Abstract
Xylella fastidiosa causes Pierce's disease of grapevine as well as several other major agricultural diseases but is a benign endophyte in most host plants. X. fastidiosa colonizes the xylem vessels of host plants and is transmitted by xylem sap-feeding insect vectors. To understand better the pattern of host colonization and its relationship to disease, we engineered X. fastidiosa to express a green fluorescent protein (Gfp) constitutively and performed confocal laser-scanning microscopic analysis of colonization in a susceptible host, Vitis vinifera. In symptomatic leaves, the fraction of vessels colonized by X. fastidiosa was fivefold higher than in nearby asymptomatic leaves. The fraction of vessels completely blocked by X. fastidiosa colonies increased 40-fold in symptomatic leaves and was the feature of colonization most dramatically linked to symptoms. Therefore, the extent of vessel blockage by bacterial colonization is highly likely to be a crucial variable in symptom expression. Intriguingly, a high proportion (>80%) of colonized vessels were not blocked in infected leaves and instead had small colonies or solitary cells, suggesting that vessel blockage is not a colonization strategy employed by the pathogen but, rather, a by-product of endophytic colonization. We present evidence for X. fastidiosa movement through bordered pits to neighboring vessels and propose that vessel-to-vessel movement is a key colonization strategy whose failure results in vessel plugging and disease.
Xylella fastidiosa is a gram-negative bacterium limited to the plant xylem vessels, a unique, nutritionally dilute habitat (27). The bacterium is transmitted to new host plants during xylem sap feeding by insect vectors such as sharpshooter leafhoppers (Hemiptera, Cicadellidae) or spittlebugs (Hemiptera, Cercopidae) (30). X. fastidiosa multiplies and spreads from the site of infection to colonize the xylem, a water transport network of vessels composed of dead, lignified cells. Vessels are interconnected by channels, called bordered pits, that allow the passage of xylem sap but block the passage of larger objects due to the presence of a pit membrane (34). Bacterial cells attach to the vessel wall and multiply, forming biofilm-like colonies that can, when sufficiently large, completely occlude xylem vessels, blocking water transport (35). Many agriculturally important plants, such as citrus, almond, coffee, and grapevines, are susceptible to diseases caused by X. fastidiosa (20). In susceptible plants, leaf scorching, fruit shriveling, and other symptoms result, probably due to the increased stress of xylem blockage as colonization ensues. However, within the majority of host plants, X. fastidiosa behaves as a harmless endophyte (11, 31). X. fastidiosa population comparisons between grapevines resistant and susceptible to Pierce's disease (PD) demonstrate a positive correlation between high populations and symptom expression (12). However, we still lack an understanding of the process of colonization and what specific aspects of large populations of X. fastidiosa lead to symptom expression.
Many studies of X. fastidiosa colonization have involved grinding infected plant tissue and detecting bacteria by culture (1, 17), enzyme-linked immunosorbent assay (6, 21), or PCR (23); with these methods, the distribution of X. fastidiosa within the tissue was unknown. For example, it is not known whether large populations seen in plant macerates of susceptible plants correspond to many small communities or a few large communities of X. fastidiosa. However, the size, distribution, and behavior of X. fastidiosa colonies within the xylem network may be important factors in the generation of PD symptoms. Understanding the pattern of X. fastidiosa colonization of the xylem and relating this pattern to symptom expression will further our progress in understanding PD as well as the endophytic life-style of the pathogen. To this end, several other studies have analyzed X. fastidiosa in planta using various types of microscopy, including light microscopy (13, 19), scanning electron microscopy (35), transmission electron microscopy (24), and immunofluorescence microscopy (4).
In these studies, it was determined that the frequency of vessels colonized and blocked by X. fastidiosa in grapes is positively correlated with disease symptom development over the growing season (19) and within individual plants (35). X. fastidiosa colonies in the xylem were reported to be of different sizes(35), to be either distributed evenly throughout the vessel or appressed against the vessel wall (24), and sometimes to be accompanied by a matrix presumed to be a gel of either plant or bacterial origin; however, no quantitative analysis of these observations has been reported (4, 13, 24, 35). One drawback to the methods used in these studies is that they required extensive preparation of the sample prior to microscopy, such that only small regions of the plant could be examined. In addition, dissection and preparation of samples is fraught with procedural issues, leading to uncertainty about the original spatial distribution of the pathogen.
In this study, we characterized X. fastidiosa colonization of grapevine in a quantitative manner and related patterns of colonization to symptom development in the plant tissue. To conduct this analysis, we engineered a strain of X. fastidiosa that constitutively expresses a green fluorescent protein (Gfp). Fluorescent X. fastidiosa cells were visualized directly in the plant by confocal laser-scanning microscopy (CLSM). This type of microscopy captures images from within a sample, allowing visualization of unperturbed X. fastidiosa cells in intact xylem vessels. Fixation, washing, and staining are not needed, and sample dissection is minimal, eliminating the potential for artifacts that can affect other types of microscopy used for in planta analysis of X. fastidiosa. We chose to limit our study to petioles, where high levels of colonization of the xylem occur in symptomatic plants (19).
MATERIALS AND METHODS
Bacterial culture.
X. fastidiosa strain Temecula (ATCC 700964), which recently has been sequenced (36), was used for all experiments (Table 1). The cultures were incubated at 28°C. Shaking at 160 rpm was provided for liquid cultures. Liquid and solid media for X. fastidiosa were PW and PWG, respectively (9, 16), with 21.6 μg of natamycin (Gist-Brocades, Delft, The Netherlands) per ml. Selective media contained 30 μg of kanamycin per ml.
TABLE 1.
X. fastidiosa strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| X. fastidiosa | ||
| Temecula | Wild type, PD | ATCC 700964 |
| KLN59.3 | Gfp+, Kanr | This study |
| Plasmids | ||
| pGEM-5Zf(+) | Ampr | Promega |
| pKLN2 | X. fastidiosa oriC in pGEM-5Zf(+), Ampr | This study |
| pKLN41 | X. fastidiosa predicted untranscribed region in pGEM-5Zf(+), Ampr | This study |
| pKLN43 | X. fastidiosa predicted untranscribed region, X. fastidiosa oriC, Ampr | This study |
| pFAK | kan-2 source | 10 |
| pKLN56 | X. fastidiosa kan-2 integration allelic exchange plasmid, X. fastidiosa oriC, Ampr Kanr | This study |
| pPROBE-gfp[tagless] | gfp and transcriptional terminator source, Kanr | 22 |
| pKLN59 | X. fastidiosa kan-gfp integration allelic exchange plasmid, X. fastidiosa oriC, Ampr Kanr | This study |
Ampr and Kanr, resistance to ampicillin and kanamycin, respectively.
Construction of plasmids and strains.
X. fastidiosa oriC was amplified from Temecula genomic DNA with primers OriC F PvuI and OriC R PvuI (Table 2), A-tailed, and ligated into pGEM-T (Promega, Madison, Wis.) to make pKLN2 (Table 1). A region of the X. fastidiosa genome predicted to be untranscribed (36) was amplified from Temecula genomic DNA with primers Untxb2 F PstI and Untxb2 R PstI, A-tailed, and ligated into pGEM-T (Promega) to make pKLN41. This insert was excised from pKLN41 with PstI and ligated into pKLN2 cut with PstI to make pKLN43. kan-2 was amplified from pFAK (10) with primers Kan-2 F RI Sma and Kan-2 R MCS, digested with EcoRI and MfeI, and ligated into pKLN43 cut with EcoRI to make pKLN56. The gfp gene and terminator were excised from pPROBE-gfp[tagless] (22) with BamHI and EcoRV and ligated into pKLN56 cut with BamHI and partially blunted to make pKLN59. DNA sequences of inserts and junctions were determined for each cloning step (data not shown). All amplifications were performed using Pfu polymerase (Stratagene, La Jolla, Calif.), as recommended by the manufacturer. Plasmids were introduced into X. fastidiosa by electroporation as follows. Electrocompetent X. fastidiosa cells were freshly prepared for each transformation by resuspending colonies from 7-day-old plates in water. Cells were washed by pelleting and resuspending once with water and twice with 10% glycerol before being resuspended in 10% glycerol to a density of ∼109 CFU/ml. Solutions were chilled and centrifugations were carried out at 4°C. A 2-μg portion of each plasmid in a volume of 10 μl was electroporated into 40 μl of electrocompetent X. fastidiosa cells in a 0.1-cm-gap cuvette at 1.8 kV, 200 Ω, and a capacitance of 25 μF in a GenePulser (Bio-Rad, Hercules, Calif.) with time constants of about 4 ms. Electroporated cells were recovered for 24 h in PW, plated on selective PWG, and incubated at 28°C for 7 to 21 days. The resulting colonies were restreaked onto fresh selective plates and grown for 7 days before further use. The three green-fluorescing pKLN59 transformants were named KLN59.1, KLN59.2, and KLN59.3. Genomic DNA from strains KLN59.1 and KLN59.3 were digested with NdeI, PvuII, and SmaI, Southern blotted, and probed with the 1.5-kb insert of pKLN41 by using digoxigenin labeling and chemiluminescence-based detection (Boehringer GmbH, Mannheim, Germany) as specified by the manufacturer.
TABLE 2.
Synthetic oligonucleotides used in this study
| Name | DNA sequence (5′ to 3′) |
|---|---|
| OriC F PvuI | CTCGATCGAGACTGGGATAAACTAATGCGAA |
| OriC R PvuI | ATCGATCGATGCGCTGTAATCTGAAACGCATTTGGT |
| Untxb2 F PstI | AACTGCAGACCGTCGTGAAAAGGGAAACAACC |
| Untxb2 R PstI | AACTGCAGACAAACACCGCCGTCGATATGAACTCTT |
| Kan-2 F RI Sma | GGAATTCCCGGGCTGTCTCTTATACACATCTCAACCA |
| Kan-2 R MCS | ATATCAATTGAAGCTTGCATGCCTGCAGGTCGACTCTAGAGGATCCAACCAATTCTGATTAGAAAAACTCA |
Pathogenicity assays.
Temecula and KLN59.3 were inoculated via a needle into greenhouse-grown Vitis vinifera seedlings of the susceptible variety Cabernet sauvignon. Two points on each plant were pricked five times through a 5 μl drop of an X. fastidiosa suspension of ∼109 CFU/ml (17). Ten seedlings each were inoculated with Temecula, KLN59.3, or sterile buffer (mock inoculation). Development of leaf scorch symptoms was scored at weekly intervals. X. fastidiosa populations from one symptomatic leaf petiole from each plant were quantified by dilution plating on PWG (16). Data from population counts were transformed using the equation y = 1/log x and analyzed using Student's t test for significance. Colonies cultured from plants were examined under blue light and then restreaked on selective media to verify the genotype of the strain. These cells were reinoculated into healthy greenhouse-grown Cabernet sauvignon grafted cuttings by the same procedure described above. Finally, X. fastidiosa was isolated from petioles of symptomatic leaves from the latter inoculated plants, and its identity was verified as above.
Transmission test.
Blue-green sharpshooter leafhoppers, Graphocephala atropunctata (Signoret) (Hemiptera, Cicadellidae), were collected on wild grapevines as adults and late-instar nymphs at the University of California Botanical Garden in Berkeley and kept on mugwort and basil before being used in transmission tests. G. atropunctata is a very efficient vector for transmission of X. fastidiosa to grapevines (16). To test for natural infectivity of the vector, G. atropunctata adults were caged for 4 days on healthy grapevine seedlings prior to the experiment; none of these plants became infected, and we assumed that the insects were X. fastidiosa free. Four adults were caged on each plant for a 4-day acquisition access period to acquire X. fastidiosa on seedlings previously used for pathogenicity assays. These four insects were then divided into two groups of two individuals and transferred to healthy seedlings for a 4-day inoculation access period. Fifteen test plants were inoculated per treatment (Temecula, KLN59.3, and mock inoculation). The plants were maintained in the greenhouse for 2 to 3 months, when samples were collected for X. fastidiosa identification. Transmission results were analyzed using a χ2 analysis of a 2 by 2 table of the data.
Microscopy.
Grapevine cuttings used in pathogenicity assays were also used for microscopy. Leaves were pulled from plants immediately prior to sectioning. For each leaf, the petiole was hand sectioned with a thin razor, mounted on a 4% gelatin pad with 50% glycerol, and immediately examined under the microscope. Insect heads were dissected so that the two pharynges were separated from each other and the precibarium was visible (2) and were mounted as above. Images were captured using a Zeiss 510 confocal laser-scanning microscope or an Applied Precision Deltavision Spectris DV4 deconvolution microscope at the University of California College of Natural Resources Biological Imaging Facility. For confocal images, Gfp was excited using the 488-nm wavelength of an argon ion laser. A 505- to 550-nm bandpass (BP) emission filter was placed in front of the detector. UV autofluorescence from the sample was excited using 364-nm light from a Coherent UV laser. Emissions were collected using a 385- to 470-nm BP filter. Images were captured using Zeiss 510 software and subsequently processed using Adobe Photoshop v. 7 for the Macintosh. For deconvolution images, samples were imaged at 0.2-μm intervals using the Olympus PlanApo 100x objective with 1.4 NA. The pixel size was 0.06721 × 0.200. The Gfp was visualized using the Deltavision FITC filter set (excitation = 490/20; emission = 528/38). The UV autofluorescence was visualized using the Deltavision DAPI filter set (excitation = 360/40; emission = 457/50). Subsequent image stacks were deconvolved using Softworx 3.2.2 from Applied Precision. Images were exported as TIFFs to Adobe Photoshop.
Vessel colonization analysis.
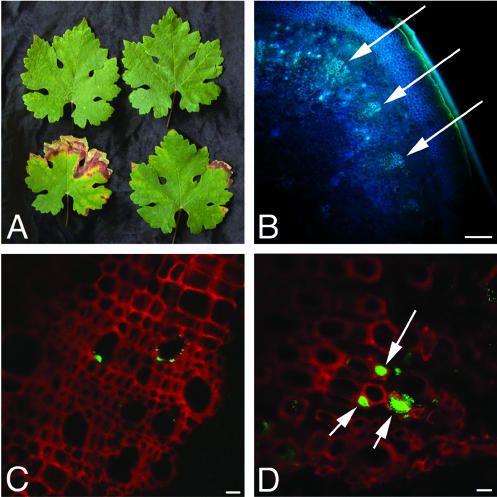
For each group (symptomatic and asymptomatic), four petioles from two different plants were analyzed as follows. The total number of vascular bundles from a single hand section of the basal end of the petiole was recorded (Fig. 1B). Each bundle in that section was examined at ×40 using confocal laser scanning microscopy (CLSM) to determine whether any colonization was present (Fig. 1C and D). Small colonies can be difficult to identify when scanning vessels. To ensure an accurate count of small colonies, every vessel was systematically observed at a magnification that allows the resolution of individual cells. For each colonized bundle, the total number of vessels was counted. Each colonized vessel was then counted, and the colony size, as visible in one 0.8- to 1.0-μm focal plane, was noted, as well as whether the vessel was completely occluded by the colony. Because the cut surface of the sample potentially was subject to artifactual spreading of X. fastidiosa cells during cutting, images for analysis were captured from the deepest optical section that still afforded adequate resolution for colony size determination (about 15 μm deep). To determine the total number of vessels for each petiole, an average number of vessels per bundle was calculated for each petiole based on the data from bundles that had been closely examined. This average was multiplied by the total number of bundles observed.
FIG. 1.
Examples of asymptomatic and symptomatic leaves used for analysis. (A) The top row contains asymptomatic leaves; the bottom row contains symptomatic leaves. (B) Low-magnification view of a hand section of a grapevine petiole, similar to those used in the analysis. Arrows indicate the vascular bundles, which are groups of adjacent xylem vessels. (C and D) Representative examples of colonized bundles from an asymptomatic leaf (C) and a symptomatic leaf (D). Plant xylem is depicted in red; X. fastidiosa cells are green. Arrows indicate occluded vessels. Bars, 100 μm (B) and 10 μm (C and D).
RESULTS
Construction of a stable green-fluorescent strain of X. fastidiosa.
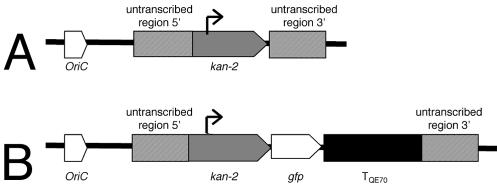
We sought to engineer a strain of X. fastidiosa that would be useful for in planta fluorescence microscopy. Such a strain should strongly express Gfp, and expression should be stable in the absence of selection. Therefore we decided to express gfp from a strong promoter and to integrate the cassette directly into the X. fastidiosa chromosome. A gfp gene followed by a transcriptional terminator was transcriptionally fused to the kan-2 kanamycin resistance gene, creating an artificial operon. This kan-gfp cassette was ligated into an allelic exchange plasmid designed for chromosomal integration (pKLN59 [see Materials and Methods]) (Fig. 2B). Plasmid pKLN59, which carries the gfp gene, and the parent plasmid pKLN56, which lacks the gfp gene, were electroporated into the Temecula strain of X. fastidiosa. In three independent electroporation experiments, only seven kanamycin-resistant transformants were obtained from pKLN59 and only three of these transformants were green fluorescent. Significantly, in the same experiments, a total of 20,240 kanamycin-resistant transformants were obtained with the control plasmid, pKLN56. The fact that transformation with pKLN59 is 3 orders of magnitude less efficient than with pKLN56 suggests that the kan-gfp construct may impair viability under our selection conditions. Alternatively, a lower chromosomal integration frequency may occur as the length of mismatched DNA increases. Further experimentation is required to resolve this. Transformant KLN59.3 was used for further study.
FIG. 2.
Constructs. (A) pKLN56 allelic exchange plasmid construct, in which the kan-2 gene is inserted into X. fastidiosa genomic DNA predicted to be untranscribed. (B) pKLN59 allelic exchange plasmid construct, in which a gfp gene and transcriptional terminator were inserted into pKLN56 after the kan-2 gene in a transcriptional fusion.
Stability and chromosomal location of the Gfp expression cassette.
We have never observed reversion of KLN59.3 cells to a nonfluorescent phenotype. KLN59.3 cells have been recovered onto nonselective media from grapevines up to 6 months after inoculation and have yielded only green-fluorescent colonies. To assess the stability of the kan-gfp cassette in vitro, single colonies of KLN59.3 were chosen from selective medium and restreaked onto nonselective medium. At 7-day intervals, KLN59.3 colonies were scored for green fluorescence and restreaked again onto nonselective medium. After three rounds of restreaking, representing an estimated 90 generations, all colonies were green fluorescent. Because reversion of a KLN59.3 has never been observed in these or other experiments, we conclude that the kan-gfp cassette in KLN59.3 is highly stable.
To verify the chromosomal location of the kan-gfp cassette in KLN59.3, a Southern blot analysis was performed using the 1.5-kb untranscribed genomic fragment as a probe (data not shown). Surprisingly, both the bands expected for the wild type and KLN59.3 were seen for the KLN59.3 samples. Closer inspection of the Temecula genome revealed that the 1.5-kb region we chose is 100% identical to two separate loci. These data indicate that the kan-gfp cassette is located in only one of the repeated regions and that disruption of gene function is highly unlikely due to the presence of a second copy of the sequence.
KLN59.3 behaves like the parent strain.
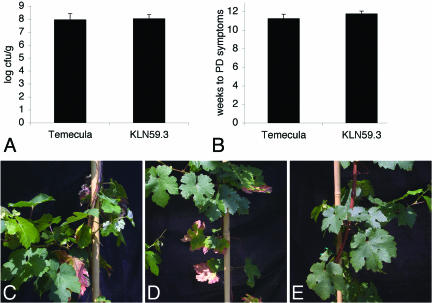
No significant differences were detected between KLN59.3 and the parent strain, Temecula, during growth on PWG (data not shown). Average bacterial populations in grapevines also were similar: 1.42 × 108 CFU/g for KLN59.3 and 1.02 × 108 CFU/g for Temecula (Fig. 3A). These differences are not significant, according to a t test (P = 0.71). KLN59.3 and the wild type caused similar symptoms, with leaf scorch of more than one leaf appearing an average of 11.75 weeks after inoculation compared to 11.25 weeks for Temecula (Fig. 3B to E). These differences are not significant, according to a t test of the average (P = 0.39), indicating that KLN59.3 is a virulent strain.
FIG. 3.
Comparison of strain KLN59.3 to the wild type. (A) Average bacterial populations in grapevines (n = 10). (B) PD symptom expression as measured by the average number of weeks elapsed from inoculation until multiple leaves exhibited leaf scorch (n = 8). Error bars in panels A and B represent the standard error. (C to E) Grapevines inoculated with Temecula (C), KLN59.3 (D), or sterile buffer (E). No differences in symptom expression were noted between panels C and D.
To fulfill Koch's postulates with KLN59.3, we recovered it from each of 5 symptomatic grapevines and reinoculated the mutant into 2 healthy grapevine cuttings per recovery (total of 10 plants). Temecula wild-type and mock inoculations also were performed. All of the vines inoculated with KLN59.3 or Temecula developed PD. No disease was observed in mock-inoculated vines. These data confirm that KLN59.3 causes PD despite the expression of foreign proteins.
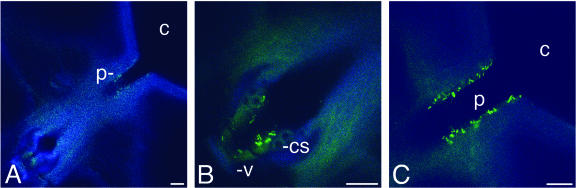
To establish whether transmission of X. fastidiosa is impaired by Gfp expression, we tested transmission of KLN59.3 by the leafhopper vector G. atropunctata, a principal vector for X. fastidiosa in coastal California (18, 28). Temecula was transmitted 11 times in 15 replicates, and KLN59.3 was transmitted 6 times in 15 replicates. This difference is not significant (χ2 = 3.4; degrees of freedom = 1; P = 0.07), suggesting that transmission of KLN59.3 is comparable to that of the wild type. No transmission from the buffer-inoculated negative control plants was observed. Furthermore, we were able to observe green-fluorescent cells in the heads of insects (n = 4 [Fig. 4]). Cells were present in areas where X. fastidiosa colonization has been observed previously by scanning electron microscopy (5, 29; R. P. P. Almeida and A. H. Purcell, unpublished data), demonstrating that vector colonization by KLN59.3 is comparable to that by the wild type.
FIG. 4.
KLN59.3-colonized G. atropunctata epipharynx. (A) View of the epipharynx showing the precibarium (p) and part of the cibarium (c). The stylets are to the lower left. (B) Higher magnification of panel A showing X. fastidiosa attached near the valve (v) and the chemosensory organs (cs). (C) Higher magnification of panel A showing X. fastidiosa attached to the precibarium wall. Bars, 10 μm.
Colony size distribution in grapevine petioles.
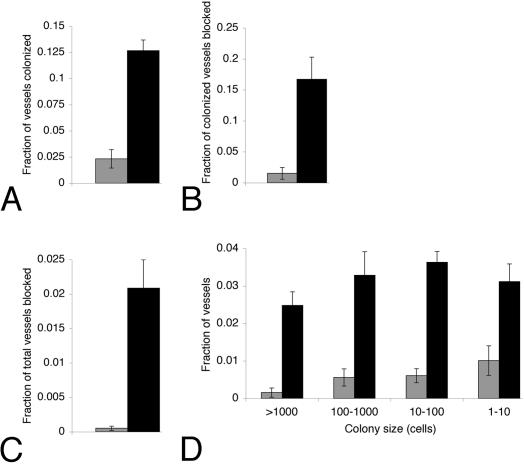
To determine whether any relationship between the incidence or pattern of xylem vessel colonization and symptom formation was present, colonization of the petioles of leaves exhibiting leaf scorch was compared to that of asymptomatic leaves located adjacent to symptomatic leaves on the same cane (Fig. 1A; see Materials and Methods). Several differences in colonization were noted between symptomatic and asymptomatic leaves. First, the fraction of vessels colonized was more than fivefold higher for symptomatic leaves (13%) than for asymptomatic leaves (2.3%) (Fig. 5A). Furthermore, of the colonized vessels, more than a 10-fold-higher proportion was blocked for symptomatic (17%) than for asymptomatic (1.5%) leaves (Fig. 5B). Despite this increase, even in symptomatic leaves 83% of colonized vessels were not blocked, demonstrating that the pathogen exists largely in colonies that do not cause blockage. A comparison of the fraction of total vessels blocked shows that vessels of symptomatic leaves are blocked 40-fold more frequently (2.1%) than those of asymptomatic leaves (0.053%) (Fig. 5C). These data show a correlation between the extent of colonization of the petiole and symptom development in the leaf and suggest that higher proportions of colonized and blocked vessels lead to disease. We also examined the distribution of colony sizes in symptomatic and asymptomatic leaf petioles (Fig. 5D). Regardless of symptom expression, most vessels (over 80%) had fewer than 1,000 cells per section and more than half had fewer than 100 cells per section. While symptomatic leaves showed a 3-fold increase in the number of small colonies (1 to 10 cells per section) over asymptomatic leaves, a dramatic (20-fold) increase in the number large colonies (1,000 or more cells per section) was observed. These data indicate that the frequency of occurrence of large colonies, most of which occlude the vessel, is associated with disease.
FIG. 5.
Analysis of colonization of vessels in four asymptomatic and four symptomatic leaf petioles, representing 3,784 and 7,963 total vessels, respectively. Gray bars represent asymptomatic leaves; black bars represent symptomatic leaves. (A) Average fraction of vessels per petiole in which colonization was observed in the section examined. (B and C) Average fraction of colonized vessels (B) or total vessels (C) per petiole that were completely plugged by bacteria in the section examined. (D) Colony sizes and average frequency observed per petiole. Note that colony size refers to the number of cells visible in one 0.8- to 1-μm section. Error bars represent the standard error.
Qualitative analysis of colonization.
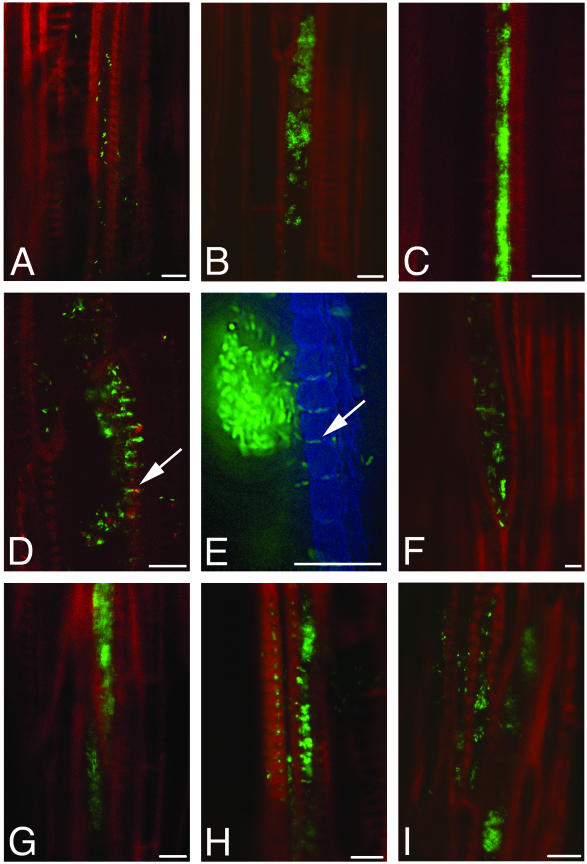
To develop a clear understanding of X. fastidiosa colonization, we compiled a series of 165 images of undisturbed X. fastidiosa colonies inside intact vessels of symptomatic leaves. Because of the disruptive nature of cross sectioning the xylem vessel prior to observation, we instead elected to section 1-cm-long petiole segments longitudinally and, using CLSM, focus inside the sample onto X. fastidiosa colonies inside unopened vessels. Because larger colonies are easier to identify when searching through uncut xylem vessels, this collection of images is biased against small colonies, and therefore no quantification of frequency of observation of each type was performed. Colonies observed fell into three general groups (Fig. 6A to C); (i) single cells or small colonies (up to 100 cells per section [Fig. 6A]), (ii) larger colonies (>100 cells per section [Fig. 6B]), and (iii) colonies completely filling the vessel (Fig. 6C). Colonies were found in four situations; (i) contained in a single vessel (Fig. 6B and C), (ii) traversing pit membranes into adjacent vessels (Fig. 6D and E), (iii) abutting a vessel end (Fig. 6F), and (iv) traversing the vessel end (Fig. 6G). Colonized vessels were either isolated (Fig. 6B, C, and F) or adjacent to other colonized vessels (Fig. 6A, D, E, H, and I). Colonies were sometimes accompanied by a haze (Fig. 6D and E), appeared to be floating in the vessel lumen (Fig. 6A, F, and I), or had blank space between individual cells (Fig. 6B), which may indicate that polysaccharide slime accompanies the colonies or surrounds individual cells. The passage of cells via the bordered pits was especially clear when using CLSM. Cells appeared in a large colony in one vessel, lined up single file in the pits themselves, and as solitary cells in the adjacent vessel. Although these are still images, they are consistent with the cells in the large colony having initiated movement, gained access through the pit membranes, and appeared in the adjacent vessel a few at a time.
FIG.6.
Representative longitudinal sections of infected grapevine petioles from a collection of 165 images. Plant xylem is depicted in red or blue; X. fastidiosa cells are green. (A) Small colonies and single X. fastidiosa cells. (B) Large colonies in a single xylem vessel. (C) Vessel completely packed by a colony confined to a single vessel. (D and E) X. fastidiosa cells traversing the bordered pits (arrows) and gaining access to an adjacent vessel. The image in panel E was taken on a deconvolution microscope. (F) Colony at a vessel end. (G) Colony spanning a vessel end. (H and I) Adjacent colonized vessels. Bars, 10 μm.
DISCUSSION
Because of the recent publication of the genome sequence of several strains of X. fastidiosa (3, 33, 36) and the subsequent development of molecular genetic tools (8, 14, 15, 37), X. fastidiosa research is ready to move forward at a fast pace. As researchers seek to investigate the function of individual genes by mutational analysis, a vigorous understanding of the biology of the wild-type bacterium will be required for comparison. In this work, a green-fluorescent strain of X. fastidiosa (KLN59.3) was used for in planta analysis of bacterial colonization. Strain KLN59.3 behaved like the wild type in all aspects, including virulence and transmission, making it an excellent tool for studying various aspects of PD.
We used strain KLN59.3 to assess the colony size within xylem vessels and the frequency of different colony sizes observed in tissues with and without symptoms in order to gain insight into both the mechanism of disease and the process of plant colonization. Because asymptomatic leaves adjacent to symptomatic leaves in our greenhouse-grown plants invariably developed disease symptoms, we assumed that colonized asymptomatic leaves represented an earlier stage of disease progression than symptomatic leaves. Therefore, our analysis can be considered a measure of colonization at two stages of disease progression: presymptomatic and postsymptomatic. Predictably, the fraction of vessels colonized was higher in symptomatic than asymptomatic leaves. We found that a vessel was five times more likely to be colonized if the leaf had symptoms. However, the differences in specific aspects of colonization, such as colony size distribution and vessel occlusion, between symptomatic and asymptomatic leaves were more informative.
For all infected leaves, the colony size was variable; however, we found a dramatic change in the colony size distribution between the two stages. For example, while the number of small colonies (10 or fewer cells per section) increased 3-fold in symptomatic leaves over asymptomatic leaves, the number of large colonies (more than 1,000 cells per section) increased 20-fold (Fig. 5D). This indicates that most cells in symptomatic leaves are in large colonies while this is not true in asymptomatic leaves. This shift to larger colonies in symptomatic leaves may indicate that large colonies have a more deleterious effect on the host than do smaller colonies. Small colonies in asymptomatic leaves may grow into large colonies over time; however, cells must also be dispersing to new vessels since symptomatic leaves had a fivefold increase in the fraction of vessels colonized (Fig. 5A).
The fraction of colonized vessels that were also blocked increased 10-fold in diseased leaves (Fig. 5B). This result is consistent with our observation that colonies are larger in symptomatic leaves since only large colonies can fill the entire vessel lumen. However, when we compared the fraction of the total number of vessels that were blocked, we found that symptomatic leaves had a 40-fold-higher fraction of vessels that were blocked (Fig. 5C). The magnitude of change in the fraction of total vessels that were blocked was larger than that of any other feature of X. fastidiosa colonization measured by ourselves or others and suggests that the deleterious effect of large colonies on the host is due mainly to vessel blockage. Therefore, the extent of vessel blockage by bacterial colonization is highly likely to be a crucial variable in symptom expression. Because bacterial vessel plugging was a prerequisite for disease in our study, we conclude that it is unlikely that bacterial toxins or plant-initiated vessel failure leads to disease symptoms.
These results differ in some respects from those of previous studies. Hopkins (19) found frequencies of colonization and blockage in symptomatic leaf petioles similar to ours but saw little difference between symptomatic and asymptomatic leaves. Tyson et al (35) saw much higher frequencies of colonization and blockage than we did and saw only marginally lower frequencies in asymptomatic tissue. Two key experimental factors may explain these differences. First, the previous studies used V. lambrusca and a French-American grapevine hybrid as plant hosts, both of which would be expected to be less susceptible to PD symptoms than would the V. vinifera Cabernet sauvignon used in this study (25, 26). Second, our vines grew in the greenhouse, where water stress is common due to limitations on root mass in pots, while the previous studies used field-grown specimens. The environmental differences may have contributed to a difference in symptom expression.
While most attention has been directed to vessel plugging, which is the most obvious feature of X. fastidiosa colonization of xylem, until now there was little appreciation of the significance of the extensive colonization of vessels by small, nonplugging bacterial communities. The limitations of previous methods of examination of vessels, such as scanning electron microscopy (which restricts the amount of tissue that can practically be examined and leads to uncertainty about the original location of cells observed), probably has led to an emphasis on vessel blockage during infection. While the extent of this blockage is well correlated with disease, our quantitative study reveals clearly that such vessel occlusion is relatively uncommon for X. fastiodiosa colonies. Indeed, we found that the large majority of colonized vessels contained relatively few cells of X. fastidiosa (Fig. 5B). Such a finding implies that several features may characterize the process of vessel colonization by X. fastidiosa. If one assumes that the growth of X. fastidiosa in vessels mimics that of a bacterial biofilm in a flowing liquid, whereby cells acquire nutrients from dilute solutions, then acquisition of nutrients would be dependent on the flow of nutrients past adhering cells. The low nutrient content of xylem fluids would be expected to supply the needed nutrients only if replenished by flow past X. fastidiosa cells. Thus, vessel blockage would be expected to be a self-limiting process whereby further multiplication of the pathogen would not be possible due to a lack of nutrients. In addition to the problem of nutrient limitation, vessel blockage probably interferes with the transmission of cells in plugged vessels to new plants because insects avoid feeding from blocked vessels. In such a self-limiting environment, escape of cells to vessels in which fluids continue to flow would be strongly selected.
Dispersion of X. fastidiosa through the xylem network probably follows the natural course of the xylem stream through the plant but would be expected to require a mechanism for accessing vessels connected only by bordered pits because pit membranes do not readily allow the passage of objects 20 nm in diameter or larger (7). Pits can be damaged or broken by bacterium-independent mechanisms, and this breach of pit membrane integrity may be sufficient for bacterial colonization of the xylem network. Alternatively, X. fastidiosa may require a pit membrane-degrading activity in order to traverse bordered pits. Indeed, X. fastidiosa has been shown to express genes predicted to encode pit membrane-degrading enzymes in vitro (32). As shown by the data presented here, whether or not X. fastidiosa requires pit membrane degradation activity to colonize the xylem network, it probably does possess this capability because colonies can be observed to traverse pits adjacent to the colony and to enter new vessels (Fig. 6D and E). These examples of pit transit are probably due to bacterial degradation and not just a stochastic encounter with a damaged pit, because all pits adjacent to the colony have bacterial cells moving through them (Fig. 6D and E), whereas if a colony randomly encountered a broken pit, we would expect to see only one pit containing cells.
The finding that most vessels are only sparsely colonized by X. fastidiosa would be expected if it was primarily an endophytic colonist of plants that only accidentally caused sufficient blockage of vessels to induce water stress in plants under certain conditions. We might also surmise that X. fastidiosa is efficient in movement between vessels since only relatively few vessels in the plant are occluded while many more contain cells of the pathogen (Fig. 5B). While such dispersal clearly would benefit a commensal endophytic interaction with the plant, only when colonization becomes excessive in plant species particularly amenable to colonization, such as grapevine, would such an interaction become pathogenic. This hypothesis can be tested by comparative analysis of colonization in susceptible hosts and plants that do not develop symptoms when colonized by X. fastidiosa.
This analysis of X. fastidiosa colonization of a susceptible host can serve as a baseline with which to compare colonization by other strains and in other hosts. For example, this strain will be useful in comparisons between susceptible and resistant or tolerant hosts to identify characteristics of colonization that differ among these types of hosts. Use of Gfp-marked mutants will greatly advance the in planta and in insecta characterization of mutant strains. KLN59.3 can be used for more in-depth microscopic analyses of the wild type. Finally, the use of Gfp as a reporter for gene expression in X. fastidiosa will enable us to understand the importance of various genes during colonization and symptom expression.
Acknowledgments
We thank Ed Norberg and Dick Hoenisch for grapevines; Christina Wistrom, Denise Schichnes, and Sky Rashby for technical assistance; and Gale Wichmann for helpful comments on the manuscript.
This material is based on work supported by the National Science Foundation under a grant awarded to K.L.N. in 2002 and by funding from the American Vineyard Foundation and the California Competitive Grant Program for Research in Viticulture and Enology.
REFERENCES
- 1.Almeida, R. P. P., E. F. Pereira, A. H. Purcell, and J. R. S. Lopes. 2001. Multiplication and movement of a citrus strain of Xylella fastidiosa within sweet orange. Plant Dis. 85:382-386. [DOI] [PubMed] [Google Scholar]
- 2.Backus, E. A., and D. L. McLean. 1982. The sensory systems and feeding behavior of leafhoppers 1. The aster leafhopper Macrosteles fascifrons Stal (Homoptera, Cicadellidae). J. Morphol. 172:361-379. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya, A., S. Stilwagen, N. Ivanova, M. D'Souza, A. Bernal, A. Lykidis, V. Kapatral, I. Anderson, N. Larsen, T. Los, G. Reznik, E. Selkov, Jr., T. L. Walunas, H. Fell, W. S. Feil, A. Purcell, J.-L. Lassez, T. L. Hawkins, R. Haselkorn, R. Overbeek, P. F. Predki, and N. C. Kyrpides. 2002. Whole-genome comparative analysis of three phytopathogenic Xylella fastidiosa strains. Proc. Nat. Acad. Sci. USA 99:12403-12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brlansky, R. H., R. F. Lee, L. W. Timmer, D. E. Purcifull, and B. C. Raju. 1982. Immunofluorescent detection of xylem-limited bacteria in situ. Phytopathology 72:1444-1448. [Google Scholar]
- 5.Brlansky, R. H., L. W. Timmer, R. E. McCoy, and W. J. French. 1983. Colonization of the sharpshooter vectors Oncometopia nigricans and Homalodisca coagulata by xylem limited bacteria. Phytopathology 73:530-535. [Google Scholar]
- 6.Chang, C. J., M. Garnier, L. Zreik, V. Rossetti, and J. M. Bove. 1993. Culture and serological detection of the xylem-limited bacterium causing citrus variegated chlorosis and its identification as a strain of Xylella fastidiosa. Curr. Microbiol. 27:137-142. [DOI] [PubMed] [Google Scholar]
- 7.Choat, B., M. Ball, J. Luly, and J. Holtum. 2003. Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species. Plant Physiol 131:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva Neto, J. F., T. Koide, S. L. Gomes, and M. V. Marques. 2002. Site-directed gene disruption in Xylella fastidiosa. FEMS Microbiol. Lett. 210:105-110. [DOI] [PubMed] [Google Scholar]
- 9.Davis, M. J., W. J. French, and N. W. Schaad. 1981. Isolation and culture of the bacteria associated with phony peach disease and plum leaf scald. Phytopathology 71:869-870. [Google Scholar]
- 10.Feil, H., W. S. Fell, J. C. Detter, A. H. Purcell, and S. E. Lindow. 2003. Site-directed disruption of the fimA and fimF fimbrial genes of Xylella fastidiosa. Phytopathology 93:675-682. [DOI] [PubMed] [Google Scholar]
- 11.Freitag, A. H. 1951. Host range of Pierce's disease virus of grapes as determined by insect transmission. Phytopathology 41:920-934. [Google Scholar]
- 12.Fry, S. M., and R. D. Milholland. 1990. Multiplication and translocation of Xylella fastidiosa in petioles and stems of grapevine resistant, tolerant, and susceptible to Pierce's disease. Phytopathology 80:61-65. [Google Scholar]
- 13.Fry, S. M., and R. D. Milholland. 1990. Response of resistant, tolerant, and susceptible grapevine tissues to invasion by the Pierce's disease bacterium, Xylella fastidiosa. Phytopathology 80:66-69. [Google Scholar]
- 14.Gaurivaud, P., L. C. A. Souza, A. C. D. Virgilio, A. G. Mariano, R. Palma, and B. P. Monteiro. 2002. Gene disruption by homologous recombination in the Xylella fastidiosa citrus variegated chlorosis strain. Appl. Environ. Microbiol. 68:4658-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilhabert, M. R., L. M. Hoffman, D. A. Mills, and B. C. Kirkpatrick. 2001. Transposon mutagenesis of Xylella fastidiosa by electroporation of Tn5 synaptic complexes. Mol. Plant-Microbe Interact. 14:701-706. [DOI] [PubMed] [Google Scholar]
- 16.Hill, B. L., and A. H. Purcell. 1995. Acquisition and retention of Xylella fastidiosa by an efficient vector, Graphocephala atropunctata. Phytopathology 85:209-212. [Google Scholar]
- 17.Hill, B. L., and A. H. Purcell. 1995. Multiplication and movement of Xylella fastidiosa within grapevine and four other plants. Phytopathology 85:1368-1372. [Google Scholar]
- 18.Hill, B. L., and A. H. Purcell. 1997. Populations of Xylella fastidiosa in plants required for transmission by an efficient vector. Phytopathology 87:1197-1201. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins, D. L. 1981. Seasonal concentration of the Pierce's disease bacterium in grapevine stems, petioles, and leaf veins. Phytopathology 71:415-418. [Google Scholar]
- 20.Hopkins, D. L. 1989. Xylella fastidiosa: xylem-limited bacterial pathogen of plants. Annu. Rev. Phytopathol. 27:271-290. [Google Scholar]
- 21.Li, W. B., C. H. Prou, W. D. Pria, C. Teixeira, V. S. Miranda, E. O. Pereira, A. J. Ayres, C. X. He, P. I. Costa, and J. S. Hartung. 2002. Citrus and coffee strains of Xylella fastidiosa induce Pierce's disease in grapevine. Plant Dis. 86:1206-1210. [DOI] [PubMed] [Google Scholar]
- 22.Miller, W. G., J. H. J. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 23.Minsavage, G. V., C. M. Thompson, D. L. Hopkins, R. Leite, and R. E. Stall. 1994. Development of a polymerase chain reaction protocol for detection of Xylella fastidiosa in plant tissue. Phytopathology 84:456-461. [Google Scholar]
- 24.Mollenhauer, H. H., and D. L. Hopkins. 1974. Ultrastructural study of Pierce's disease bacterium in grape xylem tissue. J. Bacteriol. 119:612-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollenhauer, H. H., and D. L. Hopkins. 1976. Xylem morphology of Pierce's disease infected grapevines with different levels of tolerance. Physiol. Plant Pathol. 9:95-100. [Google Scholar]
- 26.Mortensen, J. A., L. H. Stover, and C. F. Balerdi. 1977. Sources of resistance to Pierce's disease in Vitis. J. Am. Soc. Hort. Sci. 102:695-697. [Google Scholar]
- 27.Press, M. C., and J. B. Whittaker. 1993. Exploitation of the xylem stream by parasitic organisms. Philos. Trans. R. Soc. London Ser. B 341:101-111. [Google Scholar]
- 28.Purcell, A. H. 1975. Role of the blue-green sharpshooter Hordnia circellata in the epidemiology of Pierce's disease of grapevines. Environ. Entomol. 4:745-752. [Google Scholar]
- 29.Purcell, A. H., A. H. Finlay, and D. L. McLean. 1979. Pierce's disease bacterium mechanism of transmission by leafhopper vectors. Science 206:839-841. [DOI] [PubMed] [Google Scholar]
- 30.Purcell, A. H., and D. L. Hopkins. 1996. Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 34:131-151. [DOI] [PubMed] [Google Scholar]
- 31.Purcell, A. H., and S. R. Saunders. 1999. Fate of Pierce's disease strains of Xylella fastidiosa in common riparian plants in California. Plant Dis. 83:825-830. [DOI] [PubMed] [Google Scholar]
- 32.Scarpari, L. M., M. R. Lambais, D. S. Silva, D. M. Carraro, and H. Carrer. 2003. Expression of putative pathogenicity-related genes in Xylella fastidiosa grown at low and high cell density conditions in vitro. FEMS Microbiol. Lett. 222:83-92. [DOI] [PubMed] [Google Scholar]
- 33.Simpson, A. J. G., F. C. Reinach, P. Arruda, F. A. Abreu, M. Acencio, R. Alvarenga, L. M. C. Alves, J. E. Araya, G. S. Baia, C. S. Baptista, M. H. Barros, E. D. Bonaccorsi, S. Bordin, J. M. Bove, M. R. S. Briones, M. R. P. Bueno, A. A. Camargo, L. E. A. Camargo, D. M. Carraro, H. Carrer, N. B. Colauto, C. Colombo, F. F. Costa, M. C. R. Costa, C. M. Costa-Neto, L. L. Coutinho, M. Cristofani, E. Dias-Neto, C. Docena, H. El-Dorry, A. P. Facincani, A. J. S. Ferreira, V. C. A. Ferreira, J. A. Ferro, J. S. Fraga, S. C. Franca, M. C. Franco, M. Frohme, L. R. Furlan, M. Garnier, G. H. Goldman, M. H. S. Goldman, S. L. Gomes, A. Gruber, P. L. Ho, J. D. Hoheisel, M. L. Junqueira, E. L. Kemper, J. P. Kitajima, J. E. Krieger, E. E. Kuramae, F. Laigret, M. R. Lambais, L. C. C. Leite, E. G. M. Lemos, M. V. F. Lemos, S. A. Lopes, C. R. Lopes, J. A. Machado, M. A. Machado, A. Madeira, H. M. F. Madeira, C. L. Marino, M. V. Marques, E. A. L. Martins, E. M. F. Martins, A. Y. Matsukuma, C. F. M. Menck, E. C. Miracca, C. Y. Miyaki, C. B. Monteiro-Vitorello, D. H. Moon, M. A. Nagai, A. Nascimento, L. E. S. Netto, A. Nhani, F. G. Nobrega, L. R. Nunes, M. A. Oliveira, M. C. de Oliveira, R. C. de Oliveira, D. A. Palmieri, A. Paris, B. R. Peixoto, G. A. G. Pereira, H. A. Pereira, J. B. Pesquero, R. B. Quaggio, P. G. Roberto, V. Rodrigues, A. J. D. Rosa, V. E. de Rosa, R. G. de Sa, R. V. Santelli, H. E. Sawasaki, A. C. R. da Silva, A. M. da Silva, F. R. da Silva, W. A. Silva, J. F. da Silveira, M. L. Silvestri, W. J. Siqueira, A. A. de Souza, A. P. de Souza, M. F. Terenzi, D. Truffi, S. M. Tsai, M. H. Tsuhako, H. Vallada, M. A. Van Slugs, S. Verjovski-Almeida, A. L. Vettore, M. A. Zago, M. Zatz, J. Meidanis, and J. C. Setubal. 2000. The genome sequence of the plant pathogen Xylella fastidiosa. Nature 406:151-157. [DOI] [PubMed] [Google Scholar]
- 34.Tyree, M. T., and M. H. Zimmermann. 2002. Xylem structure and the ascent of sap. Springer-Verlag, New York, N.Y.
- 35.Tyson, G. E., B. J. Stojanovic, R. F. Kuklinski, T. J. Divittorio, and M. L. Sullivan. 1985. Scanning electron microscopy of Pierce's disease bacterium in petiolar xylem of grape leaves. Phytopathology 75:264-269. [Google Scholar]
- 36.Van Sluys, M. A., M. C. de Oliveira, C. B. Monteiro-Vitorello, C. Y. Miyaki, L. R. Furlan, L. E. Camargo, A. C. da Silva, D. H. Moon, M. A. Takita, E. G. Lemos, M. A. Machado, M. I. Ferro, F. R. da Silva, M. H. Goldman, G. H. Goldman, M. V. Lemos, H. El-Dorry, S. M. Tsai, H. Carrer, D. M. Carraro, R. C. de Oliveira, L. R. Nunes, W. J. Siqueira, L. L. Coutinho, E. T. Kimura, E. S. Ferro, R. Harakava, E. E. Kuramae, C. L. Marino, E. Giglioti, I. L. Abreu, L. M. Alves, A. M. do Amaral, G. S. Baia, S. R. Blanco, M. S. Brito, F. S. Cannavan, A. V. Celestino, A. F. da Cunha, R. C. Fenille, J. A. Ferro, E. F. Formighieri, L. T. Kishi, S. G. Leoni, A. R. Oliveira, V. E. Rosa, Jr., F. T. Sassaki, J. A. Sena, A. A. de Souza, D. Truffi, F. Tsukumo, G. M. Yanai, L. G. Zaros, E. L. Civerolo, A. J. Simpson, N. F. Almeida, Jr., J. C. Setubal, and J. P. Kitajima. 2003. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 185:1018-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanamala, A., R. Harakava, and D. W. Gabriel. 2002. Transformation of Xylella fastidiosa using replicative shuttle vector pUFR047. Phytopathology 92:S83. [Google Scholar]