Abstract
Objective:
To describe a patient presenting with a clinically silent, incidentally found, and pathologically confirmed active demyelinating solitary cortical lesion showing MRI gadolinium contrast enhancement, in whom biopsy was performed before the radiographic appearance of disseminated white matter lesions.
Methods:
Neurologic examination, MRI, CSF and serologic analyses, and brain biopsy were performed. Sections of formalin-fixed paraffin-embedded biopsied brain tissue were stained with histologic and immunohistochemical stains.
Results:
Biopsy revealed an inflammatory subpial lesion containing lymphocytes and myelin-laden macrophages. Recurrent relapses with dissemination of MRI-typical white matter lesions characterized the subsequent course.
Conclusions:
Our findings highlight that cortical demyelination occurs on a background of inflammation and suggest that the noninflammatory character of chronic cortical demyelination may relate to long intervals between lesion formation and autopsy. This case provides pathologic evidence of relapsing-remitting MS presenting with inflammatory cortical demyelination and emphasizes the importance of considering demyelinating disease in the differential diagnosis of patients presenting with a solitary cortical enhancing lesion.
Traditionally, multiple sclerosis (MS) has been considered a disease primarily affecting the CNS white matter. Nevertheless, gray matter involvement has been recognized for a long time.1 Recent pathologic studies have revealed that cortical demyelination is more extensive than previously appreciated,2,3 is characteristic of progressive MS,4 and is devoid of lymphocytes and macrophages.3 However, these studies have relied on postmortem tissue analysis from patients with longstanding disease. Therefore, the pathology of early cortical demyelinating MS lesions is virtually unknown. Interestingly, pathologic findings in a focal cortical experimental autoimmune encephalomyelitis (EAE) animal model have challenged the concept that cortical lesions are noninflammatory.5
Recent histopathologic studies have shown that cortical demyelination may occur spatially removed from white matter pathology, without obvious anatomic relationships.6 Therefore, it is plausible that cortical demyelination could represent the earliest pathologic event in some patients with MS and, indeed, MRI evidence of MS cortical onset has been previously reported.7
We describe a patient presenting with a clinically silent, incidentally found, and pathologically confirmed active demyelinating cortical lesion showing MRI gadolinium contrast enhancement, in whom biopsy was performed before the radiographic appearance of disseminated white matter lesions. The patient subsequently fulfilled diagnostic criteria for relapsing-remitting MS. This study provides compelling radiographic and pathologic evidence of relapsing-remitting MS presenting with inflammatory cortical demyelination.
METHODS
The biopsied brain tissue was fixed in 10%–15% formalin and embedded in paraffin. Sections were stained with hematoxylin & eosin and Luxol fast blue/periodic acid–Schiff. Immunohistochemistry was performed using an avidin–biotin technique and primary antibodies specific for proteolipid protein, 2′3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase), neurofilament, macrophages/microglia (KiM1P), CD3+ T (CD3), CD8+ T (CD8), and B lymphocytes (CD20), and plasma cells (CD138) as described previously.8
Case report.
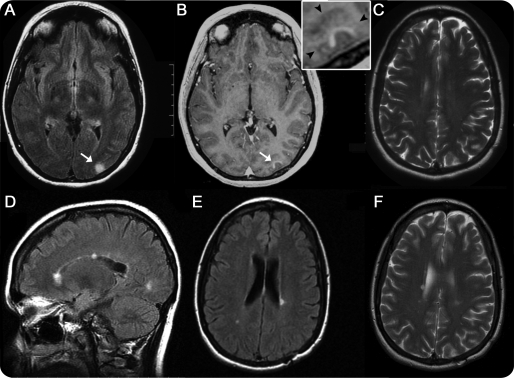
A 33-year-old Caucasian woman with a history of migraine and 4 months postpartum complained of sudden onset headache and right periorbital pain. She had an upper respiratory tract infection 1 week prior to symptom onset. Neurologic examination was normal. Brain MRI revealed a high signal intensity lesion on T2-weighted imaging involving the left visual association occipital cortex (figure 1, A– C) that enhanced with gadolinium (figure 1B, inset). No other lesions were noted. An open brain biopsy was performed 1 month after symptom onset to exclude neoplasm.
Figure 1. Brain MRI prior to biopsy (A–C) and 69 months after biopsy (D–F).
(A) Axial fluid-attenuated inversion recovery (FLAIR) image of left occipital cortical lesion (arrow). (B) Axial T1-weighted image with contrast showing enhancement of the cortical lesion (arrow); inset shows that contrast enhancement is within the cortical gray matter (arrowheads indicate the gray/white matter junction). (C) Axial T2-weighted image showing no periventricular lesions. (D) Sagittal FLAIR image showing the appearance of multiple new white matter lesions, with 2 of the lesions involving the corpus callosum. (E) Axial FLAIR image with the appearance of new periventricular lesions. (F) Axial T2-weighted image at nearly corresponding level of image C showing the appearance of new periventricular white matter lesions.
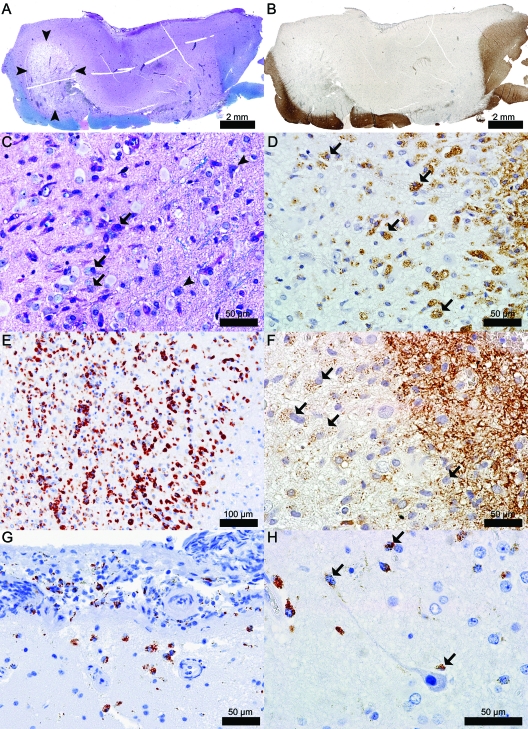
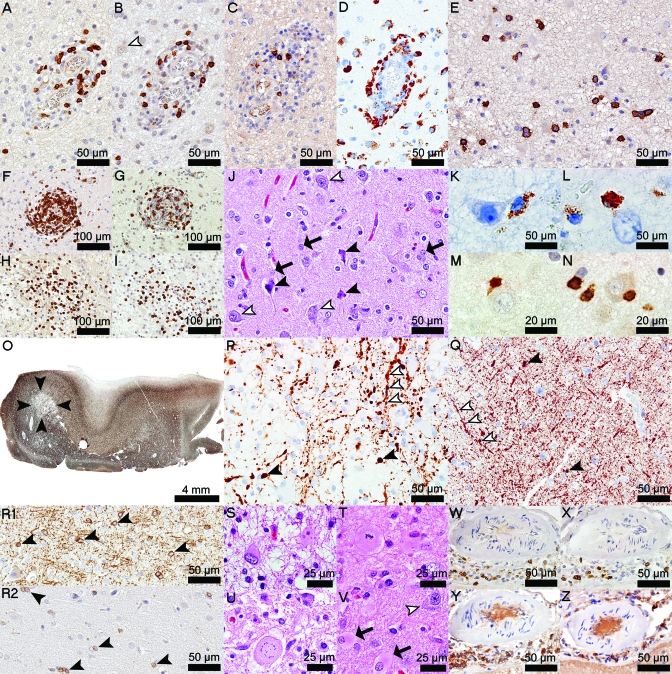
The biopsy revealed the presence of a hypercellular, focally destructive, cortical demyelinated lesion extending from the pial surface through all cortical layers, with extension into the cortex laterally and partially involving the subcortical white matter (figure 2,A and B). The lesion was infiltrated by phagocytic macrophages containing both early and late intracytoplasmic myelin degradation products, consistent with early-active ongoing cortical demyelination (figure 2, C–F).9 Marked parenchymal and perivascular (>3 cell cuffs around at least one blood vessel) cortical inflammatory infiltrates were present and composed of macrophages, microglia, CD3+ and CD8+ T cells, B lymphocytes, and plasma cells (figure 3, A–I). Perivascular and parenchymal lymphocytic infiltrates in the “pure” cortical part of this subpial lesion (figure 3, A–D), although highly inflammatory, were less cellular than infiltrates located at the gray/white matter junction or in the cavitated area (figure 3, F–I). Plasma cells were largely present in the destructive regions of the cortical plaque (figure 3E). Neuronal injury was evidenced by the presence of scattered pyknotic neurons (figure 3J). Macrophages, microglia, and T lymphocytes were often seen in close apposition to neurons (figure 2H and figure 3, K–N). Foamy macrophages were also concentrated subpially in the cortical molecular layer and subarachnoid space (figure 2G). The destructive region was cavitated, affecting mainly the cortex, with only limited involvement of the gray/white matter junction (figure 2A and figure 3, O and P). The remainder of the lesion showed relative axonal preservation. Acute axonal injury, evidenced by axonal swellings, extended beyond the lesion borders into the adjacent normal-appearing white matter and cortex (figure 3, P and Q). Oligodendrocyte density was reduced in the cortical plaque (figure 3R2) when compared to the adjacent nondemyelinated cortex (figure 3R1). A profound reactive astrogliosis was present throughout this subpial lesion (figure 3, J and S–U) extending beyond the lesion edges into the normal-appearing cortex (figure 3V). Reactive astrocytes showed a variety of morphologic changes (figure 3, J and S–U) including mitotic figures (figure 3T) and Creutzfeldt cells (figure 3U). Mild meningeal inflammation was also present and consisted of CD3+ T, CD8+ T, and B lymphocytes, and plasma cells (figure 3, W–Z).
Figure 2. Biopsy of the initial MRI enhancing lesion shown in figure 1 revealed a highly inflammatory subpial lesion with predominance of macrophages involved in active demyelination.
(A) Subpial lesion showing loss of myelin and an area (arrowheads) of cavitary changes (Luxol fast blue [LFB]/periodic acid-Schiff [PAS]). (B) Subpial lesion showing loss of immunoreactivity for myelin specific proteins (proteolipid protein [PLP]). (C) Actively demyelinating macrophages (arrows); arrowheads indicate neurons (LFB/PAS). (D) Actively demyelinating macrophages (arrows) (PLP). (E) Foamy macrophages (KiM1P). (F) Actively demyelinating macrophages (arrows) (2′3′-cyclic-nucleotide 3′-phosphodiesterase [CNPase]). (G) Subpial and CSF foamy macrophages (KiM1P). (H) Macrophages/microglia (arrows) in close apposition to neurons and neurites (KiM1P).
Figure 3. Cortical and meningeal lymphocytic infiltration, axonal and neuronal injury, loss of oligodendrocytes, and reactive astrocytosis are other neuropathologic findings in this biopsy.
(A) Perivascular CD3+ T cells (CD3). (B) Perivascular CD8+ T cells (CD8, white arrowhead shows a normal-appearing neuron). (C) Perivascular B cells (CD20). (D) Perivascular macrophages (KiM1P). (E) Parenchymal plasma cells (CD138). (F) Junctional perivascular CD3+ T cells (CD3). (G) Junctional perivascular CD8+ T cells (CD8). (H) Junctional parenchymal CD3+ T cells (CD3). (I) Junctional parenchymal CD8+ T cells (CD8). (J) Pyknotic neurons (black arrowheads) and reactive astrocytes (arrows) scattered among healthy neurons (white arrowheads) (hematoxylin & eosin [H&E]). (K, L) Macrophages in close apposition to neurons (KiM1P). (M) CD3+ T cells (CD3) and (N) CD8+ T cells (CD8) in close apposition to neurons. (O) Good axonal preservation, except in the cavitated area (arrowheads) (neurofilament [NF]). (P) Axonal swellings in the cavitated area (black arrowheads); segmental axonal swellings are seen along neurites (white arrowheads) (NF). (Q) Axonal swellings in normal-appearing gray matter (black arrowheads); white arrowheads follow a neurite with segmental axonal swellings (NF). (R1) Oligodendrocytes in normal-appearing cortex (2′3′-cyclic-nucleotide 3′-phosphodiesterase [CNPase]). (R2) Loss of oligodendrocytes in the subpial lesion (CNPase). (S) Gemistocytes (H&E). (T) Mitotic astrocytes (H&E). (U) Creutzfeldt cells (H&E). (V) Cortical reactive astrocytosis (arrows) extending beyond the lesion borders; healthy neuron (white arrowhead) (H&E). (W) Meningeal CD3+ T cells (CD3). (X) Meningeal CD8+ T cells (CD8). (Y) Meningeal B cells (CD20). (Z) Meningeal plasma cells (CD138).
Following biopsy, the patient developed an incongruous right homonymous hemianopia that slowly resolved over several months. Due to persistent headaches, a CSF analysis was performed 4 months postbiopsy and demonstrated an elevated CSF immunoglobulin G synthesis rate with normal protein, glucose, cell count, and one oligoclonal band. Lyme serology was negative. Six months after biopsy, she complained of left-sided chest numbness. Spine MRI showed 2 subtle T2-weighted lesions at C4–5 and C6 levels. Eleven months after biopsy, she developed burning dysesthesias in the lower extremities. Brain MRI revealed new periventricular T2-weighted white matter lesions. She fulfilled diagnostic criteria for relapsing-remitting MS. At last follow-up (69 months postbiopsy), brain MRI demonstrated an interval increase in the number of white matter lesions (figure 1, D–F), and she was started on glatiramer acetate.
DISCUSSION
This study provides pathologic evidence of inflammatory cortical demyelination at clinical onset of MS that preceded radiographic evidence of disseminated white matter demyelination typical of MS relapses. A recently developed cortical demyelination rodent EAE model demonstrated that cortical inflammation is early, transient, and rapidly resolving.5 Notably, this case report is based on a brain biopsy obtained early in the disease, whereas previous studies describing noninflammatory MS cortical pathology have relied on autopsy brain sections from patients who died with chronic progressive disease.3 The rapid resolution of cortical inflammation may in part explain these apparent discordant findings with respect to the presence of inflammation in early vs chronic cortical demyelinating lesions.
The presence of an inflammatory subpial cortical lesion associated with MRI gadolinium contrast enhancement that preceded the classic dissemination of white matter lesions suggests that inflammation-induced cortical blood–brain barrier damage and demyelination may be early pathogenic events in MS. Whereas double inversion recovery MRI techniques may enhance detectability of cortical lesions,10 it is also possible that there is a very short window of opportunity for detecting such cortical lesions early in MS. The transient and rapidly reversible nature of cortical inflammation and demyelination in early MS5 may preclude the development of larger, more confluent demyelinated lesions, thereby making their clinical or radiographic detection more challenging, even among symptomatic patients. These atypical presentations may result in diagnostic confusion and potentially lead to unnecessary interventions. This case emphasizes the importance of considering demyelinating disease in the differential diagnosis of patients presenting with a solitary cortical enhancing lesion.
The present case report, coupled with previously published MRI data,7 suggests that inflammatory cortical lesions at MS onset may precede the appearance of classic white matter plaques in some patients with MS. Furthermore, cortical onset MS raises interesting questions regarding the potential role of meningeal inflammation and cortical pathology in initiating and perpetuating the MS disease process. Most therapeutic, diagnostic, and research efforts have concentrated on the white matter pathology in MS. However, a better understanding of the specific factors within the cortical microenvironment that favor rapid resolution of cortical inflammation and demyelination5 may lead to new therapeutic approaches aimed at targeting early MS cortical pathology.
ACKNOWLEDGMENT
The authors thank Patricia Ziemer for technical assistance.
Footnotes
- EAE
- experimental autoimmune encephalomyelitis
- MS
- multiple sclerosis
DISCLOSURE
Dr. Popescu and Dr. Bunyan report no disclosures. Dr. Parisi serves on scientific advisory boards for the US Government Defense Health Board and the Subcommittee for Laboratory Services and Pathology; serves as a Section Editor for Neurology®; receives royalties from the publication of Principles & Practice of Neuropathology, 2nd ed. (Oxford University Press, 2003); and receives research support from the NIH. Dr. Ransohoff serves on scientific advisory boards for ChemoCentryx, Inc., Vertex Pharmaceuticals, Merck Serono, and GlaxoSmithKline; serves as an Associate Editor for Neurology®; and receives research support from the NIH and the Nancy Davis Center Without Walls (NDCWW). Dr. Lucchinetti is listed as author and receives royalties for patent re: Aquaporin-4 associated antibodies for diagnosis of neuromyelitis optica; receives royalties from the publication of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010); and receives research support from the NIH, the Guthy Jackson Charitable Foundation, and the National MS Society.
REFERENCES
- 1. Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 1962;25:315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain 1999;122:17–26 [DOI] [PubMed] [Google Scholar]
- 3. Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001;50:389–400 [DOI] [PubMed] [Google Scholar]
- 4. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;128:2705–2712 [DOI] [PubMed] [Google Scholar]
- 5. Merkler D, Ernsting T, Kerschensteiner M, Bruck W, Stadelmann C. A new focal EAE model of cortical demyelination: multiple sclerosis-like lesions with rapid resolution of inflammation and extensive remyelination. Brain 2006;129:1972–1983 [DOI] [PubMed] [Google Scholar]
- 6. Bo L, Geurts JJ, van der Valk P, Polman C, Barkhof F. Lack of correlation between cortical demyelination and white matter pathologic changes in multiple sclerosis. Arch Neurol 2007;64:76–80 [DOI] [PubMed] [Google Scholar]
- 7. Calabrese M, Gallo P. Magnetic resonance evidence of cortical onset of multiple sclerosis. Mult Scler 2009;15:933–941 [DOI] [PubMed] [Google Scholar]
- 8. Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000;47:707–717 [DOI] [PubMed] [Google Scholar]
- 9. Bruck W, Porada P, Poser S, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995;38:788–796 [DOI] [PubMed] [Google Scholar]
- 10. Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol 2009;66:1144–1150 [DOI] [PubMed] [Google Scholar]