Abstract
Objectives:
To determine the risk of epileptic seizures due to a brain arteriovenous malformation (AVM) or cavernous malformation (CM).
Methods:
In a prospective population-based study of new diagnoses of AVMs (n = 229) or CMs (n = 139) in adults in Scotland in 1999–2003, we used annual medical records surveillance, general practitioner follow-up, and patient questionnaires to quantify the risk of seizures between clinical presentation and AVM/CM treatment, last follow-up, or death.
Results:
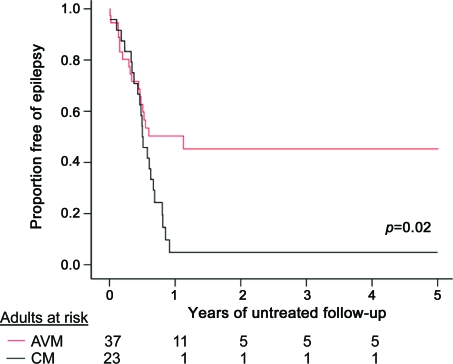
The 5-year risk of first-ever seizure after presentation was higher for AVMs presenting with intracranial hemorrhage or focal neurologic deficit (ICH/FND: n = 119; 23%, 95% confidence interval [CI] 9%–37%) than for incidental AVMs (n = 40; 8%, 95% CI 0%–20%), CMs presenting with ICH/FND (n = 38; 6%, 95% CI 0%–14%), or incidental CMs (n = 57; 4%, 95% CI 0%–10%). For adults who had never experienced ICH/FND, the 5-year risk of epilepsy after first-ever seizure was higher for CMs (n = 23; 94%, 95% CI 84%–100%) than AVMs (n = 37; 58%, 95% CI 40%–76%; p = 0.02). Among adults who never experienced ICH/FND and presented with or developed epilepsy, there was no difference in the proportions achieving 2-year seizure freedom over 5 years between AVMs (n = 43; 45%, 95% CI 20%–70%) and CMs (n = 35; 47%, 95% CI 27%–67%).
Conclusions:
AVM-related ICH confers a significantly higher risk of a first-ever seizure compared to CMs or incidental AVMs. Adults with a CM have a high risk of epilepsy after a first-ever seizure but achieve seizure freedom as frequently as those with epilepsy due to an AVM.
Intracranial vascular malformations (IVMs), which include arteriovenous malformations (AVMs) and cavernous malformations (CMs), are the most common cause of intracerebral hemorrhage (ICH) in young adults,1 but may also be detected following nonhemorrhagic focal neurologic deficits (FNDs), seizures, or brain imaging performed for other reasons.2
Seizures related to CMs seem to be attributable to surrounding hemosiderin deposition through leaky endothelial junctions.3,4 Mechanisms by which AVMs cause seizures are less clear, but in a multivariable analysis of data from a tertiary-care cohort of 100 patients referred for endovascular treatment (47% of whom presented with epilepsy), cortical location of the AVM or feeder, feeding by the middle cerebral artery, absence of aneurysms, and presence of a varix in the absence of intranidal aneurysms were all retrospectively associated with a clinical presentation of epilepsy.5
Available data on the future risk of epileptic seizures for people diagnosed with AVMs and CMs come from 2 studies at specialist referral centers: the risk of a first seizure was 2.4% per person per year during prospective follow-up of people with CMs,6 and 1.1% per person per year in a retrospective study of people with AVMs.7,8
In an attempt to minimize referral and selection biases and maximize external validity, we sought to establish the 5-year risks of a first seizure, 2 or more unprovoked seizures (epilepsy), and seizure freedom in a prospective population-based study.
METHODS
Inclusion criteria.
The Scottish IVM Study (SIVMS) is an ongoing prospective, population-based study that uses anonymized data extracts from an ongoing National Health Service national clinical audit of adults aged ≥16 years resident in Scotland (population 5.1 million) at the time of a new IVM diagnosis during 1999–2003 and 2006–2010 (The Scottish Audit of Intracranial Vascular Malformations, www.saivms.scot.nhs.uk). The audit identifies patients through multiple overlapping sources of case ascertainment that include a Scotland-wide collaborative network of neurologists, neurosurgeons, radiologists, and pathologists and central registers of hospital discharges and death certificates.9 We restricted this analysis to adults with AVMs or CMs first diagnosed in the years 1999–2003. AVMs and CMs were confirmed as definite on brain imaging studies (reviewed by our 2 study neuroradiologists) or pathologic examination. The modality used to establish definite AVM diagnoses was catheter angiography in 84%, CT with contrast enhancement or angiography in 7%, MRI in 5%, and pathologic examination of biopsy or autopsy specimens in 4% (of these 10 adults, 4 died of their presenting ICH, 4 had a history of epilepsy, and 2 died of a condition unrelated to their AVM). We established definite CM diagnoses using MRI in 96%, with reference to criteria that were similar to those subsequently published,10 and using pathologic examination in 4% (all 5 of these adults died of a condition unrelated to their CM). We included adults who entered the study because their IVM was first diagnosed as an incidental finding at autopsy in the analysis of demographic characteristics but not in the outcome analyses.
First presentation (inception).
We classified a patient's first presentation as when they developed symptoms that led to an investigation that diagnosed an AVM or CM. We defined presentation with ICH as a symptomatic event associated with evidence of intracranial blood on brain imaging, CSF, or postmortem examination.10 We defined FND as symptoms or signs of neurologic dysfunction, referable to the anatomic site of the IVM, but without evidence of ICH, cerebral infarction, epileptic seizure (Todd paresis), or migraine.10 An incidental presentation was one that could not be related to the underlying IVM. An epileptic seizure was the initial presentation if it was not symptomatic of a concomitant ICH, and symptomatic seizures were defined as those that were witnessed and occurred within 24 hours of ICH onset. We reviewed all patient records, neuroimaging, and pathology reports and attributed seizures to the AVM or CM if there was no better explanation for their epilepsy. We reviewed patient records to identify prior episodes of symptomatic ICH, FND, and seizures.
Follow-up.
Prospective follow-up started from the date of first presentation. We used annual surveillance of general (family) practitioner and hospital medical records, as well as annual questionnaires to general practitioners and consenting patients, to establish patients' medical histories, mode of clinical presentation, events during follow-up, and antiepileptic drug (AED) prescriptions. We evaluated completeness of follow-up by comparing observed to expected follow-up using these multiple sources.11 If the patient had no history of epileptic seizures prior to presentation, then their first-ever seizure was the initial one that occurred at presentation (unprovoked by ICH) or during prospective follow-up, and we determined their development of epilepsy as when they had their next seizure during prospective follow-up. We evaluated 2-year seizure freedom in adults without prior ICH or FND, who had epilepsy at presentation or developed it during follow-up. If the exact day or month of a seizure was not available, we imputed the date as the midpoint of the month or year, respectively. We summarized the use of AEDs according to their initiation after the first or second seizure in order to account for their role as a potential effect modifier. We also investigated the pattern of AED use at the end of 5-year follow-up by recording whether the patient was on monotherapy, polytherapy, had been withdrawn from AEDs, or had never been treated with AEDs.
Statistical analysis.
We grouped IVM locations as follows: lobar (cerebral hemispheres), deep (limbic, thalamus, hypothalamus, callosal, basal ganglia, and choroidal), infratentorial (brainstem and cerebellum), or multiple (if the patient had evidence of more than one IVM). We quantified the maximum linear dimension of the nidus of AVMs. We measured the size of CMs on T1-weighted MRI sequences or at autopsy (if available). We used the maximum diameter of the CM that was thought responsible for the clinical presentation in those adults with multiple CMs. We used parametric statistics for between-group comparisons when the data obeyed a normal distribution, and nonparametric statistics when they did not. We used exact tests when cell counts were <5. We performed survival analysis using life tables and Kaplan-Meier statistics, starting at the date of first presentation, and censoring on the date of interventional treatment (endovascular embolization, surgical excision, or stereotactic radiosurgery), date of death, or at the end of 5-year follow-up if an event of interest did not occur. We performed univariable comparisons using the log-rank test. We used sensitivity analyses when appropriate. All statistical tests were 2-tailed (α = 0.05) and performed using SPSS (version 14.0).
Ethical approval.
The Multicenter Research Ethics Committee for Scotland approved SIVMS (MREC/98/0/48) and all analyses were performed on anonymized extracts of a National Health Service national audit dataset.
RESULTS
We identified 368 adults first diagnosed in 1999–2003 with a definite AVM (n = 229) or CM (n = 139), for whom there was a total of 2,705 person-years of follow-up (median 8 years per person, interquartile range [IQR] 6–9 years) with a median completeness of follow-up of 95% (IQR 92%–99%) on April 30, 2010.11 Although more AVMs first presented with ICH and more CMs were first detected incidentally, one-quarter of both AVMs and CMs presented with an unprovoked seizure (table 1).
Table 1.
Baseline clinical characteristics of 368 patients with a new diagnosis of an arteriovenous malformation (AVM) or cavernous malformation (CM)

Abbreviations: IQR = interquartile range; IVH = intraventricular hemorrhage; SAH = subarachnoid hemorrhage; SDH = subdural hematoma.
Basal ganglia, choroid plexus, hypothalamus, thalamus, limbic structures, and insula.
First seizure after presentation.
For 254 adults without any history of seizures (figure e-1 on the Neurology® Web site at www.neurology.org), during 610 person-years of follow-up (median 1.5 years per person, IQR 0.2–5 years), the risk of a first-ever in a lifetime unprovoked seizure within 5 years of presentation was significantly greater for adults with an AVM presenting with ICH or FND compared to adults whose AVM had been detected incidentally (23% [95% confidence interval (CI) 9%–37%] vs 8% [95% CI 0%–20%], p = 0.046; figure 1). The 5-year risk was greater for adults with AVMs who had had a provoked seizure at the time of their ICH or FND than for those who had not suffered a provoked seizure at ICH or FND onset (48% [95% CI 19%–77%] vs 20% [95% CI 6%–34%], p = 0.002). Among adults with AVMs who presented with an ICH/FND, there was no difference in the proportion of AVMs in lobar locations between adults who developed a seizure (12/14 [86%]) compared to those who did not (72/105 [69%]; p = 0.2), nor was there a difference in the proportion of ICHs with an intraparenchymal component between adults who developed a seizure (12/14 [86%]) compared to those who did not (74/105 [70%]; p = 0.4).
Figure 1. Five-year risk of a first-ever unprovoked seizure.
The 5-year risk of a first-ever unprovoked seizure for adults with an arteriovenous malformation (AVM) or cavernous malformation (CM), who had no prior history of seizures. Analyses are stratified by whether the CM or AVM was an incidental discovery or whether it was diagnosed because of an intracerebral hemorrhage or focal neurologic deficit (ICH/FND). Seizure outcome was statistically significantly different only when comparing AVM presenting with ICH/FND to either CM presenting with ICH/FND (p = 0.04) or AVM detected incidentally (p = 0.046).
The 5-year risk of a first seizure did not differ for adults with CMs who presented with ICH or FND compared to incidentally detected CMs (6% [95% CI 0%–14%] vs 4% [95% CI 0%–10%], p = 0.6; figure 1).
There was no difference in the 5-year risk of a first-ever unprovoked seizure between AVMs and CMs that presented incidentally (p = 0.4). There was a significant difference in the 5-year risk of a first seizure between AVMs and CMs that had presented with ICH or FND (p = 0.04). This difference remained significant when adults presenting with a provoked seizure associated with their ICH or FND were censored in a sensitivity analysis (p = 0.04). There was a significant difference in the median volume of intracerebral hemorrhage between AVMs and CMs (16 cm3 vs 2 cm3, p < 0.001; table 1),12 but the proportion of adults with an intraparenchymal component was greater for CMs than AVMs (17/17 [100%] vs 90/115 [78%], p = 0.04).
Association of AVM and CM location, size, and multiplicity with the occurrence of seizures.
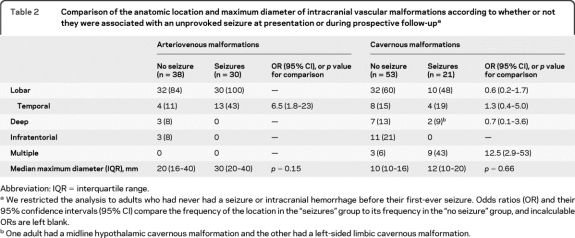
We examined the associations of AVM and CM location, their multiplicity, and maximum linear dimension with the first-ever occurrence of seizures at presentation or during prospective follow-up among untreated participants who had never had an ICH/FND. Every AVM associated with seizures was in a lobar location, as were 84% of the AVMs in adults who never experienced a seizure/ICH/FND, but AVMs were more likely to involve the temporal lobe in adults with seizures (43% vs 11%, OR 6.5 [95% CI 1.8–23]; table 2). Maximum AVM nidus size, whether comparing medians (p = 0.15; table 2) or divided into tertiles for logistic regression (p = 0.4), did not appear to influence the likelihood of seizures. We could not demonstrate any anatomic association between CM location and the occurrence of seizures, even in a sensitivity analysis attributing those with multiple CMs (all of whom possessed at least one lobar CM) to the lobar location category. However, CMs were more likely to be multiple in adults with seizures (43% vs 6%, OR 12.5 [95% CI 2.9–53], table 2). Maximum CM linear dimension, whether comparing medians (p = 0.66; table 2) or divided into tertiles in a multivariable logistic regression analysis also involving CM location (p = 0.6), did not appear to influence the likelihood of seizures.
Table 2.
Comparison of the anatomic location and maximum diameter of intracranial vascular malformations according to whether or not they were associated with an unprovoked seizure at presentation or during prospective follow-upa
Abbreviation: IQR = interquartile range.
We restricted the analysis to adults who had never had a seizure or intracranial hemorrhage before their first-ever seizure. Odds ratios (OR) and their 95% confidence intervals (95% CI) compare the frequency of the location in the “seizures” group to its frequency in the “no seizure” group, and incalculable ORs are left blank.
One adult had a midline hypothalamic cavernous malformation and the other had a left-sided limbic cavernous malformation.
Epilepsy.
For 23 adults with a CM and 37 adults with an AVM without any history of ICH/FND (figure e-2), who had a first-ever in a lifetime seizure at presentation or during prospective follow-up, the 5-year risk of developing epilepsy over 60 person-years of follow-up after their first-ever seizure (median 0.5 years per person, IQR 0.3–0.8 years) was 58% (95% CI 40%–76%) for AVMs and 94% (95% CI 84%–100%) for CMs (p = 0.02; figure 2). The low median duration of follow-up is explained by the large number of outcomes in the first year after presentation (figure 2). There was no difference in the proportion of AVMs (37/37 [100%]) and CMs (21/23 [91%]; p = 0.14) that were in lobar locations. A greater proportion of patients with AVMs were started on an AED after a first seizure but this difference was not significant (24/37 [65%] AVMs vs 10/23 [44%] CMs; p = 0.10). The numbers of outcome events precluded a reliable analysis of the risk of epilepsy following a symptomatic or delayed seizure after presenting with ICH/FND.
Figure 2. Five-year risk of developing epilepsy after a first-ever unprovoked seizure.
The 5-year risk of developing epilepsy in patients with an arteriovenous malformation (AVM; solid line) or cavernous malformation (CM; broken line) following a first-ever seizure.
Two-year seizure freedom.
For adults without any history of ICH/FND, who presented with established epilepsy (table 1; analysis started at presentation) or developed epilepsy during follow-up (figure 2; analysis started at the diagnosis of epilepsy), during 193 person-years of follow-up the chance of achieving 2-year seizure freedom was similar for the 43 adults with an AVM and for the 35 adults with a CM (45% [95% CI 20%–70%] vs 47% [95% CI 27%–67%], p = 0.8; figures 3 and e-3). The chances of achieving 2-year seizure freedom remained similar in a sensitivity analysis restricted to adults with a prospective diagnosis of epilepsy (p = 0.9). Most adults with epilepsy were prescribed AEDs (39/43 [91%] AVMs vs 34/35 [97%] CMs), but by the end of follow-up significantly more with CMs were prescribed polytherapy (16/35 [46%] vs 6/43 [14%] AVMs; p = 0.02).
Figure 3. Chance of achieving 2-year seizure freedom for adults over 5 years follow-up.
The chance of achieving 2-year seizure freedom for adults with epilepsy due to an arteriovenous malformation (AVM; solid line) or cavernous malformation (CM; broken line).
DISCUSSION
In this prospective, population-based cohort study, the risk of a first seizure was higher for adults with an AVM who had presented with an ICH or FND compared to those with incidentally detected AVMs, and compared to all modes of CM presentation (figure 1). In adults without prior ICH or FND, we found that AVMs in the temporal lobe were more frequent in adults with epileptic seizures, although this was not the case for CMs, which were more frequently multiple in adults with epileptic seizures (table 2).
The risk of developing an epileptic seizure after an incidental CM diagnosis was low (0.9% per person-year, annualized over 5 years) and after an incidental AVM diagnosis it was similar (2% per person-year, annualized over 5 years); these risks do not appear to support the prescription of prophylactic AEDs. The increased risk after AVM-related ICH/FND may be explained by intracerebral hemorrhages—which are known to predispose to seizures13,14—being larger when caused by AVMs than CMs.12 However, while there is no evidence to support the prescription of prophylactic AEDs after ICH,15 further study of their effects on the subsequent risk of epilepsy in adults with a provoked seizure secondary to AVM-ICH are warranted, ideally in randomized controlled trials (RCTs).
For those with a first seizure who were unaffected by prior ICH or FND, the 5-year risk of epilepsy was higher for adults with CMs than AVMs. Immediate AED prescription after a first seizure or early epilepsy increases the time to first and second seizure recurrence and reduces the time to 2-year seizure freedom but does not affect seizure freedom at 5 years.16 Therefore, AED prescribing patterns in Scotland might have influenced the observed risks of epilepsy after a first seizure, and similar data from other populations would help to indicate the generalizability of our findings. The risk of progressing to epilepsy was higher for CMs than for a composite group of patients with a first-ever seizure in a recent trial of AEDs for unprovoked seizures.16 Although guidelines do not suggest starting an AED for every patient after a first tonic-clonic seizure if brain imaging shows an underlying lesion,17 the high risk of epilepsy, concentrated in the first year after a first seizure, suggests that prescribing AEDs for adults with a first-ever seizure due to a CM may be warranted. Our data suggest that adults with epilepsy due to a CM or AVM have a similar chance of achieving 2-year seizure freedom but that this chance is lower than that reported for people with cryptogenic epilepsy (on whom there are few data on 2-year seizure freedom).18
In this prospective population-based study of the natural history of epileptic seizures after a new diagnosis of an AVM or CM, we used multiple overlapping sources of case ascertainment and follow-up was comprehensive (median completeness of follow-up was 97% for AVMs and 96% for CMs). The population-based design of our study sought to avoid any selection bias on the basis of participants' symptoms or feasibility of treatment. We have provided estimates of 2-year seizure freedom, which is a more robust outcome than 1-year seizure freedom and is more relevant to clinical practice since AEDs tend not to be withdrawn until a patient is at least 2 years seizure-free.19
Largely because of the logistical and financial constraints upon studying a geographically dispersed population, we relied on clinicians' evaluations in patients' medical records as well as questionnaire data, rather than scheduled study visits, and therefore may have missed some events. We extensively reviewed medical records and general practitioner notes to determine a prepresentation history of epilepsy, and thereby tried to minimize the risk of misclassifying an adult with either epilepsy or a first-ever in a lifetime seizure. Reporting bias may have existed among patients with a longstanding history of epilepsy, who may be less inclined to present to medical attention, although we tried to mitigate this by using a more robust, 2-year seizure freedom outcome. The crude detection rate of AVMs in the first 2 years of our study in Scotland was not significantly different from the pooled detection rate in a recent meta-analysis,20,21 and our detection rate of CMs was higher than in the one previous population-based study (conducted in the era before MRI was widely available).20,22
Our estimates of the risk of first-seizure over 5 years after incidental diagnoses of CMs (4%, 95% CI 0%–10%) and AVMs (8%, 95% CI 0%–20%) compare to previous studies' findings of 2.4% per person-year6 and 1.1% per person-year,7,8 respectively. However, we are likely to have underascertained incidental CMs, given their known prevalence on MRI in neurologically asymptomatic people,2 which may have overestimated the risk of first-ever seizure among incidental CMs.
Further recruitment and follow-up will improve the precision of our current estimates, and obtain sufficient numbers of outcomes to investigate factors predictive of first seizures, epilepsy, and seizure freedom. Previous data have suggested that patients with an AVM affected by epileptic seizures tend to be younger,8,23 but influences on CMs are unknown. Retrospective, uncontrolled studies of pure CM lesionectomy,24–27 extended CM lesionectomy,28,29 and multimodality AVM treatment for patients with epilepsy have all shown welcome improvements in seizure control.30 However, it will be important to determine whether interventional treatment of CMs and AVMs influences the risk of a first seizure or developing epilepsy in hospital- and population-based studies as well as in RCTs (such as ARUBA, www.arubastudy.org, ISRCTN 44013133).
Supplementary Material
ACKNOWLEDGMENT
The authors thank their collaborators (see reference9 for a collaborator listing; updates at www.saivms.scot.nhs.uk); Rosemary Anderson and Aidan Hutchinson for their support with study coordination and programming, respectively; Cavernoma Alliance UK; and the Royal College of Physicians International Sponsorship Scheme.
Editorial, page 1540
Supplemental data at www.neurology.org
- AED
- antiepileptic drug
- AVM
- arteriovenous malformation
- CI
- confidence interval
- CM
- cavernous malformation
- FND
- focal neurologic deficit
- ICH
- intracranial hemorrhage
- IQR
- interquartile range
- IVM
- intracranial vascular malformation
- RCT
- randomized controlled trial
- SIVMS
- Scottish IVM Study
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by C.B.J. and R.A.-S.S., C.B.J., and R.A.-S.S. contributed to the design and conceptualization of the study, analysis and interpretation of the data. C.B.J., J.P.L., R.D., R.C.R., C.E.C., and R.A.-S.S. all contributed to the drafting and revising of the manuscript. C.B.J., J.P.L., R.D., R.C.R., C.E.C., and R.A.-S.S. all agree to the publication of this version of the manuscript.
COINVESTIGATORS
The SAIVMs steering committee consists of Diana Beard, RGN, MBA (NHS National Services Scotland, Edinburgh, SAIVMs steering committee member); Jo J. Bhattacharya, MBBS, FRCR, MSc (Institute of Neurological Sciences, Glasgow, SAIVMs steering committee member); Carl E. Counsell; E. Jerome St. George, MD, FRCS (Institute of Neurological Sciences, Glasgow, SAIVMs steering committee member); Vaughn Ritchie, MBBS (Fauldhouse Health Centre, Fauldhouse, SAIVMs steering committee member); Richard C. Roberts; Robin J. Sellar, MBBS, DMRD, FRCP, FRCS, FRCR (Division of Clinical Neurosciences, University of Edinburgh, SAIVMs steering committee member); Charles P. Warlow, BA, MB BChir, MD, FRCP, FMedSci, FRSE (Division of Clinical Neurosciences, University of Edinburgh, SAIVMs steering committee member); and Rustam Al-Shahi Salman. See reference 9 for a collaborator listing (updates at www.saivms.scot.nhs.uk).
DISCLOSURE
Dr. Josephson reports no disclosures. Dr. Leach has served on scientific advisory boards and received speaker honoraria from GlaxoSmithKline, UCB, Janssen, and Eisai Inc. and has received research support from the MRC UK. Dr. Duncan has received funding for travel and speaker honoraria from UCB. Dr. Roberts has served on scientific advisory boards for and received funding for travel and/or speaker honoraria from Eisai Inc., Janssen, Cyberonics, Inc., GlaxoSmithKline, Pfizer Inc, and UCB; and has received research support from Eisai Inc. and UCB. Dr. Counsell has received research support from the Chief Scientist Office of the Scottish Government, Parkinson's UK, the Dystonia Society, and the NIHR/UK. Dr. Al-Shahi Salman has received research support through the MRC UK; the Chief Scientist Office of the Scottish Government, the United Kingdom Stroke Association, and Cavernoma Alliance UK.
REFERENCES
- 1. Brown RD, Jr, Flemming KD, Meyer FB, Cloft HJ, Pollock BE, Link ML. Natural history, evaluation, and management of intracranial vascular malformations. Mayo Clin Proc 2005;80:269–281 [DOI] [PubMed] [Google Scholar]
- 2. Morris Z, Whiteley WN, Longstreth WT, Jr, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2009;339:b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Awad I, Jabbour P. Cerebral cavernous malformations and epilepsy. Neurosurg Focus 2006;21:e7. [DOI] [PubMed] [Google Scholar]
- 4. Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry 2001;71:188–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turjman F, Massoud TF, Sayre JW, Vinuela F, Guglielmi G, Duckwiler G. Epilepsy associated with cerebral arteriovenous malformations: a multivariate analysis of angioarchitectural characteristics. AJNR Am J Neuroradiol 1995;16:345–350 [PMC free article] [PubMed] [Google Scholar]
- 6. Moriarity JL, Wetzel M, Clatterbuck RE, et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery 1999;44:1166–1171 [PubMed] [Google Scholar]
- 7. Crawford PM, West CR, Chadwick DW, Shaw MD. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry 1986;49:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crawford PM, West CR, Shaw MD, Chadwick DW. Cerebral arteriovenous malformations and epilepsy: factors in the development of epilepsy. Epilepsia 1986;27:270–275 [DOI] [PubMed] [Google Scholar]
- 9. Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Scottish Intracranial Vascular Malformation Study (SIVMS): evaluation of methods, ICD-10 coding, and potential sources of bias in a prospective, population-based cohort Stroke 2003;34:1156–1162 [DOI] [PubMed] [Google Scholar]
- 10. Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA. Hemorrhage from cavernous malformations of the brain: definition and reporting standards: Angioma Alliance Scientific Advisory Board. Stroke 2008;39:3222–3230 [DOI] [PubMed] [Google Scholar]
- 11. Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet 2002;359:1309–1310 [DOI] [PubMed] [Google Scholar]
- 12. Cordonnier C, Al-Shahi Salman R, Bhattacharya JJ, et al. Differences between intracranial vascular malformation types in the characteristics of their presenting haemorrhages: prospective, population-based study. J Neurol Neurosurg Psychiatry 2008;79:47–51 [DOI] [PubMed] [Google Scholar]
- 13. Faught E, Peters D, Bartolucci A, Moore L, Miller PC. Seizures after primary intracerebral hemorrhage. Neurology 1989;39:1089–1093 [DOI] [PubMed] [Google Scholar]
- 14. Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology 2007;69:1356–1365 [DOI] [PubMed] [Google Scholar]
- 15. Kwan J, Wood E. Antiepileptic drugs for the primary and secondary prevention of seizures after stroke. Cochrane Database Syst Rev 2010;CD005398. [DOI] [PubMed] [Google Scholar]
- 16. Marson A, Jacoby A, Johnson A, Kim L, Gamble C, Chadwick D. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet 2005;365:2007–2013 [DOI] [PubMed] [Google Scholar]
- 17. Scottish Intercollegiate Guidelines Network Diagnosis and Management of Epilepsy in Adults: A National Clinical Guideline. Scottish Intercollegiate Guidelines Network; 2005 [Google Scholar]
- 18. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319 [DOI] [PubMed] [Google Scholar]
- 19. Quality Standards Subcommittee of the American Academy of Neurology Practice parameter: a guideline for discontinuing antiepileptic drugs in seizure-free patients: summary statement: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1996;47:600–602 [DOI] [PubMed] [Google Scholar]
- 20. Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke 2003;34:1163–1169 [DOI] [PubMed] [Google Scholar]
- 21. Gabriel RA, Kim H, Sidney S, et al. Ten-year detection rate of brain arteriovenous malformations in a large, multiethnic, defined population. Stroke 2010;41:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown RD, Jr., Wiebers DO, Torner JC, O'Fallon WM. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology 1996;46:949–952 [DOI] [PubMed] [Google Scholar]
- 23. Stapf C, Khaw AV, Sciacca RR, et al. Effect of age on clinical and morphological characteristics in patients with brain arteriovenous malformation. Stroke 2003;34:2664–2669 [DOI] [PubMed] [Google Scholar]
- 24. Chang EF, Gabriel RA, Potts MB, Garcia PA, Barbaro NM, Lawton MT. Seizure characteristics and control after microsurgical resection of supratentorial cerebral cavernous malformations. Neurosurgery 2009;65:31–37 [DOI] [PubMed] [Google Scholar]
- 25. Casazza M, Broggi G, Franzini A, et al. Supratentorial cavernous angiomas and epileptic seizures: preoperative course and postoperative outcome. Neurosurgery 1996;39:26–32 [DOI] [PubMed] [Google Scholar]
- 26. Ferroli P, Casazza M, Marras C, Mendola C, Franzini A, Broggi G. Cerebral cavernomas and seizures: a retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci 2006;26:390–394 [DOI] [PubMed] [Google Scholar]
- 27. Cohen DS, Zubay GP, Goodman RR. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg 1995;83:237–242 [DOI] [PubMed] [Google Scholar]
- 28. Stavrou I, Baumgartner C, Frischer JM, Trattnig S, Knosp E. Long-term seizure control after resection of supratentorial cavernomas: a retrospective single-center study in 53 patients. Neurosurgery 2008;63:888–896 [DOI] [PubMed] [Google Scholar]
- 29. Baumann CR, Acciarri N, Bertalanffy H, et al. Seizure outcome after resection of supratentorial cavernous malformations: a study of 168 patients. Epilepsia 2007;48:559–563 [DOI] [PubMed] [Google Scholar]
- 30. Hoh BL, Chapman PH, Loeffler JS, Carter BS, Ogilvy CS. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizure incidence and seizure outcomes. Neurosurgery 2002;51:303–309 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.