, GE Healthcare, Ceregene, Bayer Schering Pharma, Schering-Plough Corp., TauRx Pharmaceuticals, Otsuka Pharmaceutical Co., Ltd., and Merck & Co; and receives research support from the NIH and from the Alzheimer's Association. Dr. Rabinovici serves on scientific advisory boards for Novartis and GE Healthcare and receives research support from the NIH/NIA, the Alzheimer's Association, and the John Douglas French Alzheimer's Foundation.
REFERENCES
- 1. Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer's disease. Brain 2007;130:2636–2645 [DOI] [PubMed] [Google Scholar]
- 2. Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology 2004;63:1168–1174 [DOI] [PubMed] [Google Scholar]
- 3. Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology 2004;63:1175–1180 [DOI] [PubMed] [Google Scholar]
- 4. von Gunten A, Bouras C, Kovari E, Giannakopoulos P, Hof PR. Neural substrates of cognitive and behavioral deficits in atypical Alzheimer's disease. Brain Res Rev 2006;51:176–211 [DOI] [PubMed] [Google Scholar]
- 5. Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer's disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res 1997;37:3609–3625 [DOI] [PubMed] [Google Scholar]
- 6. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol Mar 2004;55:306–319 [DOI] [PubMed] [Google Scholar]
- 7. Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain 2008;131:1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh compound B confirmed at autopsy: a case report. Arch Neurol 2007;64:431–434 [DOI] [PubMed] [Google Scholar]
- 9. Tenovuo O, Kemppainen N, Aalto S, Nagren K, Rinne JO. Posterior cortical atrophy: a rare form of dementia with in vivo evidence of amyloid-beta accumulation. J Alzheimers Dis 2008;15:351–355 [DOI] [PubMed] [Google Scholar]
- 10. Ng SY, Villemagne VL, Masters CL, Rowe CC. Evaluating atypical dementia syndromes using positron emission tomography with carbon 11 labeled Pittsburgh compound B. Arch Neurol 2007;64:1140–1144 [DOI] [PubMed] [Google Scholar]
- 11. Kambe T, Motoi Y, Ishii K, Hattori N. Posterior cortical atrophy with [C] Pittsburgh compound B accumulation in the primary visual cortex. J Neurol Epub 2009. November 14 [DOI] [PubMed] [Google Scholar]
- 12. Edison P, Archer HA, Hinz R, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PiB and [18F]FDG PET study. Neurology 2007;68:501–508 [DOI] [PubMed] [Google Scholar]
- 13. Kemppainen NM, Aalto S, Wilson IA, et al. Voxel-based analysis of PET amyloid ligand [11C]PiB uptake in Alzheimer disease. Neurology 2006;67:1575–1580 [DOI] [PubMed] [Google Scholar]
- 14. Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008;64:388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer's disease is not related to amyloid burden. Brain 2010;133:512–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003;16:211–218 [DOI] [PubMed] [Google Scholar]
- 17. Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company; 1991 [Google Scholar]
- 18. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, second ed. San Antonio, TX: The Psychological Corporation; 2000 [Google Scholar]
- 19. McMonagle P, Deering F, Berliner Y, Kertesz A. The cognitive profile of posterior cortical atrophy. Neurology 2006;66:331–338 [DOI] [PubMed] [Google Scholar]
- 20. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 21. Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain 2009;132:1310–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rabinovici GD, Furst AJ, O'Neil JP, et al. 11C-PiB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2007;68:1205–1212 [DOI] [PubMed] [Google Scholar]
- 23. Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 2005;46:1959–1972 [PubMed] [Google Scholar]
- 24. Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005;25:1528–1547 [DOI] [PubMed] [Google Scholar]
- 25. Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr 1995;19:541–547 [DOI] [PubMed] [Google Scholar]
- 26. Whitwell JL, Jack CR, Jr, Kantarci K, et al. Imaging correlates of posterior cortical atrophy. Neurobiol Aging 2007;28:1051–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Jr, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr 1990;14:561–570 [DOI] [PubMed] [Google Scholar]
- 28. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–289 [DOI] [PubMed] [Google Scholar]
- 29. Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain 2007;130:708–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology 2009;73:1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008;63:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 2009;29:1860–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009;62:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol 2008;65:244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mucke L, Masliah E, Yu GQ, et al. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 2000;20:4050–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walsh DM, Selkoe DJ. A beta oligomers: a decade of discovery. J Neurochem 2007;101:1172–1184 [DOI] [PubMed] [Google Scholar]
- 39. Kim EJ, Cho SS, Jeong Y, et al. Glucose metabolism in early onset versus late onset Alzheimer's disease: an SPM analysis of 120 patients. Brain 2005;128:1790–1801 [DOI] [PubMed] [Google Scholar]
- 40. Frisoni GB, Pievani M, Testa C, et al. The topography of grey matter involvement in early and late onset Alzheimer's disease. Brain 2007;130:720–730 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.