Abstract
Burn patients are susceptible to opportunistic infections due partly to decreased immune functions, especially Th1-driven antigen-specific responses, which are regulated by dendritic cells. The dendritic cell growth factor, fms-like tyrosine kinase-3 ligand (FL), has been shown to increase resistance to P. aeruginosa, in a dendritic cell-dependent manner, in a mouse model of burn wound infection. The specific mechanisms of protection are not known. This study tested the hypothesis that FL can enhance production of P. aeruginosa-specific antibodies following burn wound infection. Mice that had been previously exposed to P. aeruginosa were infected after burn injury by wound inoculation, or challenged by intraperitoneal injection of heat-killed P. aeruginosa. In response to wound infection, FL treatments enhanced bacterial clearance and induced a shift from IgM towards IgG and IgA. However, serum levels of neither P. aeruginosa-specific antibodies nor IFN-γ were significantly increased by FL, possibly due to decreased systemic exposure to bacteria. Following challenge with heat-killed bacteria, which ensured equal exposures, FL-treated mice produced significantly greater levels of P. aeruginosa-specific IgG2a, which correlated with an increase in serum levels of IFN-γ and enhanced opsonization capacity. IL-12, IL-10, and TGF-β were significantly increased in FL-treated mice, regardless of the type of challenge. These findings indicate that FL treatments after burn injury enhance cytokine responses to recall antigens and increase bacterial clearance. Additionally, through its ability to promote Th1-associated antigen-specific responses, FL may have potential as an immunotherapy to enhance adaptive immunity following severe burn injury.
Keywords: dendritic cells, humoral, burn, infection
Introduction
The ability of severely burned patients to resolve infections is often compromised, which can lead to complications that delay recovery and increase mortality after burn injury. While post-injury immunological responses have been studied extensively, the complexity of immune responses to infection and the large numbers of immunological alterations that have been detected have impeded the identification of suitable treatments to enhance effective immune responses to infection. Early responses of neutrophils and macrophages to a microbial challenge have been shown to be impaired following burn injury [1–4], and both of these cell types play important roles not only in elimination of microorganisms, but also in activation of other effector cells through direct interactions and secretion of cytokines [5–9]. Impairment of early innate responses not only increases microbial burden upon later acquired immune functions, but may also decrease the efficacy of subsequent responses. Indeed, T cell proliferation, functions and appropriate polarization are decreased following severe burn injury [10–13]. Additionally, antigen-specific antibody production is impaired following severe burn injury, with a large impact upon the IgG2a isotype due to decreases in the induction of Th1 response-promoting cytokines[14–17]. Impairments in production of IgG could have significant consequences since IgG is highly effective for opsonization of both gram-positive and gram-negative bacteria.
Fms-like tyrosine kinase-3 ligand (FL) is a dendritic cell growth factor that has been shown to enhance immune function under various conditions. Treatments with FL drive the production of dendritic cells, the magnitude of which is determined by the duration of treatment, that can produce cytokines and interact with effector cells to initiate numerous immune responses. In normal mice, FL-induced expansion of dendritic cells can enhance the expansion of antigen-specific T cells and antibody production [18;19]. Due to expansion of dendritic cell populations that produce and promote production of IL-12, IFN-γ, and Th1 responses, FL can enhance IgG2a production against various antigens [20–22].
Since burn injury is associated with impairments in Th1 responses and antigen-specific IgG production, processes that are induced by presentation of antigens by dendritic cells, FL may have potential for enhancing antigen-specific responses to an infection after burn injury. Restoration of specific antibody responses may improve outcome after severe burns. Specifically, in patients with P. aeruginosa bacteremia, high titers of specific IgG are associated with a significant reduction in the frequency of shock and death [23]. Additionally, induction of P. aeruginosa-specific antibodies by vaccination protects burned mice from a subsequent P. aeruginosa infection [24]. We previously reported that expansion of dendritic cells in burned mice through treatments with FL can restore Th1 cytokine responses to a primary bacterial challenge and can increase bacterial clearance and survival following infection of burn wounds with P. aeruginosa. The protective effects of FL against burn wound infection are dependent upon the presence of dendritic cells [25–27]. However, the specific mechanisms by which this dendritic cell enhancement increases resistance to burn wound infection, and the effects of FL on acquired immune responses after a severe burn injury are not known. This study was designed to determine if FL treatments can enhance antigen-specific immune responses to infections with Pseudomonas aeruginosa after a severe burn injury.
Materials and Methods
Mice
Animal protocols were consistent with the National Institutes of Health guidelines for the care and use of experimental animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. Male BALB/c mice, 6 to 8 weeks of age, were allowed to acclimate for at least one week after arrival at the animal facility. For primary immunizations, mice received an intraperitoneal injection with 104 cfu P. aeruginosa. There was no mortality associated with this dose. Mice were allowed to rest for one month after primary immunization. One month after primary exposure, mice received a full-thickness scald burn injury to 30% of the total body surface area and post-burn care as described [50;51]. Sham-injured mice were subjected to all of the procedures except immersion in water. FL (10 microgram in 0.1 ml LR) treatments were administered for 5 days by intraperitoneal injection once daily, beginning on the day of burn or sham injury. Control treatment mice received equivalent injections of LR. Recombinant human FL was kindly provided by Amgen corporation (Thousand Oaks, CA). Secondary inoculations with P. aeruginosa were administered 4 days after burn injury by application of 104 cfu to the surface of the wound. There is no relevant sham-injured, infection-only control group for burn wound infections, so responses were compared between treatment groups. In some experiments, secondary exposure was given by i.p. injection with heat-killed P. aeruginosa (HKPA) as a control for unequal systemic exposures to P. aeruginosa after wound inoculation in the control and treatment groups. The dose administered was 105 cfu HKPA prior to heat-killing. Blood was harvested 4 days after secondary exposure to P. aeruginosa for assessment of antibody responses.
Microbiology
P. aeruginosa was used because it is a common source of wound infections and pneumonia in burn patients [52–55]. P. aeruginosa was purchased from the American Type Culture Collection (ATCC #19660). Cultures were grown in tryptic soy broth and diluted in sterile saline for wound inoculation. To examine bacterial dissemination, blood was harvested 72 hours after wound inoculation, plated on tryptic soy agar and grown at 37°C overnight. HKPA was obtained by heating P. aeruginosa for one hour at 60°C. Killing was confirmed by culture prior to use.
Anti-P. aeruginosa antibody ELISA
Wells were coated by overnight incubation with heat-killed P. aeruginosa (1.85 × 106 cfu prior to killing) in 0.05M carbonate-bicarbonate buffer (pH 9.6). This was previously determined during assay optimization to be an appropriate coating concentration for signal detection with minimal background. Wells were washed four times with PBS containing 0.01% Tween-20 (pH 7), then blocked with blocking buffer (PBS + 2% bovine serum albumin) containing 0.01% Tween-20 for two hours at room temperature, then washed as before. Diluted samples in a volume of 100 ul blocking solution were incubated at room temperature for 2 hours, followed by 5 washes. For measuring antigen-specific isotypes, horseradish peroxidase-conjugated anti-mouse IgM, IgA, IgG2a or IgG1 antibodies (Zymed Laboratories, San Francisco, CA) were incubated for one hour at room temperature and wells were washed 6 times. Horseradish peroxidase substrate solution (eBiosciences, San Diego, CA) was added for 15 minutes, reactions were stopped by addition of 1M H2SO4 and absorbance read at 450nm with 540nm as a reference wavelength. Total IgG and IgM levels were measured using ELISA kits from Alpha Diagnostic (San Antonio, TX), and total IgA kits were from Bethyl Laboratories (Montgomery, TX).
Cytokine measurements
Spleens were aseptically harvested and placed in RPMI 1640 supplemented with 10% fetal bovine serum, and the cells were mechanically dispersed. The homogenate was passed through a nylon mesh strainer and incubated with red blood cell lysis buffer (Sigma-Aldrich, St. Louis, MO). Splenocytes were washed and cultured in RPMI 1640 supplemented with 10% fetal calf serum and antibiotics. Cells (5 × 106) were cultured overnight with and without additional stimulation by HKPA (107 cfu before killing), and media were harvested for measurement of cytokines by ELISA (eBiosciences).
Neutrophil isolation
Single cell suspensions from normal mouse spleens were prepared as described above and neutrophils were isolated using an anti-Ly6G (1A8) microbead kit from Miltenyi Biotec (Auburn, CA). The enriched Ly6Ghigh GR-1(Ly6G/C)high cells were used for subsequent opsonization assays.
Opsonization assay
Sera were heated at 60°C for one hour to inactivate endogenous complement and eliminate viable P. aeruginosa possibly remaining from immunizations. Absence of viable microorganisms was confirmed by overnight cultures. Serum samples were incubated in a 96-well plate with 103 P. aeruginosa on a shaking platform for 15 minutes at 37°C, followed by addition of baby rabbit complement (12.5% final concentration) and 105 neutrophils. Samples were incubated for 2 hours at 37°C, and aliquots were plated on tryptic soy agar plates and incubated at 37°C overnight. The number of cfu/plate were compared between groups. As a positive control, 2 ug purified anti-P. aeruginosa antibodies (AbCam, Cambridge, MA) were used instead of serum. Data are expressed as % killing: (cfu in “no sera” control group – cfu in experimental group)/cfu in “no sera” control group.
Statistics
Comparisons between two groups were made with an unpaired, two-tailed student’s t test. Comparisons between multiple groups were performed with one-way ANOVA and Tukey-Kramer multiple comparison test. All tests were performed using GraphPad Prism version 4.00 for Windows, GraphPad Software (San Diego, California). A p≤0.05 was considered statistically significant. All data shown are mean ± SEM.
Results
Burn injury suppresses the secondary P. aeruginosa-specific IgG response
Earlier studies have indicated that FL treatments enhance innate immune responses in a clinically relevant model of burn wound infection {Bohannon, 2008 412 /id;Toliver-Kinsky, 2003 326 /id;Toliver-Kinsky, 2005 352 /id}. The present study was designed to determine if acquired immune responses to a burn wound infection can also be enhanced by FL treatments after a severe burn injury. In our mouse model, burn wounds heal rapidly in the absence of an infection, and the eschar sloughs between 10 to 14 days post-burn. Therefore, burn wounds are inoculated with P. aeruginosa four days after injury, before significant healing occurs, and subsequent mortalities begin to occur 3 days after inoculation in the absence of FL treatments {Bohannon, 2008 412 /id}. This time frame of 3 days is not sufficient for an antigen-specific antibody response to a primary exposure through the burn wound. Therefore, all mice were immunized with P. aeruginosa one month before the study began, and the recall response to infection after burn injury was examined.
Others have reported that burn injury suppresses induction of antigen-specific immune responses [28;29]. We first confirmed that P. aeruginosa-specific IgG was also suppressed in our model of burn injury. Since there is no comparable sham, or non-injured, control for a burn wound infection, mice received a secondary exposure to P. aeruginosa by intraperitoneal injection four days after burn injury. Burn-injured mice had significantly lower levels of P. aeruginosa-specific IgG than did the sham, non-injured mice (p<0.05; figure 1).
Figure 1. P. aeruginosa-specific IgG following burn injury.
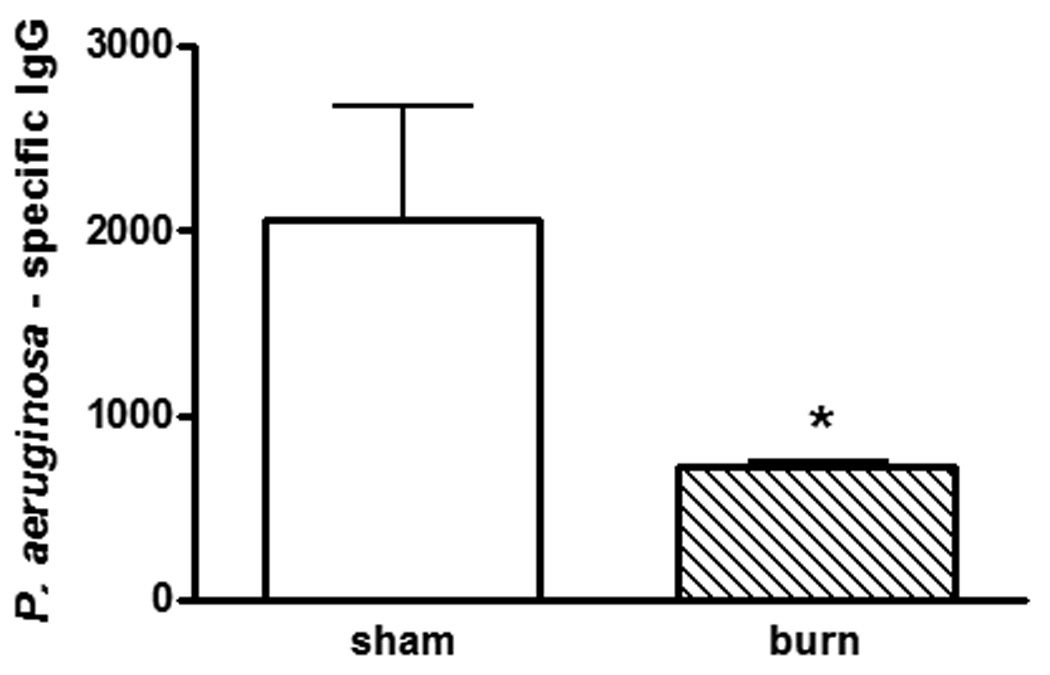
Previously immunized mice were rechallenged with HKPA by intraperitoneal injection 4 days after burn or sham injuries. Sera were harvested 4 days after bacterial challenge. n=4 mice/group, representative of two experiments. *significantly different from sham group, p<0.05.
Antibody responses to burn wound infection
Levels of P. aeruginosa-specific IgG2a and IgG1 were measured as indicators of Th1- or Th2-polarized responses, respectively, after wound infection. As shown in figure 2A, there were no significant differences in serum levels of P. aeruginosa-specific IgG2a or IgG1. Similarly, serum levels of total IgG were not different between the two treatment groups. There were also no significant differences in P. aeruginosa-specific and total IgA levels between mice receiving FL or control treatment (figure 2B). However, serum levels of both P. aeruginosa-specific and total IgM were significantly lower in mice that had been treated with FL, compared to mice that received control treatment (figure 2C).
Figure 2. Effects of FL on antibody responses following burn wound infection.
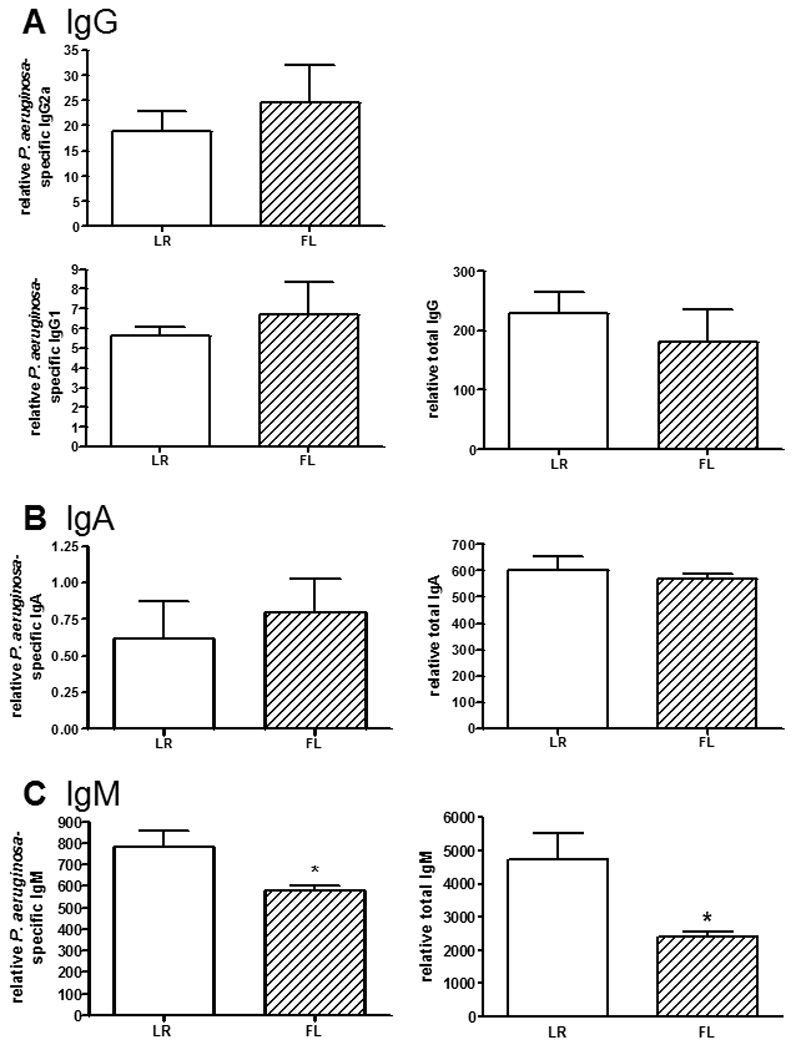
Previously immunized mice were rechallenged by inoculation of burn wounds with P. aeruginosa 4 days after burn injury. Mice received control treatment (LR) or FL for 5 days, beginning on the day of injury. Sera were harvested 4 days after infection for assessment of P. aeruginosa-specific or total (A) IgG, (B) IgA, and (C) IgM antibodies by ELISA. Relative values are shown, expressed as O.D. × sample dilution factor. n=13 mice/group, representative of 3 separate experiments. *significantly different than LR group, p<0.05.
Antibody responses to non-infectious P. aeruginosa challenge
Since FL-treated mice control bacterial growth and dissemination better than control-treated mice (figure 5), antibody production in response to a noninfectious challenge was also examined, to control for potential differences in the dose of the secondary exposure. Four days following an intraperitoneal injection of HKPA, mice that had been treated with FL had significantly higher serum levels of P. aeruginosa-specific IgG2a, compared to the control-treated mice (figure 3A). However, there were no significant differences in specific IgG1 or total IgG between the treatment groups. Similarly, there were no significant differences in specific or total IgA levels in sera (figure 3B). Unlike the response to wound infection, serum P. aeruginosa-specific IgM and total IgM were not different between treatment groups following challenge with HKPA (figure 3C).
Figure 5. Effects of FL on systemic dissemination of P. aeruginosa from the burn wound.
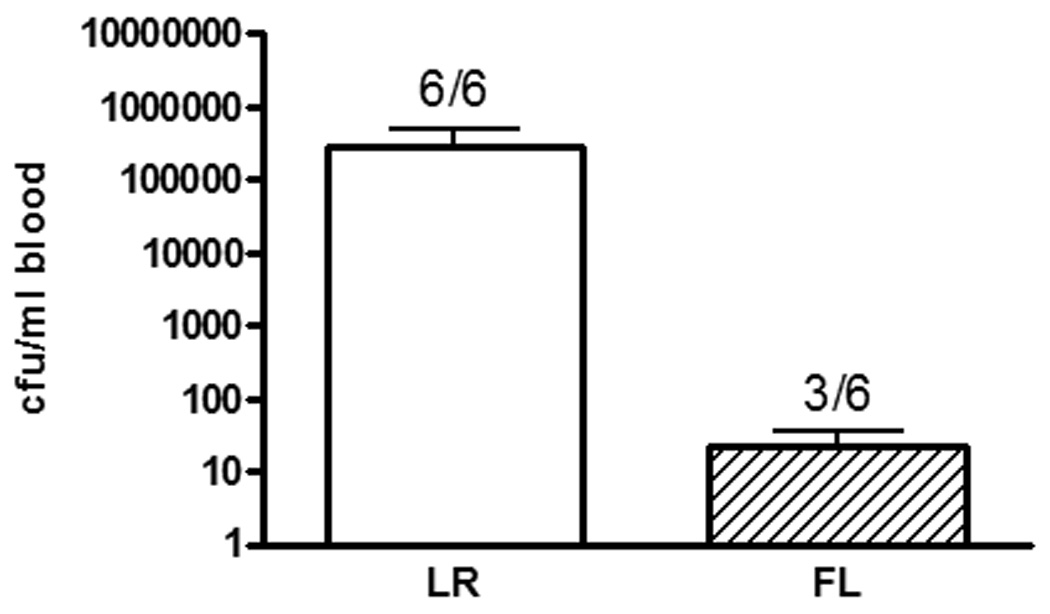
Mice were treated as in figure 2, and sera were harvested 72 hours after inoculation for overnight culture. Ratios on the graph represent the proportion of mice with positive blood cultures. n = 6 mice/group, representative of two experiments, p>0.05.
Figure 3. Effects of FL on antibody responses following noninfectious challenge.
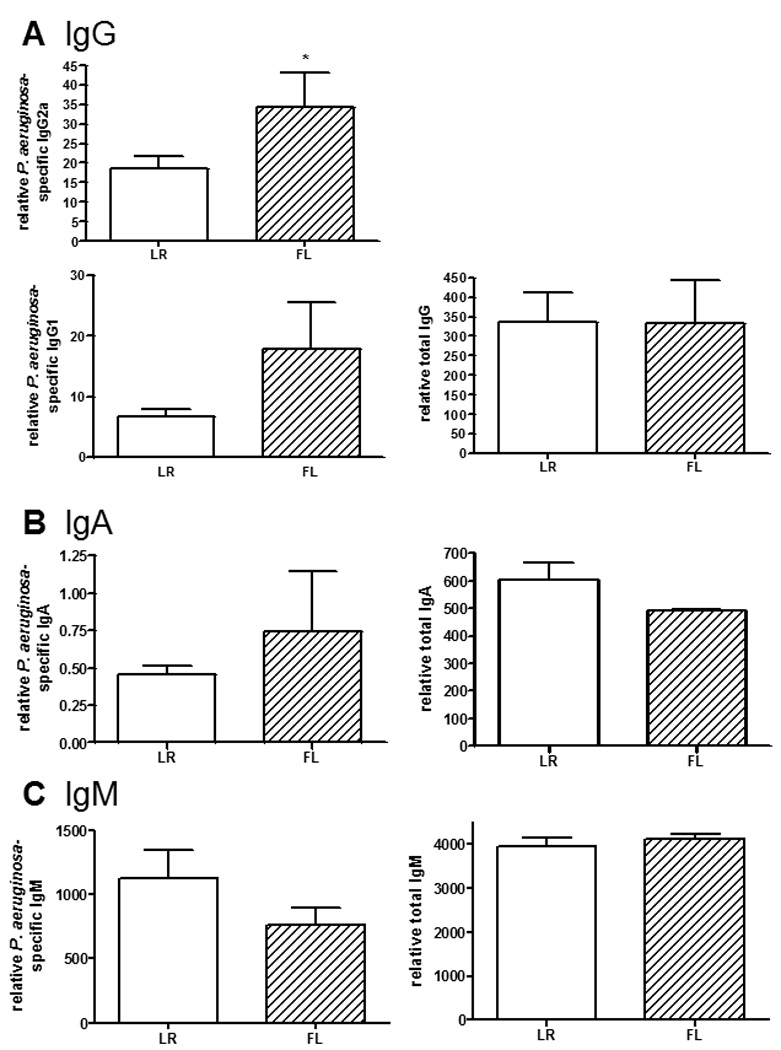
Previously immunized mice were rechallenged by intraperitoneal injection of HKPA 4 days after burn injury. Mice received control treatment (LR) or FL for 5 days, beginning on the day of injury. Sera were harvested 4 days after infection for assessment of P. aeruginosa-specific or total (A) IgG, (B) IgA, and (C) IgM antibodies by ELISA. Relative values are shown, expressed as O.D. × sample dilution factor. n=5 mice/group, representative of 2 experiments. *significantly different than LR group, p<0.05.
Cytokine responses to secondary P. aeruginosa exposure
Soluble cytokines can regulate antibody production and isotype switching, so in vitro cytokine production by spleen leukocytes isolated from mice following secondary exposure to P. aeruginosa was assessed. Following a burn wound infection, production of IL-12 p70 was significantly enhanced in spleens from FL-treated mice compared to control-treated mice (figure 4). Additionally, IL-10 and TGF-β levels were also significantly enhanced by FL treatments. Surprisingly, IFN-γ production was not enhanced by FL treatments when mice had been subjected to a burn wound infection. However, when mice had been challenged by intraperitoneal injection of HKPA, production of IFN-γ, IL-12 p70, IL-10 and TGF-β by splenocytes from FL-treated mice was significantly higher compared to the control treatment groups (figure 4). The data in figure 4 are from overnight cultures restimulated in vitro with HKPA. The same cultures were performed without additional in vitro stimulation, and the same differences between groups were detected, but with a slight decrease in magnitude. IFN-γ, however, could not be detected unless additional stimulation in vitro was provided. IL-4 was measured in all groups and could not be detected.
Figure 4. Effects of FL on splenic cytokine production following secondary exposure to P. aeruginosa.
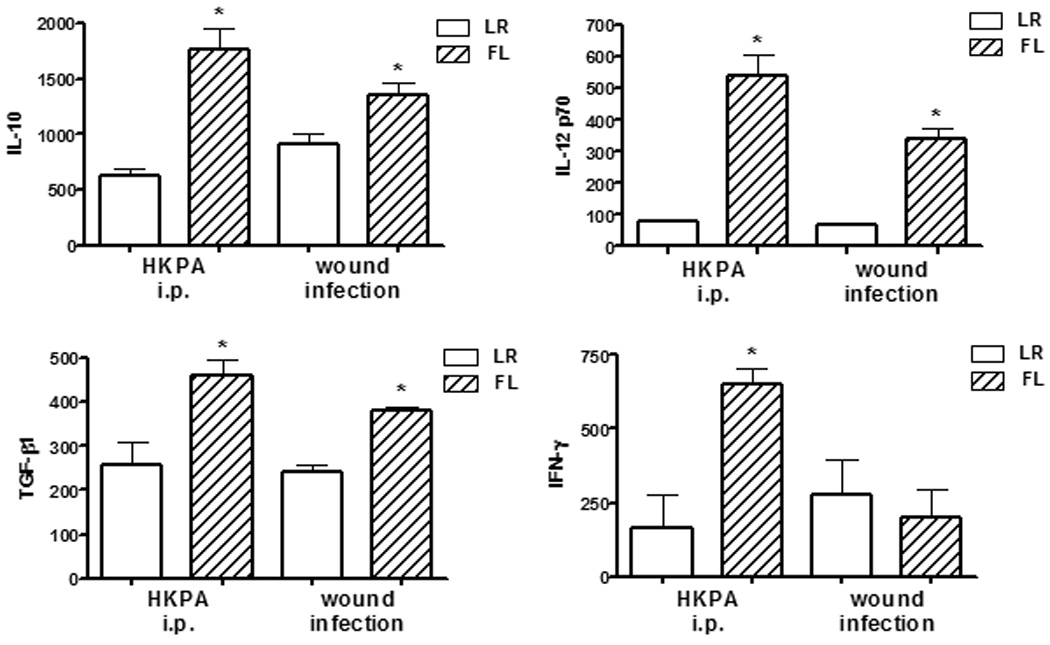
Mice were treated as in figures 2 (wound infection) and 3 (HKPA i.p.), and spleen leukocytes were cultured overnight with additional HKPA stimulation. Secreted cytokines were measured by ELISA. n=5 mice/group, representative of two experiments. *significantly different than corresponding control (LR) group, p<0.05.
Effect of FL on bacterial clearance and opsonization
Three days following secondary exposure to P. aeruginosa by burn wound inoculation, the mice treated with FL had lower levels of bacteria in blood. Specifically, only 50% of the FL-treated mice had positive blood cultures, whereas 100% of the control-treated mice had positive cultures. Additionally, although not significantly different (p=0.18) the mean cfu/ml was negligible in the FL-treated mice with positive cultures, specifically, 104-fold lower than in the control-treated mice (figure 5). To compare the ability of sera from FL-treated mice to opsonize bacteria, facilitation of neutrophil-mediated killing of P. aeruginosa in vitro was examined. Since systemic dissemination of bacteria following wound infection was different between treatment groups, sera from mice that had received secondary exposure by intraperitoneal injection of HKPA were used since equivalent systemic doses of primary and secondary exposures were certain. Bacterial killing was significantly greater in cultures containing sera from FL-treated mice, compared to sera from control-treated mice (figure 6). The difference was not due to differences in complement or bacterial contamination of serum at the time of harvest, since samples were heated for inactivation of endogenous complement and killing of potential contamination prior to the opsonization assay.
Figure 6. Facilitation of neutrophil-mediated bacterial killing by sera from immunized mice.
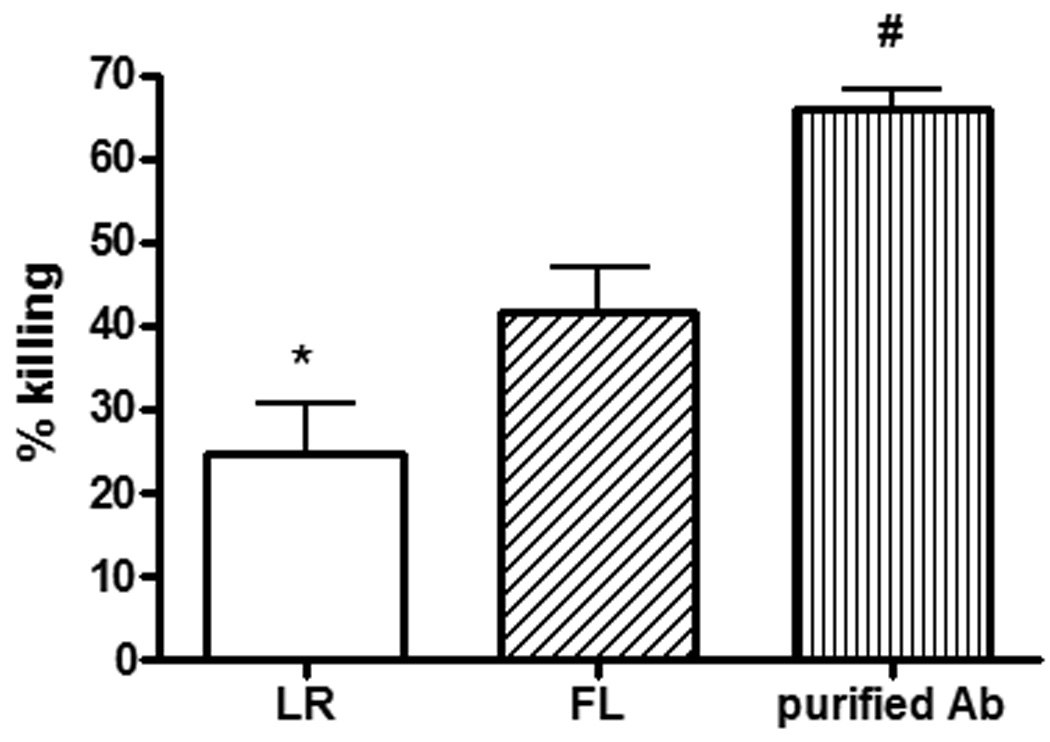
Mice were treated as in figure 3, and heat-inactivated sera were incubated in vitro with P. aeruginosa, external complement, and neutrophils for two hours, then plated on tryptic soy agar for determination of cfu. n = 5 mice/group, with each serum sample assayed in duplicate; purified antibody control performed in triplicate. #,*significantly different than other groups, p<0.05.
Discussion
The ability of FL to enhance humoral responses in normal mice has been previously reported. Expansion of dendritic cells by FL promotes expansion of antigen-specific T cells and an increase in antigen-specific antibody production, particularly of the IgG2a isotype [30;31]. This enhancement is due to the ability of FL to expand DC populations that promote the induction of IL-12, IFN-γ, T cell proliferation and Th1 differentiation [32;33].
Similarly, FL can expand DC populations and promote induction of Th1-associated cytokines, such as IL-12 and IFN-γ, after a severe burn injury. We previously reported that induction of these cytokines after a primary challenge with HKPA is no longer suppressed after burn injury if mice have been treated with FL [34]. Likewise, the current study indicates that induction of IL-12 and IFN-γ is significantly enhanced in burn-injured mice treated with FL when the same challenge is given as a secondary exposure. Interestingly, induction of the Th2 cytokine, IL-10, and the Th3 cytokine, TGF-β1, was also enhanced. Although the induction of all of these cytokines was enhanced by FL treatments, the relative differences between the two treatment groups were greater for IL-12 (6.9-fold difference) and IFN-γ (3.9-fold), compared to IL-10 (2.8-fold) and TGF-β (1.79-fold). Since cytokine release was assessed in cultures containing equivalent numbers of splenic leukocytes, enhanced cytokine production is likely due to the relatively greater proportion of DCs in the cultures from FL-treated mice. As previously reported, the proportion of DCs that comprise the splenic leukocyte population in the FL-treated, burn-injured mice is approximately three times greater than in the control-treated mice {Toliver-Kinsky, 2003 326 /id}. Since DCs are abundant sources of cytokines, especially IL-12 and IFN-γ, the higher proportion of DCs in the FL cultures likely contributes to increased cytokine levels in vitro, similarly as in vivo where DC numbers are enhanced in multiple tissues. Consistent with a Th-1-enhanced response to HKPA, P. aeruginosa-specific IgG2a was significantly increased with FL treatment, whereas significant differences between treatment groups were not detected for any of the other antibodies measured. This is consistent with studies in normal animals, in which only antigen-specific, but not natural, antibodies are increased by FL [35].
Examination of responses to a noninfectious challenge was used to control for the dose of the secondary challenge. This was necessary because there is less bacterial growth and systemic dissemination following inoculation of burn wounds with live P. aeruginosa in mice treated with FL, and this may impact the degree and/or nature of the cytokine and antibody responses. However, a P. aeruginosa intraperitoneal infection is of minimal clinical significance, and our primary interest is to determine the clinical applicability of FL as a potential immune adjuvant to enhance resistance to infections after severe burn injury. A well-established model of P. aeruginosa burn wound infection was therefore employed for mice that had been previously immunized with live organisms. There were no treatment-associated differences in antigen-specific or total IgG or IgA levels, but both P. aeruginosa-specific and total IgM levels were significantly lower in mice that had been treated with FL after burn injury, compared to mice that received control treatment after injury.
The reason for decreased levels of serum IgM in response to a burn wound infection in FL-treated mice is not known. However, the relatively higher ratios of antigen-specific non-IgM antibodies to IgM in FL-treated mice suggest that class switching to IgG and IgA isotypes in response to burn wound infection may be enhanced. Ratios of P. aeruginosa-specific non-IgM antibodies, especially IgG2a, to IgM were also increased in FL-treated mice following a noninfectious challenge with HKPA, again suggesting that FL treatments somehow promote IgG and IgA class switching during a recall response to P. aeruginosa.
Dendritic cells alone, in the absence of exogenous cytokines, can induce IgA switching. In the presence of exogenous cytokines, such as IL-10 and TGF-β, DC-induced switching is further enhanced [36]. Following CD40 ligation, dendritic cells significantly increase IgG and IgA secretion by memory B cells [37]. Therefore, enhanced dendritic cell numbers in FL-treated mice could promote class switching from IgM during the secondary response to infection.
Cytokines are important regulators of antibody isotype class switching. IFN-γ induces switching from IgM to IgG2a, IL-4 induces switching to IgG1 and IgE, and TGF-β and IL-10 induce switching to IgA and IgG2b. However, the response to infection is complex and antibody-secreting cells can be exposed to mixtures of these cytokines. Others have examined the effects of cytokine mixtures on isotype switching and found that IFN-γ has a greater influence on this process than IL-4, and both of these cytokines have a greater influence than TGF-β [38]. Following challenge with HKPA, splenic production of all cytokines examined was significantly enhanced by FL treatments, but relative differences between treatment groups were greater for IL-12 p70 and IFN-γ, cytokines associated with Th-1 responses. This cytokine response correlated with a significant increase in antigen-specific IgG2a, indicating that FL treatments can enhance Th1 responses and antigen-specific IgG2a production after severe burn injury, similarly as in normal mice. Functionally, enhanced IgG2a levels should increase the ability of the FL-treated host to opsonize and facilitate elimination of bacteria. Indeed, sera from FL-treated mice were more efficient at facilitation of neutrophil-mediated killing of P. aeruginosa in vitro.
The finding that FL treatments did not affect IFN-γ production following secondary exposure to P. aeruginosa through the burn wound, despite enhanced production of IL-10, TGF-β, and the IFN-γ-inducing factor IL-12p70, was surprising and could be responsible for the lack of significantly enhanced IgG2a production in these same mice. Specifically, there appeared to be a correlation between IFN-γ induction and enhancement of IgG2a production. Following wound infection, neither IFN-γ nor IgG2a was increased in FL-treated mice. On the contrary, IFN-γ was significantly increased in FL-treated mice following intraperitoneal challenge with HKPA, as was IgG2a. Yet, the FL-treated mice are capable of clearing bacteria following wound inoculation. These findings are consistent with other models of infection. During retroviral infection, IFN-γ-deficient, but not IL-4- or IL-12-deficient, mice are defective at controlling a primary infection. However, if mice are previously immunized prior to infection, as in this study, IFN-γ-deficient mice can control the infection, despite an inability to class-switch to IgG [39]. Therefore, while IFN-γ is important during a primary response to infection, it appears to be dispensable during the secondary, or recall, response.
The reasons for the differing recall responses of FL-treated mice, when comparing a live wound infection with a noninfectious peritoneal challenge, are not known. A major difference between these two models is that the wound infection permits a gradual, local growth of bacteria that culminates in systemic dissemination over a period of several days, whereas the intraperitoneal injection of HKPA provides a rapid, high-dose exposure to bacterial components that may induce a response that is more representative of an acute systemic inflammatory condition than a typical host response to infection. Additionally, during the wound infection, live P. aeruginosa produce virulence factors that can modify the host response to infection, whereas these factors are not expressed after heat killing of the organisms. While both of these factors may contribute to the different responses seen with the wound infection versus intraperitoneal challenge models, the contribution of these, and other factors, to the decreased IgM and lack of IFN-γ response after wound infection in FL-treated mice remains to be determined.
Nonetheless, mice treated with FL have a significantly increased resistance to burn wound infection, indicated by decreased systemic dissemination of bacteria from the burn wound (figure 5) and increased survival [40]. Although antigen-specific responses were modulated by FL in this study, it appears unlikely that antibody-mediated clearance of bacteria plays a large role in the protective effects of FL in our wound infection model. Mice treated with FL are resistant to burn wound infection, even when they have not been previously immunized as in this study. Enhanced bacterial clearance in FL-treated mice becomes apparent within three days of burn wound inoculation. Within this time frame, mice produce negligible amounts of P. aeruginosa-specific antibodies in the absence of a prior immunization. Therefore, FL-induced resistance to infection in our burn wound infection model is likely due to responses of rapidly activated innate immune functions, currently under investigation in our laboratory.
Whereas immunization with P. aeruginosa before injury was used in this study to control experimental conditions, vaccination of burn patients with P. aeruginosa proteins or polysaccharides after injury is being explored by others. Induction of P. aeruginosa-specific antibodies following vaccination of burn patients has been demonstrated [41] and may have potential for reducing the frequency and/or severity of P. aeruginosa infections after burn injury [42;43]. In this context, FL may be useful as an adjuvant for vaccination or other immunomodulatory interventions in burn patients.
Unfortunately, mortalities in non-treated mice following infection of burn wounds {Bohannon, 2008 412 /id} preclude examination of an antigen-specific antibody response following a primary exposure through the wound. Consequently, this study is restricted to analysis of antibody responses to a previously recognized microorganism. However, others have demonstrated enhancement of primary antibody responses in non-injured mice by FL {Pabst, 2003 78 /id}, so it is likely that primary humoral responses would similarly be enhanced by FL after burn injury.
In conclusion, this study suggests that FL may have potential for enhancing acquired immune responses to burn-associated infections in the clinical scenario. Earlier studies established that severe burns can impact humoral immune responses by polarizing antigen-specific CD4+ T cells away from a Th1, and towards a Th2, phenotype [44], causing an impairment in antigen-specific IgG and IgG2a production [45] that can be prevented by exogenous Interleukin-12 [46]. The ability of FL to enhance IL-12 in both the wound infection and intraperitoneal HKPA models suggests that FL may be able to prevent some of the burn-associated alterations in antigen-specific responses. Additionally, the relative increase in antigen-specific IgG2a in response to wound infection and the absolute increase in antigen-specific IgG2a production after intraperitoneal challenge in burned mice treated with FL suggest that FL may be able to prevent defects in humoral immunity in response to infections originating from various tissue sources. These data, combined with earlier studies demonstrating restoration of innate cytokine responses following a primary challenge with HKPA, enhanced bacterial clearance following both intraperitoneal and wound inoculations with live P. aeruginosa, and increased survival in response to an otherwise lethal wound infection [47–49], suggest that prophylactic treatments with FL after severe burn injury may be able to decrease susceptibility to various types of infections that adversely affect patients recovering from severe burn injuries.
Acknowledgments
This project was supported by research grants R01 GM072810 from the National Institutes of Health and 8810 from the Shriners Hospitals for Children.
Abbreviations
- FL
fms-like tyrosine kinase-3 ligand
- HKPA
heat-killed Pseudomonas aeruginosa
- LR
Lactated Ringers solution
References
- 1.Holzheimer RG, Molloy R, Mendez MV, O'Riordain D, Curley P, Nestor M, Collins K, Saproschetz I, Mannick JA, Rodrick ML. Multiple system organ failure may be influenced by macrophage hypoactivation as well as hyperactivation--importance of the double challenge. Eur.J.Surg. 1995;161:795–803. [PubMed] [Google Scholar]
- 2.Loose LD, Megirian R, Turinsky J. Biochemical and functional alterations in macrophages after thermal injury. Infect.Immun. 1984;44:554–558. doi: 10.1128/iai.44.3.554-558.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogle CK, Johnson C, Guo XL, Ogle JD, Solomkin JS, Alexander JW. Production and release of C3 by cultured monocytes/macrophages isolated from burned, trauma, and septic patients. J.Trauma. 1989;29:189–194. doi: 10.1097/00005373-198902000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Rodeberg DA, Meyer JG, Babcock GF. Heat shock response: presence and effects in burn patient neutrophils. J.Leukoc.Biol. 1999;66:773–780. doi: 10.1002/jlb.66.5.773. [DOI] [PubMed] [Google Scholar]
- 5.Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 2007;15:87–92. doi: 10.1016/j.tim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Bliss SK, Zhang Y, Denkers EY. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-gamma-independent IL-12. J.Immunol. 1999;163:2081–2088. [PubMed] [Google Scholar]
- 7.Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J.Immunol. 1999;162:7369–7375. [PubMed] [Google Scholar]
- 8.Egan CE, Sukhumavasi W, Bierly AL, Denkers EY. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunol.Res. 2008;40:35–48. doi: 10.1007/s12026-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. Journal of Experimental Medicine. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hultman CS, Cairns BA, Yamamoto H, DeSerres S, Frelinger JA, Meyer AA. The 1995 Moyer Award. The effect of burn injury on allograft rejection, alloantigen processing, and cytotoxic T-lymphocyte sensitization. J.Burn Care Rehabil. 1995;16:573–580. [PubMed] [Google Scholar]
- 11.Hunt JP, Hunter CT, Brownstein MR, Giannopoulos A, Hultman CS, DeSerres S, Bracey L, Frelinger J, Meyer AA. The effector component of the cytotoxic T-lymphocyte response has a biphasic pattern after burn injury. J.Surg.Res. 1998;80:243–251. doi: 10.1006/jsre.1998.5488. [DOI] [PubMed] [Google Scholar]
- 12.Schwacha MG, Ayala A, Chaudry IH. Insights into the role of gammadelta T lymphocytes in the immunopathogenic response to thermal injury. J.Leukoc.Biol. 2000;67:644–650. [PubMed] [Google Scholar]
- 13.Teodorczyk-Injeyan JA, Sparkes BG, Mills GB, Peters WJ, Falk RE. Impairment of T cell activation in burn patients: a possible mechanism of thermal injury-induced immunosuppression. Clin.Exp.Immunol. 1986;65:570–581. [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly JL, Lyons A, Soberg CC, Mannick JA, Lederer JA. Anti-interleukin-10 antibody restores burn-induced defects in T-cell function. Surgery. 1997;122:146–152. doi: 10.1016/s0039-6060(97)90003-9. [DOI] [PubMed] [Google Scholar]
- 15.Kelly JL, O'Suilleabhain CB, Soberg CC, Mannick JA, Lederer JA. Severe injury triggers antigen-specific T-helper cell dysfunction. Shock. 1999;12:39–45. doi: 10.1097/00024382-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Molloy RG, Nestor M, Collins KH, Holzheimer RG, Mannick JA, Rodrick ML. The humoral immune response after thermal injury: an experimental model. Surgery. 1994;115:341–348. [PubMed] [Google Scholar]
- 18.Pabst R, Luhrmann A, Steinmetz I, Tschernig T. A single intratracheal dose of the growth factor Fms-like tyrosine kinase receptor-3 ligand induces a rapid differential increase of dendritic cells and lymphocyte subsets in lung tissue and bronchoalveolar lavage, resulting in an increased local antibody production. J.Immunol. 2003;171:325–330. doi: 10.4049/jimmunol.171.1.325. [DOI] [PubMed] [Google Scholar]
- 19.Pulendran B, Smith JL, Jenkins M, Schoenborn M, Maraskovsky E, Maliszewski CR. Prevention of peripheral tolerance by a dendritic cell growth factor: flt3 ligand as an adjuvant. J.Exp.Med. 1998;188:2075–2082. doi: 10.1084/jem.188.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal DK, Hopfenspirger MT, Chavez J, Talmadge JE. Flt3 ligand: a novel cytokine prevents allergic asthma in a mouse model. Int.Immunopharmacol. 2001;1:2081–2089. doi: 10.1016/s1567-5769(01)00122-9. [DOI] [PubMed] [Google Scholar]
- 21.Mosley RL, Parajuli P, Pisarev V, Chavez J, Meeks A, Steffel A, Leutzinger C, Talmadge JE. Flt3 ligand augmentation of T cell mitogenesis and expansion of type 1 effector/memory T cells. Int.Immunopharmacol. 2002;2:925–940. doi: 10.1016/s1567-5769(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 22.Parajuli P, Mosley RL, Pisarev V, Chavez J, Ulrich A, Varney M, Singh RK, Talmadge JE. Flt3 ligand and granulocyte-macrophage colony-stimulating factor preferentially expand and stimulate different dendritic and T-cell subsets. Exp.Hematol. 2001;29:1185–1193. doi: 10.1016/s0301-472x(01)00722-6. [DOI] [PubMed] [Google Scholar]
- 23.Pollack M, Callahan LT, III, Taylor NS. Neutralizing antibody to Pseudomonas aeruginosa exotoxin in human sera: evidence for in vivo toxin production during infection. Infect.Immun. 1976;14:942–947. doi: 10.1128/iai.14.4.942-947.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cryz SJ, Jr, Furer E, Germanier R. Protection against fatal Pseudomonas aeruginosa burn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect.Immun. 1984;43:795–799. doi: 10.1128/iai.43.3.795-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J.Immunol. 2008;180:3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 26.Toliver-Kinsky TE, Lin CY, Herndon DN, Sherwood ER. Stimulation of hematopoiesis by the Fms-like tyrosine kinase 3 ligand restores bacterial induction of Th1 cytokines in thermally injured mice. Infection & Immunity. 2003;71:3058–3067. doi: 10.1128/IAI.71.6.3058-3067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J.Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 28.Kelly JL, O'Suilleabhain CB, Soberg CC, Mannick JA, Lederer JA. Severe injury triggers antigen-specific T-helper cell dysfunction. Shock. 1999;12:39–45. doi: 10.1097/00024382-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Molloy RG, Nestor M, Collins KH, Holzheimer RG, Mannick JA, Rodrick ML. The humoral immune response after thermal injury: an experimental model. Surgery. 1994;115:341–348. [PubMed] [Google Scholar]
- 30.Pabst R, Luhrmann A, Steinmetz I, Tschernig T. A single intratracheal dose of the growth factor Fms-like tyrosine kinase receptor-3 ligand induces a rapid differential increase of dendritic cells and lymphocyte subsets in lung tissue and bronchoalveolar lavage, resulting in an increased local antibody production. J.Immunol. 2003;171:325–330. doi: 10.4049/jimmunol.171.1.325. [DOI] [PubMed] [Google Scholar]
- 31.Pulendran B, Smith JL, Jenkins M, Schoenborn M, Maraskovsky E, Maliszewski CR. Prevention of peripheral tolerance by a dendritic cell growth factor: flt3 ligand as an adjuvant. J.Exp.Med. 1998;188:2075–2082. doi: 10.1084/jem.188.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosley RL, Parajuli P, Pisarev V, Chavez J, Meeks A, Steffel A, Leutzinger C, Talmadge JE. Flt3 ligand augmentation of T cell mitogenesis and expansion of type 1 effector/memory T cells. Int.Immunopharmacol. 2002;2:925–940. doi: 10.1016/s1567-5769(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 33.Parajuli P, Mosley RL, Pisarev V, Chavez J, Ulrich A, Varney M, Singh RK, Talmadge JE. Flt3 ligand and granulocyte-macrophage colony-stimulating factor preferentially expand and stimulate different dendritic and T-cell subsets. Exp.Hematol. 2001;29:1185–1193. doi: 10.1016/s0301-472x(01)00722-6. [DOI] [PubMed] [Google Scholar]
- 34.Toliver-Kinsky TE, Lin CY, Herndon DN, Sherwood ER. Stimulation of hematopoiesis by the Fms-like tyrosine kinase 3 ligand restores bacterial induction of Th1 cytokines in thermally injured mice. Infection & Immunity. 2003;71:3058–3067. doi: 10.1128/IAI.71.6.3058-3067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulendran B, Smith JL, Jenkins M, Schoenborn M, Maraskovsky E, Maliszewski CR. Prevention of peripheral tolerance by a dendritic cell growth factor: flt3 ligand as an adjuvant. J.Exp.Med. 1998;188:2075–2082. doi: 10.1084/jem.188.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fayette J, Dubois B, Vandenabeele S, Bridon JM, Vanbervliet B, Durand I, Banchereau J, Caux C, Briere F. Human dendritic cells skew isotype switching of CD40-activated naive B cells towards IgA1 and IgA2. J.Exp.Med. 1997;185:1909–1918. doi: 10.1084/jem.185.11.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van KC, Briere F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J.Exp.Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deenick EK, Hasbold J, Hodgkin PD. Decision criteria for resolving isotype switching conflicts by B cells. Eur.J.Immunol. 2005;35:2949–2955. doi: 10.1002/eji.200425719. [DOI] [PubMed] [Google Scholar]
- 39.Dittmer U, Peterson KE, Messer R, Stromnes IM, Race B, Hasenkrug KJ. Role of interleukin-4 (IL-4), IL-12, and gamma interferon in primary and vaccine-primed immune responses to Friend retrovirus infection. J.Virol. 2001;75:654–660. doi: 10.1128/JVI.75.2.654-660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J.Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 41.Baumann U, Mansouri E, von Specht BU. Recombinant OprF-OprI as a vaccine against Pseudomonas aeruginosa infections. Vaccine. 2004;22:840–847. doi: 10.1016/j.vaccine.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 42.Cryz SJ, Jr, Furer E, Germanier R. Protection against fatal Pseudomonas aeruginosa burn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect.Immun. 1984;43:795–799. doi: 10.1128/iai.43.3.795-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holder IA, Neely AN, Frank DW. PcrV immunization enhances survival of burned Pseudomonas aeruginosa-infected mice. Infect.Immun. 2001;69:5908–5910. doi: 10.1128/IAI.69.9.5908-5910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Z, Kavanagh E, Zang Y, Dolan SM, Kriynovich SJ, Mannick JA, Lederer JA. Burn injury promotes antigen-driven Th2-type responses in vivo. J.Immunol. 2003;171:3983–3990. doi: 10.4049/jimmunol.171.8.3983. [DOI] [PubMed] [Google Scholar]
- 45.Molloy RG, Nestor M, Collins KH, Holzheimer RG, Mannick JA, Rodrick ML. The humoral immune response after thermal injury: an experimental model. Surgery. 1994;115:341–348. [PubMed] [Google Scholar]
- 46.Kelly JL, Lyons A, Soberg CC, Mannick JA, Lederer JA. Anti-interleukin-10 antibody restores burn-induced defects in T-cell function. Surgery. 1997;122:146–152. doi: 10.1016/s0039-6060(97)90003-9. [DOI] [PubMed] [Google Scholar]
- 47.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J.Immunol. 2008;180:3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 48.Toliver-Kinsky TE, Lin CY, Herndon DN, Sherwood ER. Stimulation of hematopoiesis by the Fms-like tyrosine kinase 3 ligand restores bacterial induction of Th1 cytokines in thermally injured mice. Infection & Immunity. 2003;71:3058–3067. doi: 10.1128/IAI.71.6.3058-3067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J.Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 50.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J.Immunol. 2008;180:3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 51.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J.Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 52.Armour AD, Shankowsky HA, Swanson T, Lee J, Tredget EE. The impact of nosocomially-acquired resistant Pseudomonas aeruginosa infection in a burn unit. J.Trauma. 2007;63:164–171. doi: 10.1097/01.ta.0000240175.18189.af. [DOI] [PubMed] [Google Scholar]
- 53.Dalley AJ, Lipman J, Venkatesh B, Rudd M, Roberts MS, Cross SE. Inadequate antimicrobial prophylaxis during surgery: a study of {beta}-lactam levels during burn debridement. J.Antimicrob.Chemother. 2007;60:166–169. doi: 10.1093/jac/dkm128. [DOI] [PubMed] [Google Scholar]
- 54.Rodgers GL, Mortensen J, Fisher MC, Lo A, Cresswell A, Long SS. Predictors of infectious complications after burn injuries in children. Pediatric Infectious Disease Journal. 2000;19:990–995. doi: 10.1097/00006454-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Still J, Newton T, Friedman B, Furhman S, Law E, Dawson J. Experience with pneumonia in acutely burned patients requiring ventilator support. American Surgeon. 2000;66:206–209. [PubMed] [Google Scholar]


