Abstract
Fragile X syndrome (FXS) is the most common inherited form of mental retardation and a leading known cause of autism. It is caused by loss of expression of the fragile X mental retardation protein (FMRP), an RNA-binding protein that negatively regulates protein synthesis. In neurons, multiple lines of evidence suggest that protein synthesis at synapses is triggered by activation of group 1 metabotropic glutamate receptors (Gp1 mGluRs) and that many functional consequences of activating these receptors are altered in the absence of FMRP. These observations have led to the theory that exaggerated protein synthesis downstream of Gp1 mGluRs is a core pathogenic mechanism in FXS. This excess can be corrected by reducing signaling by Gp1 mGluRs, and numerous studies have shown that inhibition of mGluR5, in particular, can ameliorate multiple mutant phenotypes in animal models of FXS. Clinical trials based on this therapeutic strategy are currently under way. FXS is therefore poised to be the first neurobehavioral disorder in which corrective treatments have been developed from the bottom up: from gene identification to pathophysiology in animals to novel therapeutics in humans. The insights gained from FXS and other autism-related single-gene disorders may also assist in identifying molecular mechanisms and potential treatment approaches for idiopathic autism.
Keywords: FMRP, metabotropic glutamate receptor, autism, mental retardation, protein synthesis, long-term depression
INTRODUCTION
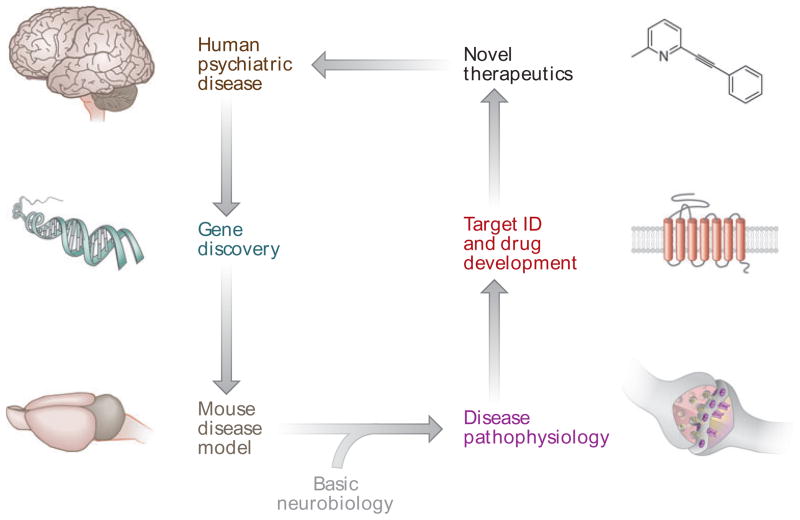
Combining insights obtained from basic biological research with genetic and clinical findings can paint a detailed picture of the molecular events that result in disease, and suggest ways these mechanisms can be targeted with corrective interventions. Not surprisingly, application of such “molecular medicine” approaches to psychiatric and neurodevelopmental disorders has been slow and difficult owing to the daunting biological complexity of the brain and the complex genetics that underlie most of these diseases (1, 2). However, in recent years there have been significant advances in understanding the pathophysiology of several single-gene disorders of brain development associated with intellectual impairment and autism (see side-bar “The Promise of Molecular Medicine in Brain Disorders” and Figure 1). The encouraging prospects for developing new corrective treatments based on these insights have generated considerable excitement, particularly in light of reports that the prevalence of autism spectrum disorders (ASDs) is rising sharply (3). In this review, we describe recent progress in the study of one of these disorders, fragile X syndrome (FXS), and discuss the implications of these data for autism and other related neurodevelopmental disorders.
Figure 1.
The promise of molecular medicine in psychiatric and neurodevelopmental disorders (see sidebar “The Promise of Molecular Medicine in Brain Disorders” for explanation).
THE PROMISE OF MOLECULAR MEDICINE IN BRAIN DISORDERS.
Understanding the genetic basis of psychiatric and neurological disorders eventually will lead to insights into how the brain functions differently in these diseases. Understanding their pathophysiology, in turn, will suggest molecular targets for therapeutic interventions. This process begins with careful phenotypic stratification of patients followed by gene-variant discovery efforts. Psychiatry, in particular, faces major obstacles in applying this strategy because (a) many behavioral disorders fall on a spectrum, making diagnosis and stratification difficult; (b) there is often a large contribution of environmental influences to disease progression and outcome; and (c) most disorders do not have a single major genetic cause but are the result of a plethora of individual mutations and gene copy number variations (2). Single-gene disorders such as FXS are therefore particularly valuable as models for more genetically complex disorders such as autism. Once a disease-associated gene has been identified, animal models of the disorder can be generated through genetic manipulation to reproduce the underlying genetic deficits. These animal models enable further study of the cellular, physiological, and behavioral consequences of aberrant gene expression. It is here that basic neurobiology research becomes an essential partner in the drug discovery process, allowing the observations made in animal models to be interpreted in the context of a vast background of knowledge on brain structure and function. Based on the convergence of information from these sources, hypotheses can be formulated and tested to generate a plausible model of disease pathophysiology. This model, in turn, can be used to identify potential drug targets, which then provide the basis for the development of novel therapeutic strategies that can finally be validated in clinical trials. Although the causes vary, many autism spectrum disorders may share pathophysiological mechanisms with FXS and therefore respond to the same treatments.
MIXING THE STREAMS OF GENETICS AND NEUROBIOLOGY IN FRAGILE X SYNDROME
Fragile X Syndrome
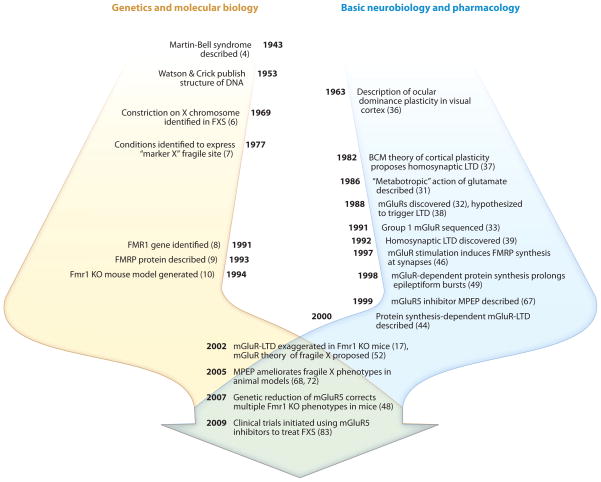
FXS, originally known as Martin-Bell syndrome (4), was first described in 1943 as an X-linked form of inherited mental retardation (Figure 2) (5). The first evidence regarding the molecular origin of FXS was obtained in 1969, when an unusual constriction, or fragile site, was observed at the end of the X chromosome in several affected individuals (6, 7). It was not until 1991, however, that the fragile site was mapped to a specific location in the genome (8). This fragile site coincided with an expanded CGG trinucleotide repeat in the 5′ untranslated region of a novel gene, subsequently named fragile X mental retardation 1 (FMR1). Trinucleotide repeat expansions consisting of >200 CGG repeats were found to cause hypermethylation of the surrounding sequence, resulting in transcriptional silencing of FMR1 (for an extensive review of this subject, see Reference 5). In 1993, the protein encoded by FMR1, fragile X mental retardation protein (FMRP), was characterized and found to be an RNA binding protein that is expressed predominantly in the brain and testes (9). Shortly thereafter, an Fmr1 knockout mouse model (Fmr1 KO) was generated, in which part of the Fmr1 gene was deleted to mimic the loss of FMRP expression seen in FXS (10). Since then, these Fmr1 KO mice and other animal models have been extensively characterized, and in conjunction with further in vitro studies of FMRP function, they have been instrumental in providing insights into potential roles for FMRP in the brain as well as the pathophysiology of FXS.
Figure 2.
Some milestones in defining the pathophysiology of fragile X syndrome (FXS). The current therapeutic efforts in FXS originate from the mixing of two independent lines of research: genetic research on FXS (left timeline, yellow) and basic neurobiology research on mGluR-dependent synaptic plasticity (right timeline, blue). The discovery that mGluR-LTD is exaggerated in Fmr1 knockout (KO) mice (17) led to the mGluR theory of FXS pathophysiology, culminating in the initiation of clinical trials to test the efficacy of mGluR5 antagonists in the treatment of FXS. Numbers in parentheses are reference citations. Fmr1, fragile X mental retardation 1; FMRP, fragile X mental retardation protein; mGluR, metabotropic glutamate receptor; LTD, long-term depression; MPEP, 2-methyl-6-(phenylethynyl)-pyridine.
FMRP Function and Loss of Function in FXS
One important clue regarding the function of FMRP came from the observation that it is associated with polyribosomes, implying a role in the regulation of protein synthesis (11–13). Early biochemical studies yielded contradictory conclusions regarding the precise nature of this role (see 14 for a review of the early literature); however, there is now broad support for the hypothesis that FMRP functions as a translational repressor of target mRNAs (15–17), as we discuss further below. In addition to its role in regulating protein synthesis, FMRP has also been implicated in the transport and localization of mRNAs to dendrites and synapses. The mechanisms by which FMRP may regulate protein synthesis and transport remain under active investigation, and a detailed discussion of the current state of this field can be found elsewhere (18, 19).
The observation that FMRP may be linked to the protein synthesis machinery, together with the fact that both FMRP and mRNA are expressed in the dendrites and dendritic spines of neurons, suggested that it may play an important role in local protein synthesis at synapses (18, 19). These findings were of particular interest in light of the increasing evidence linking local protein synthesis to synapse maturation and synaptic plasticity (20), and it was hypothesized that loss of FMRP expression might result in disruptions of synaptic structure and function. Consistent with this notion, one of the most prominent morphological phenotypes observed in both FXS patients and Fmr1 KO mice is an increase in dendritic spine density and the presence of abnormally long and tortuous spines (21–23). Cultured neurons from Fmr1 KO mice mimic this phenotype, displaying an increased number of structural synapses (24). In addition, loss of FMRP in animal models has also been shown to affect synaptic plasticity: Fmr1 KO mice show exaggerated forms of long-term depression (LTD) in hippocampus (17) and cerebellum (25), discussed in further detail below. Moreover, several groups have reported alterations in long-term potentiation (LTP) in the cortex and hippocampus of Fmr1 KO mice (26–30). Together, these findings suggested that the absence of FMRP may alter synaptic plasticity throughout the brain, which may be important in the pathogenesis of FXS.
Metabotropic Glutamate Receptors and Plasticity
It was shortly after the discovery that FMRP plays a role in protein synthesis that the FXS field crossed paths with emerging lines of research on metabotropic glutamate receptors and activity-dependent synaptic plasticity, leading to the discoveries that gave rise to current therapeutic efforts in FXS (Figure 2).
Metabotropic glutamate receptors (mGluRs) are G protein–coupled receptors that link to intracellular signaling pathways, including the Gq/PLC pathway [group 1 (Gp1) mGluRs] and Gi/Go pathways (groups 2 and 3 mGluRs). Their existence was predicted in 1986, when it was observed that agonists of glutamate receptors, thought to be ion channels exclusively at the time, could also stimulate phosphatidylinositide (PI) turnover (31). In 1988, the first mGluR mRNAs were isolated (32), and the first corresponding gene was cloned in 1991 (33). These findings caused a major shift in the way people thought about glutamate as a neurotransmitter (34), suggesting that it may act as a neuromodulator in addition to its role in fast excitatory neurotransmission. In 1993, Weiler & Greenough presented the first evidence that one consequence of activating Gp1 mGluRs, comprising mGluR1 and mGluR5, is increased protein synthesis at synapses (35).
The explosion of knowledge about glutamate receptors in the 1980s also made possible the formulation of detailed hypotheses about how excitatory synapses in the brain are bidirectionally modified by experience to store information. One powerful in vivo model of experience-dependent plasticity is the visual cortex. Temporarily degrading image formation in one eye sets in motion synaptic changes in the visual cortex that render neurons unresponsive to the deprived eye (36). Various theories of synaptic modification were developed to account for these and related modifications. The influential BCM theory (37) posited that the loss of strength of deprived-eye synapses was not caused by the loss of activity from the deprived retina but rather by the presence of stochastic afferent activity (registered in the cortex as glutamate release) that no longer correlates with strong postsynaptic responses. On the basis of this idea and the observation that glutamate-stimulated PI turnover was exaggerated in visual cortex at the age of maximal plasticity, the hypothesis was put forth that Gp1 mGluRs might serve as a trigger for synaptic weakening (38). Homosynaptic LTD, triggered by weak activation of glutamate receptors, was subsequently discovered in the CA1 region of hippocampus (39), and one type of homosynaptic LTD was later shown to be triggered by activation of mGluR5 (40–42) and to require synaptic protein synthesis (43, 44). For a recent detailed review of the molecular mechanisms underlying mGluR-LTD, see Reference 45.
The mGluR Theory of Fragile X Syndrome
In 1997, while conducting a screen for synaptic mRNAs that are translated in response to Gp1 mGluR activation, Weiler et al. demonstrated that FMRP is synthesized following application of the receptor agonist DHPG (dihydroxyphenylglycine) in synaptoneurosomes (46). As described above, another line of investigation showed that DHPG, acting through mGluR5, can trigger LTD that requires translation of preexisting mRNA (40, 44). Thus, FMRP was at the top of the list of candidate proteins that are synthesized in response to mGluR5 activation to stabilize LTD. The simple hypothesis that FMRP is required for mGluR-LTD was tested using the Fmr1 KO mouse (17). The results showed, however, that instead of impaired LTD, there was exaggerated LTD in the Fmr1 KO.
These findings did not fit the prevailing model of the time, in which synaptic protein synthesis was impeded by the loss of FMRP in FXS (46), nor the hypothesis that FMRP stabilizes LTD. Rather, the data were consistent with the notion that FMRP, by binding directly to synaptic mRNAs, functions as a repressor of synaptic protein synthesis (15, 16). Thus, Huber et al. (17) suggested a model in which FMRP normally serves to limit expression of LTD by inhibiting mGluR-dependent translation of other synaptic mRNAs encoding the hypothetical “LTD protein(s).” According to this idea, FMRP synthesis in response to mGluR5 activation normally serves as an important brake on synthesis of other proteins—in the absence of FMRP, there is runaway or poorly regulated synaptic protein synthesis. The hypothesis that cerebral protein synthesis is elevated in FXS was later confirmed by metabolic experiments in the Fmr1 KO mouse, both in vivo and in vitro (47, 48).
Gp1 mGluRs participate in many brain circuits and serve diverse functions in addition to LTD. By the end of 2002, there were indications in the literature that some other lasting consequences of activating mGluR1 and mGluR5 require mRNA translation (49–51), consistent with the early biochemical finding that mGluR activation stimulates protein synthesis (35). The realization that many of the symptoms of FXS might plausibly be explained by excessive protein synthesis downstream of mGluR1/5 led to a formal proposal entitled “the mGluR theory of fragile X,” which was first publicly presented in 2002 and published in 2004 (52).
The mGluR theory made two important predictions that could and would be extensively tested in future experiments. First, it implied that other consequences of mGluR activation should be altered in the absence of FMRP. Second, and more significantly, it suggested that a reduction of mGluR activity might restore normal synaptic protein synthesis in the absence of FMRP and therefore reverse some mutant phenotypes in FXS. This latter prediction generated considerable excitement in the FXS field because it hinted at the possibility of a targeted treatment strategy that did not rely on replacement of the FMRP molecule itself.
Testing the mGluR Theory: Evidence for Altered Consequences of mGluR Activation
Following the original discovery of exaggerated mGluR-LTD, a wave of studies focused on further investigating this and other mGluR-related phenomena in Fmr1 KO mice. In agreement with the assumptions of the theory, mGluR1-dependent cerebellar LTD was observed to be exaggerated in the KO (25), as was mGluR1/5-dependent prolongation of epileptiform bursts in area CA3 of hippocampus (53). One of the mechanisms underlying mGluR-LTD is the protein synthesis–dependent loss of surface AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate) receptors (43), and it was hypothesized that the excess LTD in the Fmr1 KO mice might be due to an excess internalization of AMPA receptors (52). This hypothesis was confirmed using knockdown of FMRP levels by short interfering RNAs in hippocampal cultures (54).
A particularly striking discovery was that hippocampal mGluR-LTD in the Fmr1 KO mice no longer requires acute stimulation of protein synthesis at the time of induction, consistent with the notion that “LTD proteins” are constitutively overexpressed and no longer rate-limiting for LTD in the KO (55, 56). These findings inspired examination of another mGluR- and protein synthesis–dependent phenomenon that occurs at the same population of hippocampal synapses, called LTP priming (30). Priming is an enhancement of LTP that occurs when Gp1 mGluRs are briefly and weakly stimulated prior to delivery of LTP-inducing tetanic stimulation (51). Like mGluR-LTD, LTP priming normally requires mGluR-dependent translation of mRNA in wild-type mice but not in the Fmr1 KO (30). Because it is not known if “LTP priming proteins” and “LTD proteins” are the same or different, it is probably better to conceptualize these products of mGluR-stimulated mRNA translation as “plasticity gating proteins.” These gating proteins are apparently overexpressed in the Fmr1 KO, leading to diverse consequences.
A number of studies have also investigated the role of signaling pathways downstream of Gp1 mGluRs in protein synthesis and LTD phenotypes in Fmr1 KO mice. mGluR5 receptors were shown to be less tightly coupled with synapses and Homer scaffolding proteins in Fmr1 KO mice (57), and the Homer-dependent activation of mTOR (mammalian target of rapamycin) signaling in response to mGluR5 stimulation was absent altogether (58). Other groups have reported a slight basal increase in ERK (extracellular signal-regulated kinase) activity (56), an aberrant mGluR-induced inactivation of ERK (59), a basal increase in the Akt/mTOR pathway (60) and an excess of PI3K (phosphoinositide 3-kinase) activity (61). However, many of these signaling alterations appear to be highly dependent on the experimental preparation and are not observed under all conditions. For example, neither Akt/mTOR nor ERK signaling pathways were altered in hippocampal slices confirmed to have increased basal protein synthesis in the Fmr1 KO (62). These data suggest that altered Akt/mTOR and ERK signaling may not be a cause (but may be a consequence) of aberrant protein synthesis in Fmr1 KO mice. We favor the hypothesis that the protein synthesis phenotype is due to hypersensitivity of the mRNA translation machinery to normal levels of mGluR signaling, rather than hyperactivity of the mGluR signaling pathways themselves (62). It is likely, however, that the aberrant signaling pathways observed in some preparations do contribute to Fmr1 KO phenotypes, regardless of whether this is proximal or distal to excess global protein synthesis.
Finally, an important line of research has focused on identifying target proteins whose synthesis is regulated by both mGluR5 and FMRP and that are differentially expressed basally and/or in response to mGluR stimulation in Fmr1 KO mice. A number of interesting candidates have been proposed to date, including MAP1B, eEF1A, Arc, CaMKIIα, PSD-95, SAPAP3, and APP (for recent detailed reviews, see 18, 63). The roles of these candidate proteins in mGluR-related phenotypes in Fmr1 KO mice are currently under investigation.
Reversal of FXS-Related Phenotypes by Reduction or Antagonism of Gp1 mGluRs
Experiments investigating the effects of mGluR inhibition on phenotypes induced by the loss of FMRP have two essential benefits: They test the validity of the biological principles underlying the mGluR theory of fragile X, and they provide information on the potential for targeting mGluRs as a therapeutic strategy in FXS. Accordingly, a significant body of literature has emerged on this topic in the past five years.
In an extensive proof-of-principle study, Dolen et al. used a genetic strategy to investigate the effect of reducing mGluR5 levels on FXS-related phenotypes in Fmr1 KO mice (48). Mice heterozygous for the Grm5 gene (which encodes mGluR5) were crossed with Fmr1 KO mice, and the resulting 50% reduction in mGluR5 protein levels led to the correction (or prevention) of 7 out of the 8 FXS-related phenotypes assessed. A significant decrease was observed in the induction of audiogenic seizures, a model for the epilepsy frequently observed in FXS patients. In addition, the increase in dendritic spines seen in visual cortex of Fmr1 KO mice, the increase in protein synthesis in hippocampus, and the increase in total body weight observed in young Fmr1 KO mice were all reversed. Moreover, the genetic reduction of mGluR5 corrected the excessive hippocampal LTD and abnormal experience-dependent plasticity of visual cortex. Finally, the cross rescued an enhanced extinction phenotype in an inhibitory avoidance paradigm, a mouse model for learning and memory. The only phenotype not rescued in this study was macroorchidism, suggesting that other pathways (possibly mGluR1) are involved in the testicular phenotype. Similarly, a genetic approach was used in a Drosophila model of FXS, in which the Drosophila homolog of the Fmr1 gene, known as dFmr1 or dfxr1, was disrupted. Using a double KO of dFmr1 and the only Drosophila mGluR, known as dmGluRA, it was shown that dFmr1 and dmGluRA pathways converge to regulate multiple phenotypes including glutamate receptor trafficking, synaptic plasticity, presynaptic ultrastructure, and coordinated motor behavior (64–66). Based on these observations, the authors concluded that loss of the receptor at least partially corrects defects caused by impaired translational regulation and vice versa.
The genetic rescue experiments provide essential validation, not only for the theory but also for the concept that drugs targeting mGluR5 could treat multiple aspects of the disease. The therapeutic potential of mGluR5 inhibitors has also been investigated on various phenotypes in FXS animal models. Most of these studies have relied on MPEP [2-methyl-6-(phenylethynyl)-pyridine], a potent negative allosteric modulator of mGluR5 that crosses the blood-brain barrier. MPEP has proven to be a critical tool for studying mGluR5 function (67). In the first study to investigate the effects of pharmacological antagonism, acute administration of MPEP in Fmr1 KO mice was found to reduce the abnormal response of these mice in an open field test, an experimental measure commonly used to assess anxiety-like phenotypes in mouse models (68). In addition, like the genetic rescue, MPEP significantly reduced the probability of audiogenic seizures in these mice.
Subsequently, other groups have demonstrated that MPEP reverses a large number of phenotypes in Fmr1 KO mice, including prolonged epileptiform discharges in hippocampal slices (53), deficits in prepulse inhibition of startle (69), decreased mRNA granule expression (70), excess protein synthesis in hippocampal slices (62), increased density of dendritic filopodia in hippocampal cultures (69), and hyperactivity of glycogen synthase kinase-3 (71). Similarly, MPEP was able to reverse several phenotypes in dFmr1 mutant flies, including abnormalities in behavioral and structural measures of courtship-related learning and memory (72), deficits in olfactory memory (73), and increased embryonic lethality due to excitotoxicity (74). Finally, one group generated Fmr1 knockdown zebrafish embryos using the morpholino antisense oligonucleotide technology. By developing these embryos in medium containing MPEP, the authors were able to reverse disruptions in neurite morphology in the hindbrain and spinal cord of the embryos, as well as in craniofacial development (75). Together, these data provide compelling evidence that manipulating mGluR5 signaling can reverse fragile X–related phenotypes across species, indicating that mGluR5 may indeed provide a viable target for the treatment of FXS.
It is worth emphasizing that the interaction between FMRP and mGluRs seems to be highly conserved in evolution, appearing across the phylogenetic tree from invertebrates to mammals. This interplay between a repressor and an activator of protein synthesis may thus represent an essential core mechanism by which synaptic plasticity is regulated at glutamatergic synapses, which in turn may explain how its disruption can cause such widespread and severe pathological alterations in FXS. This striking degree of evolutionary conservation also boosts confidence that pharmacological approaches that have been successful in animals have great potential to succeed in humans.
Limitations of the mGluR Theory
Although a large number of studies have provided evidence in support of the mGluR theory, not all findings are consistent with the simple notion that excessive mGluR-dependent protein synthesis and synaptic plasticity in the absence of FMRP accounts for mutant phenotypes in the Fmr1 KO mouse. For example, some of the proposed FMRP target proteins do not show the expected basal upregulation in KO mice. The synaptic scaffold protein PSD-95, in particular, was reported to be downregulated in hippocampus owing to an alteration in mRNA stability, with mGluR stimulation resulting in stabilization of PSD-95 mRNA in wild-type but not in KO mice (76). Additionally, in cortex (28) and amygdala (77), forms of LTP that depend on mGluR5 activation in wild-type mice were found to be absent, rather than exaggerated, in the KO. Furthermore, it was observed that MPEP injected once daily for several days during early postnatal development actually accentuated the immature appearance of dendritic spines imaged in vivo in somatosensory cortex of KO mice (78).
In interpreting these studies, it is important to distinguish manifestations of an ongoing excess of mGluR signaling from the manifestations of synaptic development that has been altered because of elevated mGluR signaling. For example, the absence of mGluR5-dependent LTP in the cortex could reflect the fact that this LTP mechanism has already been saturated in vivo as a consequence of exaggerated mGluR5 function during development. In the case of the amygdala, a substantial deficit in basal transmission was also reported at the same synapses that showed impaired LTP. Reduced synaptic connectivity might have caused the defective LTP and might have arisen as a consequence of increased mGluR5-dependent protein synthesis during the development of amygdala circuitry. These alternative interpretations will need to be explored before rejecting the relevance of the mGluR theory to the fragile X LTP phenotypes in cortex and amygdala.
It is also important to understand the limitations of the tools that are available to inhibit mGluR5 signaling. Most pharmacological studies published to date have relied on the mGluR5 negative allosteric modulator MPEP. However, MPEP is an extremely short-acting antagonist in vivo, with maximal receptor occupancy lasting only ~15 min in mouse brain (79). It is therefore virtually impossible to study the drug’s effect on phenotypes that may require chronic mGluR5 antagonism during brain development. Single MPEP injections will not produce chronic inhibition and will likely cause transient rebound increases in mGluR5 function as the drug wears off. An alternative is the “genetic rescue” approach, implemented by crossing Grm5 heterozygotes with Fmr1 KO mice (48). This approach can overcome pharmacokinetic limitations, but only a single “dose” can be tested, namely a 50% reduction in mGluR5 protein. Novel pharmacological agents with long-lasting pharmacokinetic properties will be necessary to fully address these issues, and their development is currently under way.
Although the mGluR theory may withstand the challenges presented by the aforementioned studies, it nevertheless seems very likely that FMRP has functions that are unrelated to mGluRs. Accordingly, it will be just as informative to identify phenotypes that are not corrected by mGluR1/5 antagonism as to investigate those that are, since this distinction may lead to important mechanistic insights that will further direct the development of successful therapeutic strategies.
Testing the mGluR Theory in Humans
Encouraged by the exciting findings arising from preclinical research, a number of clinical trials have been initiated to test the efficacy of compounds directly or indirectly related to mGluR signaling in treating FXS (80, 81). To date, none of these compounds are specifically approved for the treatment of FXS, but promising preliminary results have been obtained.
The first Gp1 mGluR inhibitor to be tested in clinical trials was fenobam, a compound that was originally developed as an anxiolytic with an unknown molecular target and was subsequently demonstrated to be a selective mGluR5 antagonist (82). An open-label phase II clinical trial was recently completed, in which 12 adult patients with FXS received a single dose of fenobam to assess drug safety, pharmacokinetics, and a small number of cognitive and behavioral effects. In this trial, fenobam was reported to reduce anxiety and hyperarousal and to improve prepulse inhibition of startle and accuracy on a continuous performance task (a measure of sustained attention and impulsivity) in a subset of patients (80, 83). Although these results are encouraging, it is important to note that the study was not performed blind and was not placebo controlled. The study also revealed highly variable plasma levels of fenobam after oral dosing, making this compound problematic as a potential therapeutic and an inadequate agent to test the mGluR theory in humans.
In another small open-label trial, three young adult patients with FXS were treated with acamprosate, a drug with mGluR antagonist properties that is approved for maintenance of abstinence from alcohol (84). In all three patients, acamprosate was associated with improved linguistic communication and global clinical benefit as assessed by the CGI-I (clinical global impression-improvement) scale.
New, highly potent and selective mGluR5 negative allosteric modulators have shown promising results in preclinical studies and are currently in clinical trials in FXS (see http://www.clinicaltrials.gov). These include STX107 (Seaside Therapeutics, phase I trial initiated in the United States), AFQ056 (Novartis, phase II trial recently completed in France, Italy, and Switzerland), and RO4917523 (Hoffman-LaRoche, phase II trial initiated in the United States). The results of these studies are anxiously awaited, as they clearly represent the best tests to date of the applicability of the mGluR theory to humans.
In addition to targeting mGluRs directly, another approach to reducing excessive mGluR-mediated plasticity is to target the signaling pathways downstream or upstream of mGluRs. This approach is exemplified by the recent interest in lithium, which is already clinically approved for the treatment of mood disorders. Lithium targets multiple intracellular signaling pathways, including phospholipase C and glycogen synthase kinase-3, which have been linked to Gp1 mGluR signaling and FXS, respectively (71, 85). In a pilot trial on 15 patients with FXS, lithium treatment for two months was found to have positive effects on behavioral adaptive skills and one cognitive measure (85). A second approach that is currently in clinical trials aims at reducing the presynaptic release of glutamate and hence the activation of postsynaptic mGluR5. It has been previously shown that GABA-B receptor agonists such as baclofen inhibit glutamate release, and baclofen has been shown to reduce audiogenic seizures in Fmr1 KO mice (86). Based on this information, a placebo-controlled double-blind phase II study was conducted using arbaclofen (also known as STX209), the R-isomer of baclofen. This study has been completed and it is anticipated that the results will soon be available.
Finally, clinical trials are also under way for minocycline, a tetracycline analog that (among other things) inhibits matrix metalloproteinase-9 (MMP-9). It was previously shown that levels of MMP-9 are elevated in Fmr1 KO mice, possibly as a consequence of excess mGluR5 signaling, and that minocycline reverses several phenotypes in these mice (87).
Window of Opportunity for Therapeutic Interventions
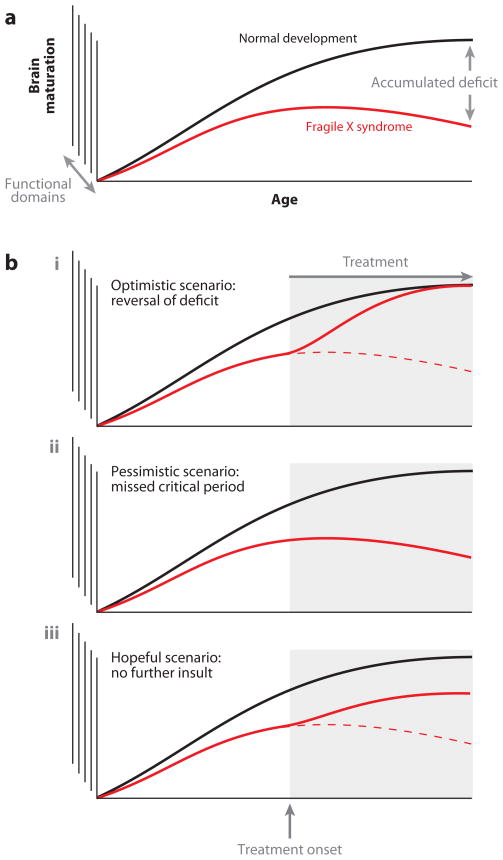
An important issue for the design of successful treatment strategies concerns the developmental time window available for intervention. Mutations such as those underlying FXS presumably alter the trajectory of normal brain development by disrupting the mechanisms of activity-dependent circuit formation and plasticity (Figure 3a). The key question then is whether these alterations are reversible, or whether the aberrant connectivity, once established, results in a permanent dysfunction that cannot be influenced by subsequent pharmacological interventions. Given that most neurodevelopmental disorders are currently not diagnosed until well after the onset of symptoms, this issue will play an essential role in determining treatment outcomes and the need for newborn screening to facilitate early intervention.
Figure 3.
Prospects for the treatment of a developmental brain disorder. (a) Divergence of brain maturation in normal development versus development in fragile X syndrome (FXS). This continuous divergence results in an accumulated deficit in individuals with FXS that increases with age. (b) Prospects for treatment of this accumulated deficit in FXS. Three scenarios are conceivable with respect to interventions that occur following symptom onset: (i ) The optimistic view assumes that pharmacological intervention after symptom onset results in near-complete reversal of associated phenotypes. (ii ) The pessimistic view is that after an initial window of opportunity, pharmacological approaches can no longer alter the course of the disorder. (iii ) The intermediate (but still hopeful) view suggests that pharmacological intervention can slow or prevent progression of symptoms, although it may not fully correct previously established impairments.
We can consider several potential scenarios for the efficacy of drugs after symptom onset (Figure 3b). The most optimistic possibility is that intervention after symptom onset results in near-complete reversal of associated phenotypes. This scenario is conceivable, e.g., if the molecular mechanisms affected are required only for acute synapse function or for the reversible strengthening or weakening of previously existing connections. The pessimistic scenario is that, following an initial window of opportunity, pharmacological approaches can no longer alter the course or progression of the disorder. This may occur either because of large-scale irreversible structural alterations, such as aberrant axonal pathfinding or dendritic branching, or because the pathogenic molecule no longer plays a role in the later stages of development or in adulthood. An intermediate, but still hopeful, scenario is that intervention can slow or prevent progression of symptoms, although it may not fully correct previously established impairments. At the molecular level, this scenario would be likely if structural abnormalities occurred locally (e.g., the development of aberrant dendritic spines) and could be compensated for by local adjustments following drug administration.
It should also be considered that most neurodevelopmental disorders manifest as a complex combination of symptoms, each of which may involve different molecular mechanisms and developmental trajectories. Moreover, not all individuals with a given disorder will always express the same combination of symptoms. In order to optimize therapeutic strategies, it will therefore be necessary to characterize each of these phenotypes individually and identify the underlying pathogenic mechanisms and developmental time course. Extensive research in animal models will be crucial in addressing these important questions and should help guide human clinical trial design.
MOLECULAR MEDICINE APPROACHES IN OTHER NEURODEVELOPMENTAL DISORDERS
Although mGluR-based therapeutics for FXS are among the first targeted treatments to reach clinical trials for any ASD-related disorder, important progress has recently also been made in other single-gene neurodevelopmental disorders associated with autism (88).
Tuberous Sclerosis Complex
Tuberous sclerosis complex (TSC) is a multisystem disorder characterized by tumorous growths, or hamartomas, in organs including the kidneys, lung, heart, brain, and liver (89). Clinical manifestations related to the brain can include mental retardation, autism, and epilepsy. TSC is caused by heterozygous mutations in either the TSC1 or TSC2 gene, both of which encode proteins that are negative regulators of the mTOR intracellular signaling pathway. The mTOR pathway has been linked to the control of protein synthesis, and disruption of this control is thought to underlie the neuropsychiatric phenotypes observed in TSC.
Mice with a heterozygous mutation in the Tsc2 gene mimic several of the cognitive deficits observed in human TSC patients. Using this mouse model, it was shown that brief treatment with rapamycin, an inhibitor of the mTOR complex, rescues deficits in context discrimination and spatial learning (90). Clinical trials of rapamycin, also known as sirolimus, are under way in children with TSC, although to our knowledge there are no trials specifically investigating the effects of rapamycin on the cognitive and behavioral phenotypes in TSC (http://www.clinicaltrials.gov).
PTEN Mutations
Mutations in the phosphatase and tensin homolog (PTEN) gene, another negative regulator of the Akt/mTOR signaling pathway, have also been linked to a number of cases of autism with extreme macrocephaly. A mouse model in which PTEN was deleted in a subset of neurons in cortex and hippocampus displays neuron hypertrophy resulting in disruptions of hippocampal structure, as well as increased susceptibility to seizures and increased anxiety (91). Chronic (four- to six-week) administration of rapamycin was able to reverse all of these phenotypes in the mice, again suggesting that mTOR might be a relevant drug target for certain forms of autism (91). Since PTEN mutations are also associated with a greatly increased susceptibility to certain cancers, clinical trials are currently under way to examine the effects of rapamycin on tumor growth in cancer patients with PTEN mutations (http://www.clinicaltrials.gov). However, studies to examine the effects of rapamycin on the cognitive and behavioral deficits associated with PTEN mutations have yet to be initiated.
Neurofibromatosis Type 1
Neurofibromatosis type 1 (NF1) is a neurodevelopmental disorder associated with cognitive impairments, including difficulties with visuospatial skills and executive function, as well as an increased incidence of autism (88, 92). NF1 is caused by mutations in the gene encoding neurofibromin, which inhibits p21Ras function. Mice with a heterozygous deletion of the Nf1 gene show an increase in the phosphorylation of ERK1/2, one of the downstream targets of p21Ras signaling, as well as deficits in visuospatial attention, spatial learning, prepulse inhibition, and hippocampal LTP (92). Brief administration of the farnesyl transferase inhibitor lovastatin, which (among other things) decreases p21Ras activity by blocking its farnesylation and membrane association, was able to reverse all of these phenotypes, indicating that lovastatin may be a viable treatment for NF1. Clinical trials to investigate the effects of lovastatin on visual spatial learning and memory in children with NF1 have been initiated (http://www.clinicaltrials.gov), although it should be noted that simvastatin, a related farnesyl transferase inhibitor, was previously found to have no effect on cognitive functioning in children with NF1 (93).
Rett Syndrome
Rett syndrome (RTT) is a severe ASD that also includes intellectual disabilities as well as motor symptoms such as ataxia, dystonia, and respiratory dysfunction (88, 94). RTT is caused by mutations of the X-chromosomal gene encoding the transcriptional regulator MeCP2, and deletion of this gene in mice causes a severe neurological phenotype related to the symptoms observed in RTT patients, including gait abnormalities, respiratory dysfunction, hind limb clasping, and decreased survival. Two recent studies have sought to reverse these phenotypes using a genetic and a pharmacological strategy, respectively. In the former, MeCP2 function was restored during or following onset of symptoms using a tamoxifen-inducible Cre-LoxP strategy, resulting in the robust reversal of several associated phenotypes (94). This is an extremely important proof of principle that the course of this disease can be arrested and even reversed with manipulations begun in late adolescence (the optimistic scenario in Figure 3). In the second study, mice were treated with insulin-like growth factor 1 (IGF-1), based on previous observations that another growth factor, brain-derived neurotrophic factor (BDNF), was a key target of MeCP2 regulation (95). IGF-1 administration improved several phenotypes in the MeCP2 KO mice, including locomotor activity, respiratory function, dendritic spine density, and survival. Neither of these rescue strategies has resulted in the initiation of clinical trials to date, but they suggest that the development of targeted treatments for RTT may be a feasible goal in the future.
Idiopathic Autism
In addition to the providing the basis for the development of targeted treatments for specific developmental disorders, the above studies are also yielding key insights into related disorders for which few mechanistic details are currently available. Idiopathic autism has been notoriously difficult to study owing to the lack of suitable animal models. By comparing the molecular mechanisms underlying different single-gene disorders, it may be possible to discover commonalities and general principles that might hold true even for those cases in which no specific genetic cause has been identified. One such key principle arose from the observation that many of the single-gene disorders appear to affect key regulators of protein synthesis, suggesting that it may be the dysregulation of protein synthesis itself that represents one final common pathogenic mechanism (96). According to this theory, aberrant synaptic protein synthesis caused by mutations or copy-number variations in regulatory signaling pathways may lead to changes in synaptic connectivity and function, which subsequently result in the cognitive and behavioral deficits that are observed in ASDs. This notion has recently been supported by clinical studies linking autism to mutations in the gene encoding the translation initiation factor eIF4E (97) and to copy-number variations in GTPase/Ras signaling pathways (98) that may be involved in the regulation of synaptic protein synthesis. Based on these findings, treatments that successfully target protein synthesis pathways in the single-gene disorders mentioned above, including mGluR5 modulators, may very well have broader therapeutic applications in idiopathic autism.
However, it is also clear that altered synaptic protein synthesis is not the only mechanism by which autism-related mutations can cause abnormalities in synaptic function. For example, a key role is also emerging for mutations in structural proteins that are involved in synaptic development or function, including the synaptic cell adhesion molecules neuroligin-3, neuroligin-4 and neurexin-1, and the synaptic scaffolding protein SHANK3 (for recent detailed reviews, see 99–101). While structural proteins themselves are unlikely to represent useful targets for traditional small-molecule drug therapies, it may ultimately be possible to target common downstream consequences of these mutations, such as a shift in the balance of excitatory and inhibitory synaptic transmission. Indeed, the first studies investigating such strategies in mouse models are beginning to emerge (102), although significant further pre-clinical research is likely to be necessary before clinical trials can be considered.
It is already clear that diverse molecular mechanisms can contribute to the synaptic abnormalities that underlie ASDs. In order to design appropriate therapeutic strategies for idiopathic autism, it will be critical to identify biomarkers that report the pathophysiological processes at work in the brains of the affected individuals.
FUTURE ISSUES.
Is it possible to identify additional novel therapeutic targets for FXS based on insights into the molecular mechanisms of FMRP function at the synapse? Can these targets be validated in animal models of FXS and subsequently in clinical trials?
What is the developmental window of opportunity for the pharmacological treatment of FXS? Can existing symptoms be corrected or improved, or is it necessary to initiate treatment early in pre- or postnatal development prior to phenotype onset? Is newborn screening for early intervention warranted?
Can we gain new insights into idiopathic autism from comparing and contrasting the molecular mechanisms underlying different autism-associated single-gene disorders? Can this knowledge be used to develop novel treatment strategies for at least a subset of individuals with ASDs?
What peripheral biomarkers will best reflect brain pathophysiology and inform clinical trials and treatments for autism of unknown etiology?
Acknowledgments
The authors thank Emily Osterweil for valuable discussions and the FRAXA Research Foundation, the National Institute of Mental Health, and the National Institute of Child Health and Human Development for funding support.
Glossary
- ASD
autism spectrum disorder
- FXS
fragile X syndrome
- FMRP
fragile X mental retardation protein
- LTD
long-term depression
- mGluR
metabotropic glutamate receptor
- TSC
tuberous sclerosis complex
- NF1
neurofibromatosis type 1
- RTT
Rett syndrome
Footnotes
DISCLOSURE STATEMENT
Mark Bear has a financial interest in Seaside Therapeutics, Inc.
LITERATURE CITED
- 1.Hyman SE. The role of molecular biology in psychiatry. Psychosomatics. 1988;29:328–32. doi: 10.1016/S0033-3182(88)72371-3. [DOI] [PubMed] [Google Scholar]
- 2.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogan MD, Blumberg SJ, Schieve LA, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 4.Martin JP, Bell J. A pedigree of mental defect showing sex-linkage. J Neurol Psychiatry. 1943;6:154–57. doi: 10.1136/jnnp.6.3-4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile X syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–29. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 6.Lubs H. A marker X chromosome. Am J Hum Genet. 1969;21:231–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland GR. Fragile sites on human chromosomes: demonstration of their dependence on the type of tissue culture medium. Science. 1977;197:265–66. doi: 10.1126/science.877551. [DOI] [PubMed] [Google Scholar]
- 8.Verkerk AJMH, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 9.Ashley C, Wilkinson K, Reines D, et al. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–66. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- 10.Dutch Belgian Fragile X Consortium. Bakker CE, Verheij C, Willemsen R, et al. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- 11.Corbin F, Bouillon M, Fortin A. The fragile X mental retardation protein is associated with poly(A)+ mRNA in actively translating polyribosomes. Hum Mol Genet. 1997;6:1465–72. doi: 10.1093/hmg/6.9.1465. [DOI] [PubMed] [Google Scholar]
- 12.Khandjian EW, Corbin F, Woerly S, et al. The fragile X mental retardation protein is associated with ribosomes. Nat Genet. 1996;12:91–93. doi: 10.1038/ng0196-91. [DOI] [PubMed] [Google Scholar]
- 13.Tamanini F, Meijer N, Verheij C, et al. FMRP is associated to the ribosomes via RNA. Hum Mol Genet. 1996;5:809–13. doi: 10.1093/hmg/5.6.809. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–38. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- 15.Laggerbauer B, Ostareck D, Keidel EM, et al. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–38. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Zhang Y, Ku L, et al. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–83. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber KM, Gallagher SM, Warren ST, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–14. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–67. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–89. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- 21.Comery TA, Harris JB, Willems PJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94:5401–4. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinton VJ, Brown WT, Wisniewski K, et al. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–94. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 23.Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–44. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci. 2007;27:3120–30. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koekkoek SKE, Yamaguchi K, Milojkovic BA, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in fragile X syndrome. Neuron. 2005;47:339–52. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Pelletier MR, Perez Velazquez JL, et al. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci. 2002;19:138–51. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- 27.Desai NS, Casimiro TM, Gruber SM, et al. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol. 2006;96:1734–45. doi: 10.1152/jn.00221.2006. [DOI] [PubMed] [Google Scholar]
- 28.Wilson BM, Cox CL. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci USA. 2007;104:2454–59. doi: 10.1073/pnas.0610875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao MG, Toyoda H, Ko SW, et al. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–92. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auerbach BD, Bear MF. Loss of the fragile X mental retardation protein decouples metabotropic glutamate receptor dependent priming of long-term potentiation from protein synthesis. J Neurophysiol. 2010;104:1047–51. doi: 10.1152/jn.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicoletti FMJ, Iadarola MJ, Chuang DM, et al. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J Neurochem. 1986;46:40–46. doi: 10.1111/j.1471-4159.1986.tb12922.x. [DOI] [PubMed] [Google Scholar]
- 32.Hirono C, Ito I, Yamagishi S, et al. Characterization of glutamate receptors induced in Xenopus oocytes after injection of rat brain mRNA. Neurosci Res. 1988;6:106–14. doi: 10.1016/0168-0102(88)90012-0. [DOI] [PubMed] [Google Scholar]
- 33.Houamed KM, Kuijper JL, Gilbert TL, et al. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252:1318–21. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- 34.Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann NY Acad Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- 35.Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci USA. 1993;90:7168–71. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiesel T, Hubel D. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–17. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 37.Bienenstock E, Cooper L, Munro P. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bear MF. Involvement of excitatory amino acid receptor mechanisms in the experience-dependent development of visual cortex. In: Lehman J, Turski L, editors. Recent Advances in Excitatory Amino Acid Research. New York: Liss; 1988. pp. 393–401. [Google Scholar]
- 39.Dudek S, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–67. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–25. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 41.Oliet SHR, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–82. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 42.Palmer MJ, Irving AJ, Seabrook GR, et al. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–32. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- 43.Snyder EM, Philpot BD, Huber KM, et al. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–85. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 44.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–56. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 45.Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiler IJ, Irwin SA, Klintsova AY, et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA. 1997;94:5395–400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin M, Kang J, Burlin TV, et al. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the Fmr1 null mouse. J Neurosci. 2005;25:5087–95. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolen G, Osterweil E, Rao BSS, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merlin LR, Bergold PJ, Wong RK. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–93. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- 50.Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci USA. 2002;99:1639–44. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raymond CR, Thompson VL, Tate WP, et al. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–76. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–77. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Chuang SC, Zhao W, Bauchwitz R, et al. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–55. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamoto M, Nalavadi V, Epstein MP, et al. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci USA. 2007;104:15537–42. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–95. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- 56.Hou L, Antion MD, Hu D, et al. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–54. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Giuffrida R, Musumeci S, D’Antoni S, et al. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive Homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–16. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–47. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SH, Markham JA, Weiler IJ, et al. Aberrant early-phase ERK inactivation impedes neuronal function in fragile X syndrome. Proc Natl Acad Sci USA. 2008;105:4429–34. doi: 10.1073/pnas.0800257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma A, Hoeffer CA, Takayasu Y, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gross C, Nakamoto M, Yao X, et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–38. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osterweil EK, Krueger DD, Reinhold K, et al. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–27. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, fragile X. Curr Opin Neurobiol. 2009;19:319–26. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan L, Broadie KS. Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A convergently regulate the synaptic ratio of ionotropic glutamate receptor subclasses. J Neurosci. 2007;27:12378–89. doi: 10.1523/JNEUROSCI.2970-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan L, Woodruff E, III, Liang P, et al. Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol Cell Neurosci. 2008;37:747–60. doi: 10.1016/j.mcn.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Repicky S, Broadie K. Metabotropic glutamate receptor-mediated use-dependent down-regulation of synaptic excitability involves the fragile X mental retardation protein. J Neurophysiol. 2009;101:672–87. doi: 10.1152/jn.90953.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gasparini F, Lingenhöhl K, Stoehr N, et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 68.Yan QJ, Rammal M, Tranfaglia M, et al. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–66. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 69.de Vrij FMS, Levenga J, van der Linde HC, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–32. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aschrafi A, Cunningham BA, Edelman GM, et al. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci USA. 2005;102:2180–85. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Min WW, Yuskaitis CJ, Yan Q, et al. Elevated glycogen synthase kinase-3 activity in fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology. 2009;56:463–72. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McBride SMJ, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–64. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 73.Bolduc FV, Bell K, Cox H, et al. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11:1143–45. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang S, Bray SM, Li Z, et al. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4:256–63. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- 75.Tucker B, Richards RI, Lardelli M. Contribution of mGluR and Fmr1 functional pathways to neurite morphogenesis, craniofacial development and fragile X syndrome. Hum Mol Genet. 2006;15:3446–58. doi: 10.1093/hmg/ddl422. [DOI] [PubMed] [Google Scholar]
- 76.Zalfa F, Eleuteri B, Dickson KS, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10:578–87. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suvrathan A, Hoeffer CA, Wong H, et al. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci. 2010;107:11591–96. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cruz-Martin A, Crespo M, Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–66. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berry-Kravis E. Fragile X research: a status report. Natl Fragile X Found Q. 2008;31:12–16. [Google Scholar]
- 81.Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–90. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Porter RHP, Jaeschke G, Spooren W, et al. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–21. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- 83.Berry-Kravis EM, Hessl D, Coffey S, et al. A pilot open-label single-dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–71. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erickson CA, Mullett JE, McDougle CJ. Brief report: acamprosate in fragile X syndrome. J Autism Dev Disord. 2010 doi: 10.1007/s10803-010-0988-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 85.Berry-Kravis E, Sumis A, Hervey C, et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- 86.Pacey LKK, Heximer SP, Hampson DR. Increased GABAB receptor-mediated signaling reduces the susceptibility of fragile X knockout mice to audiogenic seizures. Mol Pharmacol. 2009;76:18–24. doi: 10.1124/mol.109.056127. [DOI] [PubMed] [Google Scholar]
- 87.Bilousova TV, Dansie L, Ngo M, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 88.Silva AJ, Ehninger D. Adult reversal of cognitive phenotypes in neurodevelopmental disorders. J Neurodev Disord. 2009;1:150–57. doi: 10.1007/s11689-009-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ehninger D, de Vries PJ, Silva AJ. From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res. 2009;53:838–51. doi: 10.1111/j.1365-2788.2009.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehninger D, Han S, Shilyansky C, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–48. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou J, Blundell J, Ogawa S, et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–83. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li W, Cui Y, Kushner SA, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15:1961–67. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 93.Krab LC, de Goede-Bolder A, Aarsen FK, et al. Effect of simvastatin on cognitive functioning in children with neurofibromatosis type 1: a randomized controlled trial. JAMA. 2008;300:287–94. doi: 10.1001/jama.300.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guy J, Gan J, Selfridge J, et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–47. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tropea D, Giacometti E, Wilson NR, et al. Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci USA. 2009;106:2029–34. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelleher RJ, Bear MF. The autistic neuron: troubled translation? Cell. 2008;135:401–6. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 97.Neves-Pereira M, Müller B, Massie D, et al. Deregulation of EIF4E: a novel mechanism for autism. J Med Genet. 2009;46:759–65. doi: 10.1136/jmg.2009.066852. [DOI] [PubMed] [Google Scholar]
- 98.Pinto D, Pagnamenta AT, Klei L, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–72. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. 2008;455:903–11. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toro R, Konyukh M, Delorme R, et al. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet. 2010;26:363–72. doi: 10.1016/j.tig.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 101.Betancur C, Sakurai T, Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–12. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blundell J, Blaiss CA, Etherton MR, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–29. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]