Abstract
Objective:
The relation of overweight to dementia is controversial. We aimed to examine the association of midlife overweight and obesity with dementia, Alzheimer disease (AD), and vascular dementia (VaD) in late life, and to verify the hypothesis that genetic and early-life environmental factors contribute to the observed association.
Methods:
From the Swedish Twin Registry, 8,534 twin individuals aged ≥65 (mean age 74.4) were assessed to detect dementia cases (DSM-IV criteria). Height and weight at midlife (mean age 43.4) were available in the Registry. Data were analyzed as follows: 1) unmatched case-control analysis for all twins using generalized estimating equation (GEE) models and 2) cotwin matched case-control approach for dementia-discordant twin pairs by conditional logistic regression taking into account lifespan vascular disorders and diabetes.
Results:
Among all participants, dementia was diagnosed in 350 subjects, and 114 persons had questionable dementia. Overweight (body mass index [BMI] >25–30) and obesity (BMI >30) at midlife were present in 2,541 (29.8%) individuals. In fully adjusted GEE models, compared with normal BMI (20–25), overweight and obesity at midlife were related to dementia with odds ratios (ORs) (95% CIs) of 1.71 (1.30–2.25) and 3.88 (2.12–7.11), respectively. Conditional logistic regression analysis in 137 dementia-discordant twin pairs led to an attenuated midlife BMI-dementia association. The difference in ORs from the GEE and the matched case-control analysis was statistically significant (p = 0.019).
Conclusions:
Both overweight and obesity at midlife independently increase the risk of dementia, AD, and VaD. Genetic and early-life environmental factors may contribute to the midlife high adiposity–dementia association.
Body mass index (BMI) provides an indirect measure of adiposity, and is strongly correlated with total body fat tissue.1 Adiposity may influence or be influenced by brain structures and functions, which may be involved in dementing processes.2 In the last decade, many population-based longitudinal studies have evaluated the relationship between adiposity and dementia. Several reports have shown that midlife obesity increases the risk of dementia in late life3–10; however, the effect of midlife overweight on dementia is controversial.3,5,7,9 Currently, the prevalence of overweight and obesity is over 50% among adults in the United States and Europe.11
Although adiposity may be linked to dementia through several biologically plausible pathways,12 our understanding of the mechanisms for such an association is still limited. Both obesity and dementia are complex genetic and lifestyle-related disorders. Evidence has shown that early-life environments (such as childhood socioeconomic situation) may affect the development of obesity and dementia.13,14 The life course development of the adiposity-dementia association has been suggested,15 but the contribution of genetics and early life environments to the relationship has not been investigated. In the present study, we sought to 1) verify the long-term effect of midlife overweight and obesity measured by BMI on the risk of dementia, Alzheimer disease (AD), and vascular dementia (VaD) taking into account diabetes and lifespan vascular disorders using unmatched case-control analysis; and 2) explore whether genetic and early-life environments could explain the observed association between midlife adiposity and dementia by cotwin matched case-control approach using data from the Swedish Twin Registry.
METHODS
Participants and data collection.
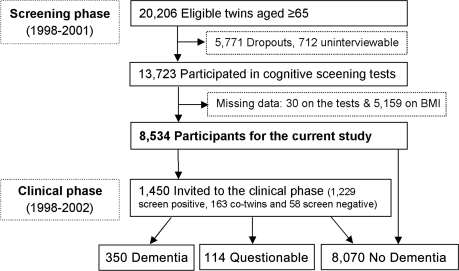
Participants were drawn from the nationwide Swedish Twin Registry.16 In 1998–2001, all living twins in the registry who were born in 1935 and earlier (aged ≥65 years) were invited to participate in a telephone interview screening for most common diseases, which included a brief cognitive assessment.17 In brief, a total of 20,206 individuals in the registry were eligible for screening. Of them, 13,693 twins completed the cognitive screening module, resulting in a participation rate of 67.8%. Information concerning demographic factors, education, current height and weight, health status and behavior, current and past diseases, and use of medications was also obtained during the interview. In addition, self-report information on height and weight at midlife (mean age 43.4) was collected for all like-sexed twin pairs by the Registry in 1967, i.e., on average 30 years preceding the screening phase. Out of the 13,693 twins, 8,534 had data on height and weight at midlife available for the current analysis (figure 1).
Figure 1. Flow chart of the study population.
BMI = body mass index.
Screening phase.
The screening test included the TELE questionnaire for participants18,19 and the Blessed Dementia Rating Scale for informants when participants performed poorly on the TELE.19,20 The TELE and BDRS were combined into an ordinal scale with scores ranging from 0 (cognitively intact) to 3 (cognitive dysfunction). Participants who screened positive (i.e., scored 3) and their cotwins were invited to a clinical examination (n = 1,450).
Clinical phase and diagnosis.
The full clinical workup consisted of a visit by an assessment team composed of a nurse and a physician using a protocol that generally followed the Consortium to Establish a Registry for Alzheimer's Disease (CERAD). The protocol included physical and neurologic examination, a review of medical history, informant interview, and a neuropsychological assessment. The neuropsychological battery included the Mini-Mental State Examination (MMSE), CERAD word list immediate and delayed recall, verbal fluency, block design, figure copying, judgment, information, symbol-digit, and prospective memory, as well as the Memory in Reality test.
The assessment team made an initial diagnosis of dementia and dementia subtypes following the DSM-IV criteria.21 All protocols were reviewed by a diagnostic board, consisting of a neurologist and a neuropsychologist, who were blind to the assessment team's preliminary diagnosis, and verified the preliminary diagnosis. More details of the clinical examination and diagnostic procedure have been reported previously.17 The cases completely fulfilling the DSM-IV criteria were diagnosed as “dementia,” in contrast with a category of “questionable dementia,” which was used for individuals who did not fulfill one of the first 3 DSM-IV diagnostic criteria, but did exhibit cognitive impairment or functional disability. Participants were first classified as no dementia, questionable, or dementia. A differential diagnosis was then given for dementia subtype according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria for AD22 and the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria for VaD.23 Through the clinical workup, dementia was diagnosed in 350 subjects including 232 AD and 74 VaD cases, and 114 had questionable dementia. Among all participants, 8,070 did not have dementia.
Data on history of diabetes and vascular diseases obtained at the screening interview were integrated with data from the Inpatient Discharge Registry (IDR), which encompasses all hospital discharges in Sweden since 1969. All discharge diagnoses for participants who were hospitalized during 1969–2001 were obtained. Each record in the IDR included up to 8 discharge diagnoses according to the International Classification of Diseases, 8th revision (ICD-8) between 1969 and 1986 and 9th revision (ICD-9) since 1987. Medical conditions derived from the inpatient register database included diabetes (ICD-8, 9 code 250), hypertension (ICD-8 codes 400–404; ICD-9 codes 401–405), ischemic heart disease (ICD-8, 9 codes 410–414), cardiac dysrhythmia, heart failure or other myocardial insufficiency (ICD-8, 9 codes 427 and 428), and stroke (ICD-8, 9 codes 430–438). Diabetes and vascular diseases were deemed present if reported by self or informant, or if recorded in the inpatient registry. Survival status from midlife to the screening phase was obtained from the Cause of Death Register.
Validation of self-reported height and weight.
A previous study indicated that the correlations between self-reported and measured height and weight were 0.97 for height and 0.95 for weight.24 The mean differences (±SD) between self-reported and measured values were 1.2 ± 2.4 cm for height and 0.8 ± 4.0 kg for weight. In the present study, the correlation between self-reported height and weight at the screening phase and measured height and weight at the clinical phase were 0.91 (p < 0.001) and 0.93 (p < 0.001, n = 864), respectively. Pearson correlation coefficients were 0.87 (p < 0.001, n = 931) for the correlation between the height at midlife recorded in the twin registry and measured height at the clinical examination.
Standard protocol approvals.
Informed consents were required from all participants during the telephone interview and again in the clinical phase. The data collection procedures were reviewed and approved by the Swedish Data Inspection Board, Stockholm Sweden, the Regional Ethics Committee at Karolinska Institutet, Stockholm, and the Institutional Review Board of the University of Southern California.
Statistical analysis.
BMI was calculated as self-reported weight in kilograms divided by self-reported height in meters squared, and categorized into 4 groups: underweight (<20), normal weight (20–25), overweight (>25–30), and obese (>30).25 Midlife BMI was used as both continuous and categorical variables in the analysis.
The characteristics of participants in different groups were compared using χ2 tests for categorical variables, one-way analysis of variance for continuous variables. Multinomial logistic regression was first performed to estimate the odds ratios (OR) and 95% confidence intervals (CI) of dementia (350 dementia cases vs 8,070 controls) and questionable dementia (114 questionable dementia cases vs 8,070 controls) separately in relation to BMI. Further, 2 analytical strategies were applied to address the following aims: 1) unmatched case-control analyses was performed in all subjects (464 dementia and questionable dementia cases vs 8,070 controls) using generalized estimating equations (GEE) models, which are conceptually equivalent to logistic regression for the analysis of classic case-control design, but control for the clustering of twins within a pair; and 2) cotwin matched case-control analyses was carried out using conditional logistic regression models among the 137 dementia-discordant twin pairs after exclusion of 3,476 single twins, and 2,357 both no dementia and 35 both dementia twin pairs. The latter strategy allows matching for unmeasured familial factors, which could reflect genetic background or early life environment. Cases and controls are comparable with respect to genetic and early life environmental history (such as prenatal and postnatal nutritional status, and childhood socioeconomic status). If the association found in GEE analyses becomes attenuated in cotwin matched case-control analyses, genetic factors or familial environments or both are likely to contribute to the association. In contrast, if a significant association remains when using cotwin matched pairs, the influences of genetic or early environmental factors on the association are likely to be marginal.
Logistic regression was used to test the difference in ORs from GEE model and conditional logistic regression by examining the difference of overweight and obesity at midlife among unmatched vs cotwin controls. Age, sex, education, zygosity, diabetes, and vascular diseases were considered as confounders in all models. The statistical analyses were performed using SAS statistical software version 9.1 and Stata SE 10.
RESULTS
Population characteristics.
Among the 8,534 participants, 350 (4.1%) were dementia cases including 232 AD and 74 VaD, and 114 (1.3%) were diagnosed with questionable dementia. Among those included in the analysis, 6.0% of the females and 4.6% of the males had dementia or questionable dementia (χ2 = 7.82, p = 0.005).
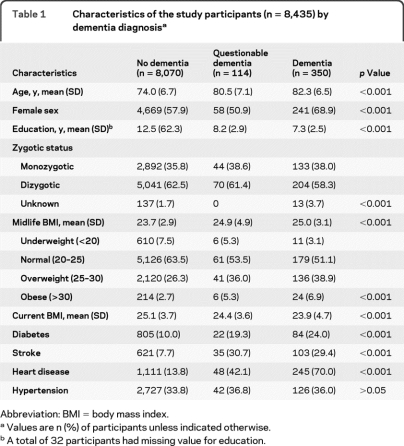
Compared to participants without dementia, twins with dementia or questionable dementia were older, had a lower level of education and current BMI, had higher midlife BMI, and were more likely to have diabetes, stroke, and heart disease. A total of 2,541 twins (29.8%) were overweight or obese at midlife (table 1).
Table 1.
Characteristics of the study participants (n = 8,435) by dementia diagnosisa
Abbreviation: BMI = body mass index.
Values are n (%) of participants unless indicated otherwise.
A total of 32 participants had missing value for education.
BMI in relation to questionable dementia and dementia.
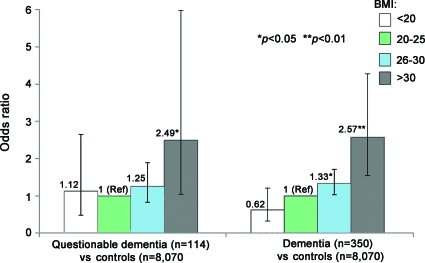
In the multinomial logistic analysis of the entire sample, midlife BMI as a continuous variable was significantly associated with an increased risk of dementia (OR 1.08, 95% CI 1.03–1.14) as well as questionable dementia (OR 1.07, 95% CI 1.04–1.11) after adjustment for potential confounders. Figure 2 shows the multi-adjusted ORs of dementia and questionable dementia related to BMI as categorical variable. As the midlife BMI-related ORs for dementia and questionable dementia were similar, these 2 categories were combined as an outcome of dementia in subsequent analyses.
Figure 2. Odds ratio (OR) and 95% confidence interval (CI) of dementia and questionable dementia related to midlife body mass index (BMI), after adjustment for age, sex, education, zygosity, diabetes, stroke, hypertension, and heart disease (results from Multinomial Logistic Regression).
Unmatched analysis.
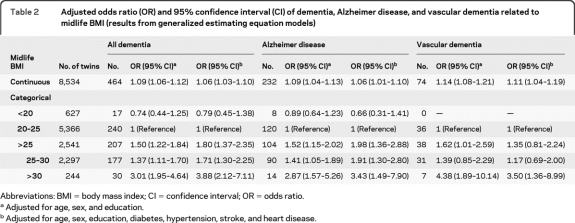
In GEE models, higher midlife BMI was associated with greater risk of all dementia, AD, and VaD using BMI as a continuous variable. When BMI was analyzed as a categorical variable, compared with normal weight, overweight was associated with an increased risk of dementia and AD, while obesity increased the risk of dementia, AD, and VaD controlling for age, sex, and education. Further, the association was not attenuated by additional adjusting for diabetes and vascular diseases (table 2). However, the statistical power was limited for the BMI-VaD association due to the few VaD cases.
Table 2.
Adjusted odds ratio (OR) and 95% confidence interval (CI) of dementia, Alzheimer disease, and vascular dementia related to midlife BMI (results from generalized estimating equation models)
Abbreviations: BMI = body mass index; CI = confidence interval; OR = odds ratio.
Adjusted for age, sex, and education.
Adjusted for age, sex, education, diabetes, hypertension, stroke, and heart disease.
Matched analysis.
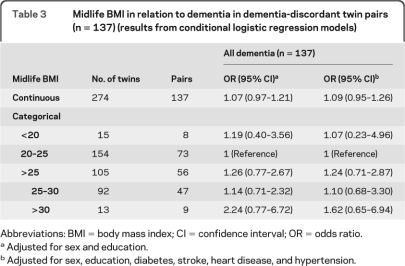
Among the 137 dementia discordant twin pairs, 44 were monozygotic (MZ), 90 were dizygotic (DZ), and 3 were unknown zygosity. In the cotwin matched case-control analyses, the association of midlife overweight and obesity (BMI >25) with dementia was attenuated, and no longer significant (table 3).
Table 3.
Midlife BMI in relation to dementia in dementia-discordant twin pairs (n = 137) (results from conditional logistic regression models)
Abbreviations: BMI = body mass index; CI = confidence interval; OR = odds ratio.
Adjusted for sex and education.
Adjusted for sex, education, diabetes, stroke, heart disease, and hypertension.
The difference between the ORs of dementia related to BMI >25 (overweight and obesity) from GEE model based on the entire sample (age- and sex-adjusted OR 1.56 [95% CI 1.28–1.91]) and from conditional logistic model based on dementia-discordant pairs (sex-adjusted OR 0.88 [95% CI 0.64–1.21]) was tested by comparing high BMI (>25) among the unmatched and matched controls. The difference was statistically significant (OR 1.62 [95% CI 1.12–2.66 p = 0.019]), indicating that genetic and family environmental factors may contribute to the high adiposity-dementia association.
Supplementary analyses.
The dropout rate in the screening phase of this study was 32.2% (6,531/20,206) mainly due to refusal (74.8%) and to death (4.6%). Multivariable GEE model showed that being a dropout was associated with ORs of 1.05 (95% CI 1.02–1.06) for old age, 1.06 (95% CI 0.86–1.39) for female gender, 0.77 (95% CI 0.72–0.81) for education (per 1-year increase), 1.05 (95% CI 0.81–1.35) for stroke, 0.95 (95% CI 0.72–1.27) for diabetes, 0.79 (95% CI 0.63–1.03) for heart disease, and 0.98 (95% CI 0.74–1.29) for hypertension.
Further, we repeated the analysis of the BMI–dementia association leaving out the twins who scored 3 at cognitive screening but were diagnosed as no dementia or not referred to clinical workup (n = 655). This produced results similar to those from the initial analysis. Finally, we examined the effect of BMI >25 on mortality from midlife to late life (the screening phase). Among all like-sexed twins born before 1936, 4,825 died before the screening phase. In the GEE model, BMI >25 (overweight and obesity) at midlife was related to elevated risk of death with multiadjusted OR of 1.30 (95% CI 1.19–1.42).
DISCUSSION
In this nationwide Swedish twin study, overweight and obesity at midlife increase the risk of all dementia, AD, and VaD, independently of lifespan diabetes and vascular diseases. Findings from the cotwin matched analyses suggest that familial factors (genetic factors and early life environments) contribute to the association between midlife high adiposity and dementia in late life.
A growing body of evidence suggests that a high level of adiposity is associated with cognitive decline and dementia10,26,27; however, the relation between BMI and dementia among people aged over 65 is controversial.28–30 Several population-based studies have reported an effect of obesity at middle age on dementia risk.3,4,7,9 Only one study reported an increased dementia risk in people with overweight.5 Despite the fact that high adiposity is associated with dementia, there remains a debate whether this concerns only AD or also VaD. Some prospective studies did find an association between obesity and increased risk of VaD,7,29,31 while others did not.9,32–34 In this Swedish twin cohort, we found that having dementia or AD was associated with more than a 70% higher odds of being overweight at midlife, while the odds of being obese at midlife were higher for those with AD as well as those with VaD. Although the effect of midlife overweight on dementia is not as substantial as that of obesity, its impact on public health and clinical practice is significant due to the fact that there are 1.6 billion overweight adults worldwide.35
Several potential biological mechanisms may explain the association between adiposity and dementia. First, higher BMI is associated with diabetes and vascular diseases, which are related to dementia risk.12 Nonetheless, in our study, the association between midlife high BMI and dementia remained significant after controlling for lifespan vascular diseases, suggesting that nonvascular pathways might play an important role in the adiposity-dementia association. Second, higher adiposity at midlife may reflect a lifetime exposure to an altered metabolic and inflammatory state. Adiposity is one component of the metabolic syndrome which has been related to cognitive decline.36 Further, adipose tissue is the largest endocrine organ and secretes inflammatory cytokines and growth hormones; some of them (such as leptin, interleukin-6, and C-reactive protein) may affect cognitive functioning. Leptin is involved in deposition of amyloid β-42, and plays a role in neurodegenerative process.37
Twin studies involving a life-course approach may help to identify genetic and environmental influences on the development of chronic diseases, as twins provide naturally matched pairs, in which confounding effects of a large number of potentially causal factors (e.g., genetics and childhood environments) may be removed when comparisons are made within twin pairs. In cotwin control analyses, the association between higher midlife BMI and dementia was significantly attenuated, and thus may be attributed in part to genetic and early environmental factors such as childhood socioeconomic situation. However, twins may also experience similar exposures even at midlife and late life. Studies have reported that early life exposure to an imbalanced nutrition and disadvantaged economic status are related to a greater risk of obesity in adult life38 and dementia in late life.39 Our findings suggest that early-life environmental and genetic factors contribute to the link between adiposity and dementia.
Some limitations of the study should be mentioned. First, the use of prevalent dementia cases may have introduced some confounding effect due to differential survival among cases and controls. In addition, midlife obesity is associated with elevated mortality in our study, which would probably lead to an underestimation of the strength of the observed association. Second, compared to participants, the nonresponders were older, less educated, and more likely to be women, but did not differ from participants in terms of vascular risk factors. As old age, low education, and female sex are risk factors for dementia, possible selection bias could arise if the prevalence of dementia in this cohort differs from that in the general population. However, the prevalence of dementia in this study was comparable with several studies of dementia prevalence in Europe and the United States.17 Third, midlife height and weight relied on self-reported information, which, however, were validated by a previous study. In addition, BMI alone might not be an ideal representation of body composition, although BMI has been widely used in population-based studies. Additional anthropometric measures, such as waist circumference, would be useful. Finally, both obesity and AD are genetically influenced disorders with substantial concordance in twins. Thus, the matched pairs could be regarded as overmatched, as twin pairs are similar on many aspects. Nevertheless, the comparison of the results from the entire sample and from matched pairs provides information about the potential role of genetic and familial influences in the observed association. Finally, in the matched analysis, both MZ and DZ twins were included due to limited number of dementia-discordant MZ twin pairs. Hence, genetic effects were not perfectly controlled for.
Our results provide further support for the important role of high adiposity at midlife in the development of dementia, and highlight the need to control body weight as early as midlife for prevention of dementia in late life. These findings have relevant implications for public health, as the risk of dementia could be reduced by midlife weight loss.40 Genetic and early-life environmental factors may contribute to the link of overweight and obesity to dementia, suggesting that the high adiposity-dementia association might develop across the lifespan.
ACKNOWLEDGMENT
The authors thank the members of the HARMONY study group for data collection and management.
Footnotes
- AD
- Alzheimer disease
- BMI
- body mass index
- CERAD
- Consortium to Establish a Registry for Alzheimer's Disease
- CI
- confidence interval
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- DZ
- dizygotic
- GEE
- generalized estimating equation
- ICD
- International Classification of Diseases
- IDR
- Inpatient Discharge Registry
- MMSE
- Mini-Mental State Examination
- MZ
- monozygotic
- OR
- odds ratio
- VaD
- vascular dementia
AUTHOR CONTRIBUTIONS
Study concept and design: W.L.X., L.F., M.G., N.L.P.; analysis and interpretation of the study: W.L.X., L.F., M.G., N.L.P.; drafting the manuscript: W.L.X.; critical revision of the manuscript: L.F., M.G., N.L.P., A.R.A., B.J. Statistical analysis was conducted by Weili Xu.
DISCLOSURE
Dr. Xu and Dr. Atti report no disclosures. Dr. Gatz received a medical education grant from Forest Laboratories, Inc.; serves on the editorial board of the Journal of Community Psychology; receives publishing royalties for Handbook of the Psychology of Aging , Fifth Edition (Academic Press, 2001) and Assessing and Treating Late-life Depression: A Casebook and Resource Guide (Basic Books, 2002); and has received research support from Forest Laboratories, Inc. and the NIH. Dr. Pedersen serves as an Associate Editor of International Journal of Molecular Epidemiology and Genetics. Dr. Johansson serves on scientific advisory boards for Aging Research Center, Stockholm, Danish Aging Research Center, Denmark, and Kavli Research Center for Ageing and Dementia, Norway; and serves on editorial advisory boards for Aging and Mental Health, European Journal of Ageing, Journal of Gerontopsychology and Geriatric Psychiatry, the Journal of Aging Research, and the Journal of European Psychology Students. Dr. Fratiglioni reports no disclosures.
REFERENCES
- 1. Taylor RW, Keil D, Gold EJ, Williams SM, Goulding A. Body mass index, waist girth, and waist-to-hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am J Clin Nutr 1998;67:44–49 [DOI] [PubMed] [Google Scholar]
- 2. Gustafson D. A life course of adiposity and dementia. Eur J Pharmacol 2008;585:163–175 [DOI] [PubMed] [Google Scholar]
- 3. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560 [DOI] [PubMed] [Google Scholar]
- 4. Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med 2005;165:321–326 [DOI] [PubMed] [Google Scholar]
- 5. Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 2005;330:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiang CJ, Yip PK, Wu SC, et al. Midlife risk factors for subtypes of dementia: a nested case-control study in Taiwan. Am J Geriatr Psychiatry 2007;15:762–771 [DOI] [PubMed] [Google Scholar]
- 7. Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 2007;4:103–109 [DOI] [PubMed] [Google Scholar]
- 8. Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008;71:1057–1064 [DOI] [PubMed] [Google Scholar]
- 9. Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: Cardiovascular Health Study. Arch Neurol 2009;66:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hassing LB, Dahl AK, Thorvaldsson V, et al. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes 2009;33:893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303:235–241 [DOI] [PubMed] [Google Scholar]
- 12. Whitmer RA. The epidemiology of adiposity and dementia. Curr Alzheimer Res 2007;4:117–122 [DOI] [PubMed] [Google Scholar]
- 13. Moceri VM, Kukull WA, Emanuel I, van Belle G, Larson EB. Early-life risk factors and the development of Alzheimer's disease. Neurology 2000;54:415–420 [DOI] [PubMed] [Google Scholar]
- 14. Teasdale TW, Sorensen TI, Stunkard AJ. Genetic and early environmental components in sociodemographic influences on adult body fatness. BMJ 1990;300:1615–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gustafson D. Adiposity indices and dementia. Lancet Neurol 2006;5:713–720 [DOI] [PubMed] [Google Scholar]
- 16. Lichtenstein P, Sullivan PF, Cnattingius S, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet 2006;9:875–882 [DOI] [PubMed] [Google Scholar]
- 17. Gatz M, Fratiglioni L, Johansson B, et al. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging 2005;26:439–447 [DOI] [PubMed] [Google Scholar]
- 18. Gatz M, Reynolds CA, John R, Johansson B, Mortimer JA, Pedersen NL. Telephone screening to identify potential dementia cases in a population-based sample of older adults. Int Psychogeriatr 2002;14:273–289 [DOI] [PubMed] [Google Scholar]
- 19. Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. Int Psychogeriatr 1995;7:429–438 [DOI] [PubMed] [Google Scholar]
- 20. Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968;114:797–811 [DOI] [PubMed] [Google Scholar]
- 21. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 22. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 23. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260 [DOI] [PubMed] [Google Scholar]
- 24. Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med 1990;322:1483–1487 [DOI] [PubMed] [Google Scholar]
- 25. Diehr P, Newman AB, Jackson SA, Kuller L, Powe N. Weight-modification trials in older adults: what should the outcome measure be? Curr Control Trials Cardiovasc Med 2002;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hassing LB, Dahl AK, Pedersen NL, Johansson B. Overweight in midlife is related to lower cognitive function 30 years later: a prospective study with longitudinal assessments. Dement Geriatr Cogn Disord 2010;29:543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gustafson DR, Backman K, Waern M, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology 2009;73:1559–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology 2007;69:739–746 [DOI] [PubMed] [Google Scholar]
- 29. Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol 2007;64:392–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Atti AR, Palmer K, Volpato S, Winblad B, De RD, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc 2008;56:111–116 [DOI] [PubMed] [Google Scholar]
- 31. Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men: The Honolulu-Asia Aging Study. Arterioscler Thromb Vasc Biol 2000;20:2255–2260 [DOI] [PubMed] [Google Scholar]
- 32. Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med 2003;163:1524–1528 [DOI] [PubMed] [Google Scholar]
- 33. Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord 2006;20:93–100 [DOI] [PubMed] [Google Scholar]
- 34. Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology 1995;45:1161–1168 [DOI] [PubMed] [Google Scholar]
- 35. WHO Obesity and overweight 2010. Available at: http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/ Accessed June 22, 2010.
- 36. Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. J Am Geriatr Soc 2007;55:758–762 [DOI] [PubMed] [Google Scholar]
- 37. Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 2009;302:2565–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salsberry PJ, Reagan PB. Comparing the influence of childhood and adult economic status on midlife obesity in Mexican American, white, and African American women. Public Health Nurs 2009;26:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20:63–72 [DOI] [PubMed] [Google Scholar]
- 40. Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer's disease. J Alzheimers Dis 2009;16:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]