Abstract
Burn patients are highly susceptible to infections due to increased exposure through wounds and impairments in a number of immune functions. Dendritic cells (DCs) are important in activation of numerous immune responses that are essential for the clearance of infections. We have found that prophylactic treatment of burn-injured mice with the DC growth factor fms-like tyrosine kinase-3 ligand (FL) significantly increases resistance to burn wound infections in a DC-dependent manner that is correlated closely with enhanced bacterial clearance. However, as DCs are not typically microbicidal, the mechanisms by which DC modulation enhances bacterial clearance are not known. Due to the rapid response of neutrophils to cutaneous wounds, and the reported interactions between DCs and neutrophils, we investigated the role of neutrophils in FL-mediated resistance to burn wound infection. This was examined both in vivo and in vitro through neutrophil depletion, supplementation of neutrophils, and assessment of neutrophil chemotaxis following FL treatment. To test the involvement of DCs, CD11c-DTR transgenic mice were utilized to deplete DCs during FL treatment. Studies revealed that neutrophils do play a critical role in FL-mediated resistance to a burn wound infection. Additionally, treatment with FL after a burn injury enhances neutrophil-mediated control of bacterial spread, neutrophil migratory capacity, and MPO production, in a DC-dependent manner. The results of this study provide new insight into immunological mechanisms that can offer protection against infection after burn injury.
Introduction
Patients with severe burn wounds are highly susceptible to opportunistic infections as a result of the loss of the protective skin barrier and numerous injury-induced immune alterations that impair the ability to control the spread of infection. Opportunistic infections remain the leading cause of death in burn patients, even with advances in antibiotic treatments and patient care 1,2. Disruption in innate immune responses following a severe burn injury include impairments in the functions of NK cells, neutrophils, and antigen-presenting cells, all of which are crucial for the establishment of a normal immune response to infection 3–7. Understanding burn injury-induced immune impairments and development of treatments to overcome these impairments are of critical importance in reducing morbidity and mortality among burn patients.
Dendritic cells (DCs) play a critical role in the recognition of infection and subsequent activation of innate and adaptive immune responses. Langerhans cells, DCs in the skin, are important in trafficking infectious antigens from wounds to the lymph nodes, where activation of immune responses occurs 8. We have previously reported that enhancement of DC numbers and functions through treatment with the hematopoietic growth factor fms-like tyrosine kinase-3 ligand (FL) leads to significantly enhanced resistance to a lethal Pseudomonas aeruginosa burn wound infection in mice. This increased resistance to infection through treatment with FL is associated with a decrease in bacterial spread 9,10. However, DCs do not play an active role in bacterial killing or clearance. Therefore, FL-induced modifications of DCs must contribute to the control of bacterial spread in an indirect manner through the activation or enhancement of other cells and bactericidal functions.
Neutrophils are among the first responders to a cutaneous injury, where they function by controlling infection through bacterial uptake and killing and production of soluble factors that initiate activation and recruitment of additional neutrophils and other immune cells to the sites of inflammation and infection 11–13. Unlike DCs, neutrophils do not express the receptor for FL, Flt3-R, and cannot be modified directly by FL 14. DCs and neutrophils can interact with each other, leading to bidirectional activation through cell-cell interactions and through secretion of activating cytokines 15. Upon interaction with neutrophils, DCs exhibit an upregulation of costimulatory molecules, and neutrophils are capable of assisting DCs in antigen-presentation and activation of T cell responses 16–18. Additionally, interactions with DCs increase the expression of activation markers on neutrophils, as well as the secretion of elastase and myeloperoxidase, and can delay neutrophil apoptosis 19. Cytokines produced by DCs can enhance bacterial uptake by neutrophils 20. Thus, interactions between DCs and neutrophils provide a potential mechanism for enhancement of bacterial clearance through DC modification by FL.
Using a model of P. aeruginosa burn wound infection, we tested the hypothesis that FL treatments enhance the ability of DCs to promote neutrophil-mediated clearance of infection. The results of this study support the hypothesis and provide new insight into immunological mechanisms that can offer protection against infection after burn injury, as well as increase understanding of the role played by interactions between DCs and neutrophils in the immune response to infection.
Materials and Methods
Mice
All animal procedures were consistent with the National Institutes of Health guidelines for the care and use of experimental animals, and were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. A full-thickness scald burn was induced as previously described 21. Briefly, male BALB/c mice, 6–8 weeks of age, were given buprenorphine (0.1 mg/kg) 30 minutes prior to burn injury for preemptive analgesia, then anesthetized with 2.5% isoflurane, and shaved with clippers on the dorsal and lateral surfaces. Mice were placed on their backs and secured in a protective template with an opening corresponding to 35% of the total body surface area. The exposed skin was immersed for 10 seconds in 97°C water. Lactated Ringers (LR) solution (2 ml) was injected i.p. immediately after burn injury for fluid resuscitation. This volume is a slight increase over that suggested by the modified Brooke’s formula (1.75 ml). Sham-injured mice were handled identically except for immersion in water. FL treatments (10 µg in 0.1 ml of LR) were administered once daily by i.p. injection. Control-treated mice received i.p. injections of the same volume of LR solution alone. Recombinant human FL was provided by Amgen (Thousand Oaks, CA). Unless otherwise noted, treatments were started immediately after burn injury. We have found that FL can be started as early as the day of injury or as late as the day of inoculation and provide a similar level of protection against wound infection 22.
Bacterial infection
Pseudomonas aeruginosa was used because it is a common source of wound infections and pneumonia in burn patients. The culture was obtained from American Type Culture Collections (Manassas, VA) (ATCC#19660) and was grown in tryptic soy broth and diluted in sterile saline solution prior to wound inoculation. Unless otherwise noted, mice were inoculated by topical application of a dose that induced approximately 80% mortality; specifically, 8 × 103 cfu applied to the surface of the burn wound at 3 days post-burn or 104 cfu if applied 4 days post-burn. Wounds were inoculated 4 days post-burn to mimic the clinical scenario in which patients typically develop infections several days to two weeks after the initial burn injury 23. This model represents a progressive wound infection that gradually develops by growing within the wound and spreading into the surrounding tissue. Approximately 3 days after inoculation, mice begin displaying symptoms of systemic infection, including positive blood and organ cultures and systemic IL-6, and treatment-associated differences in overall appearance and survival in response to infection become apparent 22,24. To examine bacterial burden, serial dilutions of tissue homogenates were grown on tryptic soy agar overnight to determine CFU per gram of tissue. For survival studies, mice were monitored daily for up to 3 weeks following wound inoculation.
FACS analysis
Spleens and wound draining lymph nodes (axillary and inguinal) were harvested from burned mice 3 days post-burn. Wound-draining lymph nodes were examined for an indication of active immune responses in the wound. Examination of draining lymph nodes as a reflection of immune activity in the tissue has been reported by others 25–28. After full-thickness injury, the wound is composed of mostly necrotic skin with a very small amount of newly-forming skin underlying the eschar. As it is difficult to accurately interpret results obtained from wound sections, measurements using the wound-draining lymph nodes provide a more reproducible indication of immune responses. The spleen and lymph nodes from individual mice were pooled for preparation of single cell suspensions as previously described 29. Leukocytes (106) were incubated with 0.5 µg of antibodies of interest for 30 minutes at 4°C, washed in PBS, and collected by centrifugation at 300 × g for 10 minutes at 4°C. Cells were reconstituted in 250 µl of 1% paraformaldehyde and analyzed by a FACScan flow cytometer (BD Biosciences, San Jose, CA). Specific staining was determined by comparison with appropriate antibody isotype controls, using FlowJo software. Fluorescence-conjugated antibodies were purchased from Caltag laboratories (Carlsbad, CA) and eBiosciences (San Diego, CA).
Neutrophil and DC depletion
To determine the role of neutrophils in natural and FL-mediated resistance to burn wound infections, neutrophils were depleted by i.p. injection of 150 µg of the 1A8 clone of the anti-Gr1/Ly6G antibody. Specifically, mice were treated with FL or LR for 4 days beginning on the day of injury, and wounds were inoculated 4 days post-burn. Anti-Ly6G antibodies were injected prior to wound inoculation. This antibody has been shown to bind specifically to Ly6G and does not cross react with Ly6C, as does the RB6-8C5 clone of Gr1 antibody 30. We have confirmed by flow cytometry that the 1A8 clone depletes neutrophils but not T cells, B cells, monocytes or DCs (not shown). The commercially available preparation contains azide, which was removed using Zebra® desalting columns per manufacturer’s directions.
For transient depletion of total DCs at the start of FL treatment, CD11c-DTR transgenic mice (C.FVB-Tg(Itgax-DTR/GFP)57Lan/J, Jackson Laboratory) were injected i.p. with 4 ng/g diphtheria toxin (DT; Sigma-Aldrich, St. Louis, MO) 1 day after burn injury. FL treatments were initiated 4 hours later and were administered for 3 days. Wounds were inoculated on the fourth day after burn injury. For depletion of DCs and the end of FL treatments, which started 1 day after burn injury and were administered for 3 days, mice were injected with 4 ng/g DT on the fourth day after burn injury, followed by wound inoculation 4 hours later. In this system, DCs are rapidly depleted and begin to gradually recover after 3–4 days 31. Depletion of DCs was confirmed by FACS analysis (not shown). For all experiments shown, tissues and cells were harvested from burn-injured mice.
Cell isolations
DCs and neutrophils were isolated from spleens and lymph nodes of burned mice using Miltenyi magnetic separation columns according to manufacturer’s directions. The use of Miltenyi microbeads to positively select cells has been extensively published and the size and biodegradable composition of these microbeads is such that they do not activate the cells or affect cell viability. Total DCs were positively selected using CD11c microbeads, and neutrophils were selected using Ly6G (1A8) microbeads. Cell enrichment was confirmed by FACS analysis.
Adoptive Transfers
4 × 106 Ly6G-positive neutrophils, harvested from spleens of donor mice, were injected i.p. into recipient mice immediately prior to wound inoculation. We have previously confirmed successful transfer of CFSE-labeled cells after i.p. injection in the spleen and blood of mice and others have reported, following direct comparison of adoptive transfer by i.p. and i.v. injection, no differences in trafficking patterns or levels of tissue reconstitution between these two methods 24,32.
Neutrophil chemotaxis
For in vitro chemotaxis studies, 105 cells were plated in the upper chambers of 24-well transwell filter plates with a filter pore size of 3 µm. For stimulation of chemotaxis, 600 µl of 2X-diluted cell-free supernatants from splenocyte cultures that had been stimulated overnight with 100 ng/ml LPS, was placed in the lower wells, and cells were incubated at 37°C for 3 hours. 7 mM EDTA was added to the bottom wells for 10 minutes to release any adhered cells from the well and filter. Cells from the lower chambers were then stained with Trypan Blue and counted on a hemocytometer. For co-cultures, DCs were isolated and pretreated overnight with FL (50 ng/ml), then washed to remove FL, and were co-cultured with neutrophils (10:1, neutrophils:DCs) overnight. Cells from co-cultures were plated in upper chambers for chemotaxis. Total numbers of migrated cells were counted, then stained with PE-conjugated anti-Ly6G and FITC-conjugated anti-CD11c for analysis by FACS to confirm neutrophil numbers. DC chemotaxis was negligible (less than 1%). All tissues and cells used came from burned mice.
Myeloperoxidase (MPO) measurements
For quantitative measurement, lymph nodes were harvested 3 days post-infection and homogenized in 3ml lysis buffer (200mM NaCl, 5mM EDTA, 10mM Tris, 10% glycerol, 1mM PMSF) per gram of tissue. MPO was measured by ELISA according to manufacturer’s directions (Calbiochem, Spring Valley, CA). For immunohistochemistry, wound-draining lymph nodes were fixed overnight in Streck fixative, processed to paraffin, and cut at 4 µm. Sections were blocked with non-immune serum, and then incubated with antibodies against myeloperoxidase, followed by secondary antibodies. Binding was visualized using DAB-chromagen.
Statistics
GraphPad Prism version 4.00 for Windows was used for all statistical analyses. Two group comparisons were made with an unpaired, two-tailed Student’s t test. Multiple group comparisons were performed using a one-way ANOVA and Tukey-Kramer multiple comparisons test. Survival curves were analyzed by log-rank test.
Results
Both DCs and neutrophils are necessary for resistance to a burn wound infection
We previously reported that FL increases resistance to a burn wound infection, and that resistance can be conferred to non-treated mice by injection of DCs from FL-treated donors. However, injection of NK cells, also increased by FL treatment, and all other leukocytes (non-DC, non-NK) from FL-treated mice, did not confer resistance to infection, indicating that FL-modified DCs can mediate the protective effects of FL 24. We confirmed the role of DCs in FL-mediated resistance to wound infection by using CD11c-DTR mice that can be transiently depleted of DCs by injection of DT. Figure 1A shows that FL significantly increased survival upon wound infection, compared to control, LR treatment. However, in mice depleted of DCs, either at the start of treatment or several days later at the time of infection, FL was no longer protective. We examined bacterial levels in spleen as an indication of systemic dissemination of bacteria from the wound 2 days after inoculation. Only 3 of 6 FL-treated mice had positive cultures, and bacterial levels in those were negligible, whereas all of the LR-treated mice had positive cultures with substantial numbers of bacteria. Similarly, all of the FL-treated mice that were depleted of DCs at the start of, or after, FL treatment had positive cultures (Figure 1B). This supports our earlier reports that FL increases resistance in a DC-dependent manner, but raised the question as to the mechanism of protection, since the predominant function of DCs is to activate other immune cells to eliminate infection.
Figure 1. DCs are necessary for FL to promote survival and control of bacterial dissemination from infected burn wounds.
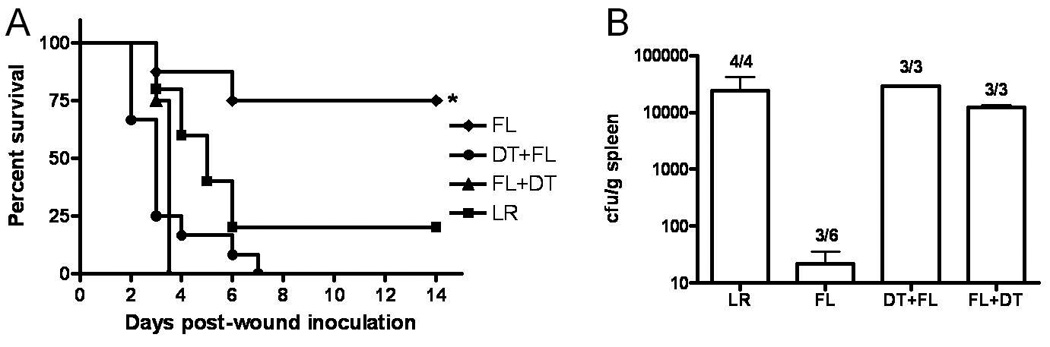
Burned mice were treated with FL (n = 8) or LR (n = 5) for 3 days beginning one day after burn injury. DCs were depleted by injection of DT one day after injury (DT + FL, n = 12) or 4 days after injury (FL + DT, n = 5). Survival was monitored for 14 days, A. *, significantly different from all others, p < 0.05. Spleens were harvested and cultured 3 days after wound inoculation.
Earlier studies implicated innate responses in FL-mediated clearance of burn wound infection 33. We examined the contribution of neutrophils to FL-induced resistance to wound infection by injecting mice with antibodies against Ly6G to deplete FL-treated mice of neutrophils on the day of wound inoculation. Again, FL significantly increased survival compared to LR treatment, but not when neutrophils were depleted at the time of infection (Figure 2A). Neutrophil depletion in burned mice did not induce mortality in the absence of a wound inoculation (not shown).
Figure 2. Neutrophils are required for FL protection following a burn wound infection.

Burned mice were treated with FL or LR as a control treatment for 4 days beginning on the day of injury. Wounds were inoculated 4 days after burn injury with a lethal dose of 8 × 103 CFU P. aeruginosa, n = 6–8 mice/group, A, or sub-lethal dose of 103 CFU, n = 5, B. Mice were injected i.p. with Ly6G antibody on the day of wound inoculation to deplete neutrophils. Survival was monitored for 21 days. *, significantly different from all other groups, p < 0.05.
Furthermore, when burned mice were injected with neutrophils from FL-treated mice on the day of wound infection, survival was increased from 50% in mice that received neutrophils from LR-treated donors, to 90% in mice that received neutrophils from FL-treated donor mice (Fig. 2B). This finding suggests that FL enhances neutrophil-mediated clearance of a lethal burn wound infection.
FL treatment enhances neutrophil responses to a burn wound infection
To examine the effects of FL on neutrophil responses to infection, wound-draining lymph nodes were harvested from FL- or LR-treated mice 3 days following inoculation of burn wounds. Single cell suspensions were stained with antibodies against Ly6G and analyzed by FACS. Following FL treatment, neutrophil numbers were significantly higher (~2X) in the lymph nodes (Fig. 3A). Figure 3A also shows representative sections of wound-draining lymph nodes stained for MPO, another neutrophil marker. MPO is not specific to neutrophils, as it can also be produced by monocytes, but the greater staining in FL-treated mice is consistent with the increase in Ly6G+ cells. Similarly, MPO levels in homogenized lymph nodes were significantly higher following burn wound infection in FL-treated versus LR-treated mice (Fig. 3B).
Figure 3. FL treatment increases MPO levels and neutrophil numbers in the wound-draining lymph nodes.

Burned mice were treated with FL or LR for 4 days beginning on the day of injury. Wounds were inoculated with a lethal dose of P. aeruginosa 3 days after burn injury. Wound-draining lymph nodes were harvested 3 days after wound inoculation and stained for MPO and Ly6G, A. MPO levels in lymph nodes were also measured by ELISA, n = 3 mice/group, measured in triplicate. Data show means +/− SEM. *, significantly different from control, p < 0.05.
To eliminate possible treatment-associated differences in local neutrophil chemoattractants, neutrophils were harvested from FL- or LR-treated burned donor mice and injected into non-treated burned mice prior to inoculation of burn wounds as in figure 2B. Levels of MPO in wound-draining lymph nodes were measured 3 days later. As shown in Figure 4A, neutrophil supplementation alone significantly increased MPO levels in wound-draining lymph nodes. However, MPO levels were significantly higher in mice that received neutrophils from FL-treated, compared to LR-treated, donor mice. Together, these data suggest that FL modifies neutrophils to increase their response to infection.
Figure 4. Adoptive transfer of neutrophils from FL-treated mice increases MPO production in burned infected mice.
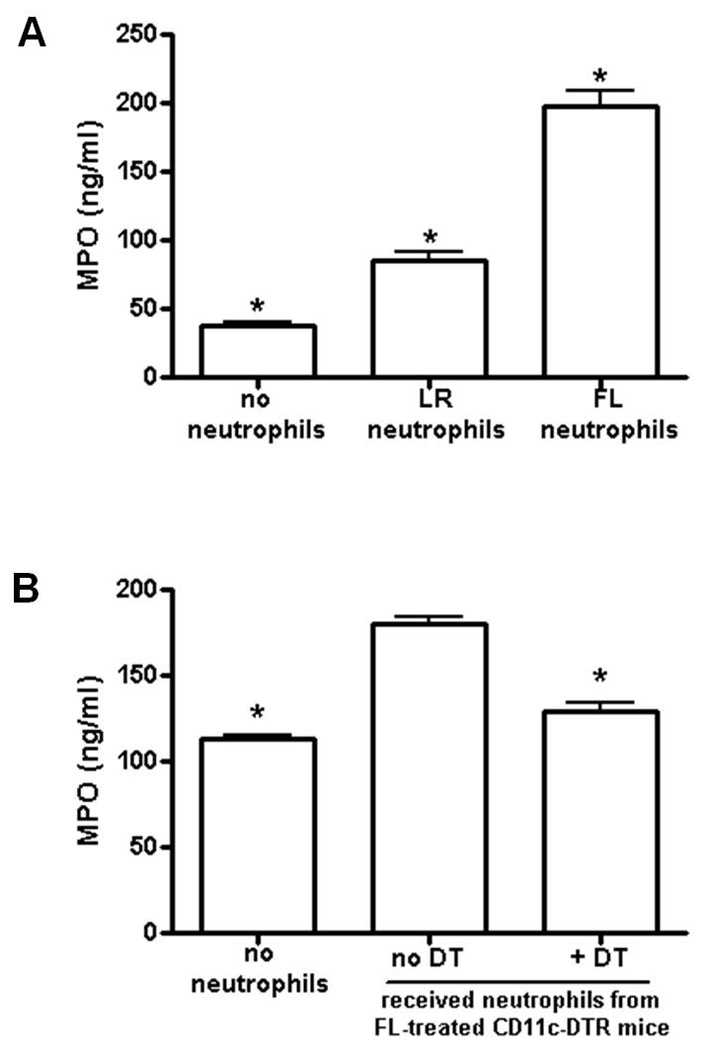
Burned mice received neutrophil adoptive transfer on the day of wound inoculation from wildtype donor FL- or LR-treated mice, A, or from CD11c-DTR FL- or LR-treated mice that had been treated with or without DT at the start of FL treatment to deplete DCs, B. Control mice received injection of PBS in lieu of neutrophils. Wounds were inoculated 4 days post-burn with P. aeruginosa. Wound-draining lymph nodes were harvested 3 days after wound inoculation, and MPO levels were measured by ELISA. Data shown are the means +/− SEM, n = 3 mice/group, measurements made in triplicate. *, significantly different from all other groups, p < 0.001.
Enhanced neutrophil responses to wound infection are DC-dependent
To determine if the effects of FL on neutrophil responses to infection are dependent upon DCs, CD11c-DTR mice were used for transient depletion of DCs during FL treatment as in figure 1A. Neutrophils were harvested from donor mice whose DCs had been depleted or left intact during FL treatment, and injected into mice as in figure 4A. As shown in Figure 4B, if donor neutrophils were harvested from mice that had been depleted of DCs during in vivo FL treatment, lymph node MPO levels were significantly decreased to levels that were similar to those in control mice that did not receive extra neutrophils. The magnitude of the difference in MPO levels between the no neutrophil-control and the neutrophils from FL-treated mice in 4B, while less than that shown in 4A due to natural variability in the live infection model, was statistically significant.
FL enhances in vitro neutrophil chemotaxis
To determine if FL enhances the ability of neutrophils to migrate, in vitro chemotaxis was measured. Specifically, neutrophils were isolated from burned mice 4 days after injury, and chemotaxis across a transwell filter towards cell-free supernatants from LPS-stimulated splenocytes was examined. Significantly more neutrophils (~4.6X) migrated across the filter when they had been harvested from FL-treated, compared to control-treated, mice (Fig. 5A). These data indicate that the neutrophils harvested from FL-treated mice had been modified in vivo such that the ability to migrate was enhanced. To eliminate potential effects from circulating mediators that may influence neutrophil responses to FL in vivo, neutrophils were isolated from cultures of total splenocytes that had been previously pretreated in vitro with or without FL. Neutrophils from FL-treated cultures also showed significantly enhanced chemotaxis (~2X) when compared to neutrophils isolated from control-treated cultures (Fig. 5B).
Figure 5. FL treatment leads to enhanced neutrophil chemotaxis in vitro.

In A, Neutrophils were isolated from the spleens of burned FL-or LR-treated mice and chemotaxis across a filter was measured. In B, total spleen and lymph node leukocytes were harvested from burned non-treated mice and were treated overnight in vitro with FL or LR. Neutrophils were then isolated for chemotaxis assay. In C, DCs were isolated from spleen and lymph node leukocytes and treated overnight with FL or LR in vitro, then co-cultured overnight with neutrophils prior to the chemotaxis assay. Data show means +/− SEM, n = 4, A; 3, B and C. *, significantly different from control, p < 0.05.
We confirmed earlier reports that neutrophils do not express the Flt3-R 14. Flt3-R was not detected on neutrophils, so the effects of FL on neutrophil responses must be mediated via another cell that is Flt3-R+, such as DCs (Fig. 6). Since the neutrophils in figures 5A&B were exposed directly to FL and numerous other cell types in vivo and in splenic cultures, respectively, we examined the ability of DCs to directly modulate neutrophils in vitro. Neutrophils were co-cultured with or without DCs that had been isolated from burned mice 4 days after injury, and pre-treated overnight in vitro with or without FL. Subsequent in vitro chemotaxis of neutrophils that had been co-cultured with control DCs was significantly higher (~1.9X) when compared with neutrophils not co-cultured with DCs. However, co-culture of neutrophils with FL-modified DCs further, and significantly, enhanced subsequent neutrophil chemotaxis (~3.2X) in vitro (Fig. 5C), further suggesting that FL-modified DCs can enhance the ability of neutrophils to migrate.
Figure 6. Flt3-R is expressed on DCs, but not neutrophils.
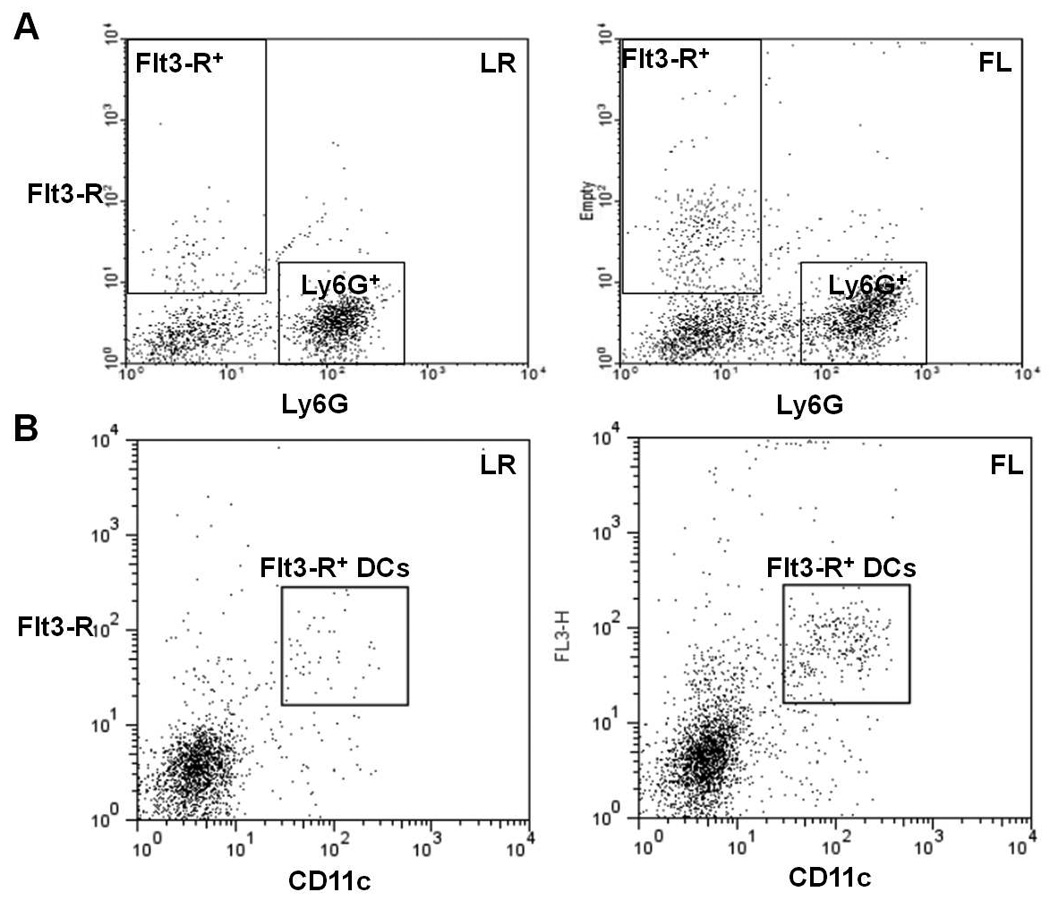
Single cell suspensions were obtained from spleens and wound-draining lymph nodes that were harvested from burned mice that had been treated with FL or LR for 4 days. A shows cells that were stained for CD135 (Flt3-R) and Ly6G. Flt3-R+ cells and Ly6G+ cells are identified in boxes. B shows MHC class II+ cells with high forward scatter, stained for CD135 (Flt3-R) and CD11c. Double positive Flt3-R+CD11c+ cells are identified in boxes. The dot plots are representative of staining observed in at least 3 mice per group, in which greater than 95% of the Ly6G-positive cells are Flt3-R-negative (in both LR and FL groups) and greater than 88% of CD11c-high cells are Flt3-R-positive in the FL group and 85% in LR. One representative of n = 3 shown.
Neutrophils from FL-treated mice promote bacterial clearance in a DC-dependent manner
We have previously reported, and confirm here (Fig. 1B), that treatment of burned mice with FL reduces systemic dissemination of infection following inoculation of burn wounds 22. Since lymph node MPO levels following intraperitoneal injection of neutrophils were greater when neutrophils had come from FL-treated donors, we sought to determine if bacterial clearance was similarly increased. As shown in Figure 7, when mice were injected with neutrophils from burned, LR-treated mice, there was no difference in the proportion of mice that had positive spleen cultures 3 days after wound inoculation when compared to control mice that did not receive extra neutrophils. However, when mice received neutrophils from FL-treated mice, only 1 out of 5 mice examined had a positive culture compared to 4 out of 5 mice that received neutrophils from LR-treated mice or no extra neutrophils at all. Compared to control mice with no neutrophil supplementation, bacterial counts were approximately 5 times lower in the group that received neutrophils from LR-treated mice and approximately 46 times lower in the group receiving neutrophils from FL-treated mice, although the differences at this sample size were not statistically significant. The same trend was seen in the blood (not shown).
Figure 7. FL treatment enhances neutrophil-mediated bacterial clearance in the spleen that is dependent upon DCs.
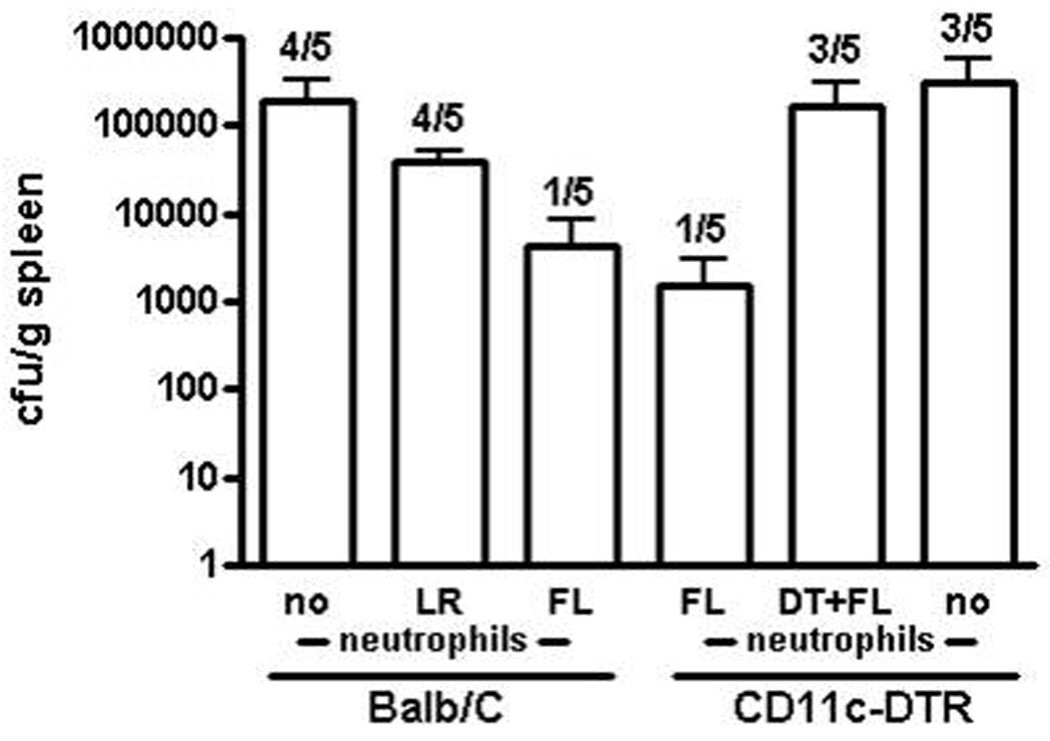
Burned mice received neutrophil adoptive transfer on the day of wound inoculation from wildtype donor FL- or LR-treated mice or from CD11c-DTR FL- or LR-treated mice that had been treated with or without DT at the start of FL treatment to deplete DCs. Control mice received injection of PBS in lieu of neutrophils. Wounds were inoculated 4 days post-burn with P. aeruginosa. Spleens were harvested and cultured 3 days after inoculation. Bacterial counts are shown (CFU/gram of tissue, wet weight) and numbers on graph represent the number of mice with positive spleen cultures of the total number examined. Data show means +/− SEM. n = 5.
When CD11c-DTR mice were used for a similar experiment, injection of neutrophils harvested from FL-treated donor mice again decreased the number of recipient mice with positive cultures to only 1 out of 5 (Fig. 7). However, when DCs were absent during FL treatment of donor mice due to injection of DT at the start of treatment, the number of recipients with positive cultures increased to 3 out of 5, which was the same as in the mice that received no neutrophil supplementation. Bacterial counts were approximately 2 times lower in the group receiving neutrophils from DC-depleted FL-treated mice and approximately 202 times lower in the group receiving neutrophils from FL-treated mice with DCs remaining intact compared with the vehicle control mice that did not receive any neutrophils.
Discussion
The results of this study provide new insight into immunological mechanisms that can provide protection against a burn wound infection. Earlier data have shown that treatment with FL after burn injury improves survival and bacterial clearance in a DC-dependent manner 34,35. While DCs express the Flt3-R, these cells do not play a primary role in bacterial killing. Therefore, we sought to determine a means by which FL-mediated modification of DCs can lead to enhanced resistance to a wound infection.
Innate immune cells, especially neutrophils and DCs, play important roles in responses to cutaneous injury and infection. Langerhans cells are involved in trafficking antigens taken up in the skin to the regional lymph nodes for processing and activation of cell-mediated immunity 8. DCs appear to respond to burn injury by migrating to the site of injury, although their specific functions in this context have not yet been identified. Corneal Langerhans cells have been shown to laterally migrate to a local thermal injury 36. Additionally, DCs localize in the dermis at the border of human burn wounds 37. Our studies suggest that FL treatment enhances natural DC responses to burn injury. Neutrophils are among the first local responders to cutaneous injuries, and are further mobilized in response to infection 11–13. Substantial influxes of neutrophils from the periphery into the site of cutaneous injury have been detected within 2 days of injury 11. Neutrophils are recruited to the skin by inflammatory mediators and also by chemotactic signals generated by bacteria themselves 38. The primary protective response of neutrophils to infection is to phagocytose and kill invading microorganisms. This study shows that both neutrophils and DCs play a vital role in mediating FL-enhanced survival following a burn wound infection (Fig. 1). Protection afforded by FL following a lethal burn wound infection is completely ablated when neutrophils or DCs are depleted (Figure 1&2). However, since neutrophils do not express the Flt3-R, their functions can not be directly affected by FL (Fig. 6). This study suggests that FL interacts with DCs that in turn enhance neutrophil responses to subsequent infection.
The specific cellular mechanisms by which FL-modified DCs promote neutrophil responses in this model of burn wound infection remain to be determined and are currently under investigation. However, other reports of DC-neutrophil interactions suggest some potential mechanisms. Human DCs and neutrophils are able to interact and activate one another through direct cell-cell contact and through production of soluble factors 39,40. DCs and neutrophils can interact through binding of Mac-1 and carcinoembryonic antigen-related cellular adhesion molecule (CEACAM1) on neutrophils to the C-type lectin DC-SIGN (CD209) on DCs 41,42. This binding leads to an increase in the expression of activation markers CD63 and CD64 on neutrophils, an increase in secretion of elastase and MPO, and can also delay neutrophil apoptosis 19. Likewise, upon interaction with neutrophils, DCs show an upregulation of costimulatory molecules CD40 and CD86 16. Furthermore, neutrophils can present antigen to DCs and assist in DC-mediated activation of T-cell responses 17,18.
This study did not look at the effect that neutrophils may have on DC responses, but did demonstrate that FL modification of DCs appears to enhance neutrophil responses to burn wound infection. The lymph nodes that drain the burn wound have an increased presence of Ly6G- and MPO-positive cells, as well as significantly higher levels of tissue MPO (Fig. 3). Increased levels of MPO following FL treatment are indicative of an enhanced immune response, but the source(s) of tissue MPO was not identified. Since monocytes can also produce MPO, enhanced lymph node levels cannot be attributed to neutrophils exclusively. However, considering that injection of neutrophils into recipient mice increases lymph node MPO, bacterial clearance, and survival, and that neutrophils are necessary for FL-mediated resistance to wound infection, it is likely that the significant effects of FL on MPO levels are due to effects on neutrophils. The enhanced responsiveness of neutrophils to a burn wound infection following FL treatment could be explained by several possible scenarios including enhanced chemotaxis of neutrophils into burn wounds and/or prolonged survival of neutrophils following interaction with FL-modified DCs. Because both MAC-1 and CEACAM1 are involved in regulation of apoptosis in neutrophils, it is likely that binding of DC-SIGN on DCs with either of these could affect neutrophil apoptosis 19,43. We did not examine the effects of FL on neutrophil survival in this study, but earlier data showed reduced percentages of leukocytes undergoing early stages of apoptosis following treatment with FL after a burn wound infection 22. Therefore, an effect of FL on neutrophil survival cannot be excluded.
It is also possible that FL treatments may increase the migratory capacity of neutrophils through modulation of chemotaxis-associated molecules or chemotactic factors. Both mature and immature human DCs can influence trafficking and recruitment of neutrophils to inflamed tissues through secreted chemokines 44. Additionally, MAC-1 and CEACAM1 receptors on neutrophils are both adhesion molecules that are involved in neutrophil attachment and rolling along the endothelium during chemotaxis. Thus, interactions of DC-SIGN with these receptors could lead to modifications affecting neutrophil migratory capacity 45,46. Future investigations will focus on the roles of cell-cell contact and secreted factors, and their receptors, on the effects reported herein and identification of specific DC-induced neutrophil alterations that regulate chemotaxis. Although the specific events directing FL and DC effects on neutrophils have not yet been determined, our data do suggest that FL can mediate enhancement of neutrophil chemotaxis both in vivo and in vitro.
Injection of mice with extra neutrophils increases MPO levels in wound-draining lymph nodes, and this result is further enhanced when neutrophils come from FL treated mice (Fig. 4). Since these neutrophils were administered to the peritoneal cavity, greater MPO in the wound-draining lymph nodes suggests that the ability of these neutrophils to migrate to infection is enhanced by FL treatment. However, this effect of FL is significantly decreased if DCs are not present during FL treatment of donor mice due to transient depletion (Fig. 4B). These data suggest a DC-dependent modification of the migratory capacity of neutrophils in vivo after FL treatment. This is further supported by the in vitro chemotaxis studies. By removing neutrophils from the animal and into controlled experimental conditions, potential treatment-associated differences in in vivo chemotactic factors were eliminated. Neutrophils harvested from FL treated mice migrate significantly better in vitro than neutrophils harvested from control treated mice (Fig. 5A). Additionally, ex vivo treatment of total splenocytes with FL also enhances subsequent chemotaxis of isolated neutrophils (Fig. 5B). Importantly, after co-culture with DCs that have been previously treated with or without FL, chemotaxis of neutrophils is significantly greater if DCs were previously exposed to FL (Fig. 5C). Since FL was washed from the DC cultures prior to co-culturing, neutrophils were never directly exposed to FL, so the observed alterations are entirely dependent upon DCs. This is the first report that FL can directly interact with and alter the functions of differentiated DCs. The effects of FL on the production and differentiation of DCs through interactions with progenitor cells have been well characterized, but the effects that FL has on Flt3-R+ DCs are not known. Our data strongly suggest that FL directly modifies DCs, which in turn can enhance the ability of neutrophils to migrate to infection or a chemotactic stimulus.
The described effects of FL on neutrophils are associated with a positive functional outcome in mice with infected burn wounds. Enhanced MPO in wound-draining lymph nodes following intraperitoneal injection of neutrophils from FL-treated donor mice was associated with an apparent decrease in systemic dissemination of bacteria from the burn wound and an increase in survival (Fig. 2&7). Therefore, enhancement of neutrophil-mediated clearance of infection by pharmacological modulation of DCs provides a novel approach for increasing resistance to infections after burn injury.
Acknowledgements
We would like to acknowledge Hal Hawkins, Ph.D. and Robert Cox, Ph.D. from the University of Texas Medical Branch and Shriner’s Hospital for Children, Galveston, Texas for their assistance with immunohistochemistry analysis.
This project was supported by research grants R01 GM072810 from the National Institutes of Health, 8810 from the Shriners Hospitals for Children, and by a Pre-doctoral Trainee Fellowship T32-07254 from the National Institute of Environmental Health Sciences.
Abbreviations
- DC
dendritic cell
- DTR
diphtheria toxin (DT) receptor
- FL
fms-like tyrosine kinase-3 ligand
- Flt3-R; receptor for FL
fms-like tyrosine kinase-3
- LR
Lactated Ringer’s solution
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
Reference List
- 1.Rodgers GL, Mortensen J, Fisher MC, Lo A, Cresswell A, Long SS. Predictors of infectious complications after burn injuries in children. Pediatric Infectious Disease Journal. 2000;19:990. doi: 10.1097/00006454-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Appelgren P, Bjornhagen V, Bragderyd K, Jonsson CE, Ransjo U. A prospective study of infections in burn patients. Burns. 2002;28:39. doi: 10.1016/s0305-4179(01)00070-5. [DOI] [PubMed] [Google Scholar]
- 3.Bender BS, Winchurch RA, Thupari JN, Proust JJ, Adler WH, Munster AM. Depressed natural killer cell function in thermally injured adults: successful in vivo and in vitro immunomodulation and the role of endotoxin. Clin. Exp. Immunol. 1988;71:120. [PMC free article] [PubMed] [Google Scholar]
- 4.Klimpel GR, Herndon DN, Fons M, Albrecht T, Asuncion MT, Chin R, Stein MD. Defective NK cell activity following thermal injury. Clin. Exp. Immunol. 1986;66:384. [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann. Surg. 1997;226:450. doi: 10.1097/00000658-199710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogle CK, Johnson C, Guo XL, Ogle JD, Solomkin JS, Alexander JW. Production and release of C3 by cultured monocytes/macrophages isolated from burned, trauma, and septic patients. J. Trauma. 1989;29:189. doi: 10.1097/00005373-198902000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Calum H, Moser C, Jensen PO, Christophersen L, Maling DS, van GM, Bjarnsholt T, Hougen HP, Givskov M, Jacobsen GK, Hoiby N. Thermal injury induces impaired function in polymorphonuclear neutrophil granulocytes and reduced control of burn wound infection. Clin. Exp. Immunol. 2009;156:102. doi: 10.1111/j.1365-2249.2008.03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmi H, Yoshino M, Yamazaki H, Naito M, Iyoda T, Omatsu Y, Shimoyama S, Letterio JJ, Nakabayashi T, Tagaya H, Yamane T, Ogawa M, Nishikawa S, Ryoke K, Inaba K, Hayashi S, Kunisada T. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int. Immunol. 2001;13:695. doi: 10.1093/intimm/13.5.695. [DOI] [PubMed] [Google Scholar]
- 9.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J. Immunol. 2005;174:404. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 10.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J. Immunol. 2008;180:3038. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 11.Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, Liu FT, Isseroff RR, Simon SI. Dynamics of Neutrophil Infiltration during Cutaneous Wound Healing and Infection Using Fluorescence Imaging. J. Invest Dermatol. 2008 doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oncul O, Yildiz S, Gurer US, Yeniiz E, Qyrdedi T, Top C, Gocer P, Akarsu B, Cevikbas A, Cavuslu S. Effect of the function of polymorphonuclear leukocytes and interleukin-1 beta on wound healing in patients with diabetic foot infections. J. Infect. 2007;54:250. doi: 10.1016/j.jinf.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Top C, Yildiz S, Oncul O, Qydedi T, Cevikbas A, Soyogul UG, Cavuslu S. Phagocytic activity of neutrophils improves over the course of therapy of diabetic foot infections. J. Infect. 2007;55:369. doi: 10.1016/j.jinf.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Antonysamy MA, Thomson AW. Flt3 ligand (FL) and its influence on immune reactivity. Cytokine. 2000;12:87. doi: 10.1006/cyto.1999.0540. [DOI] [PubMed] [Google Scholar]
- 15.van Gisbergen KP, Geijtenbeek TB, van KY. Close encounters of neutrophils and DCs. Trends Immunol. 2005;26:626. doi: 10.1016/j.it.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J. Leukoc. Biol. 2006;79:977. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- 17.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J. Immunol. 2003;171:6052. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 18.Morel C, Badell E, Abadie V, Robledo M, Setterblad N, Gluckman JC, Gicquel B, Boudaly S, Winter N. Mycobacterium bovis BCG-infected neutrophils and dendritic cells cooperate to induce specific T cell responses in humans and mice. Eur. J. Immunol. 2008;38:437. doi: 10.1002/eji.200737905. [DOI] [PubMed] [Google Scholar]
- 19.Singer BB, Klaile E, Scheffrahn I, Muller MM, Kammerer R, Reutter W, Obrink B, Lucka L. CEACAM1 (CD66a) mediates delay of spontaneous and Fas ligand-induced apoptosis in granulocytes. Eur. J. Immunol. 2005;35:1949. doi: 10.1002/eji.200425691. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa F, Miyazaki S. New biodefense strategies by neutrophils. Arch. Immunol. Ther. Exp. (Warsz.) 2005;53:226. [PubMed] [Google Scholar]
- 21.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J. Immunol. 2005;174:404. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 22.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J. Immunol. 2008;180:3038. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 23.Fitzwater J, Purdue GF, Hunt JL, O'Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. Journal of Trauma-Injury Infection & Critical Care. 2003;54:959. doi: 10.1097/01.TA.0000029382.26295.AB. [DOI] [PubMed] [Google Scholar]
- 24.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J. Immunol. 2005;174:404. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 25.Baldwin T, Henri S, Curtis J, O'Keeffe M, Vremec D, Shortman K, Handman E. Dendritic cell populations in Leishmania major-infected skin and draining lymph nodes. Infect. Immun. 2004;72:1991. doi: 10.1128/IAI.72.4.1991-2001.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabashima K, Sakata D, Nagamachi M, Miyachi Y, Inaba K, Narumiya S. Prostaglandin E2-EP4 signaling initiates skin immune responses by promoting migration and maturation of Langerhans cells. Nat. Med. 2003;9:744. doi: 10.1038/nm872. [DOI] [PubMed] [Google Scholar]
- 27.Vuylsteke RJ, van Leeuwen PA, Meijer S, Wijnands PG, Statius Muller MG, Busch DH, Scheper RJ, de Gruijl TD. Sampling tumor-draining lymph nodes for phenotypic and functional analysis of dendritic cells and T cells. Am. J. Pathol. 2002;161:19. doi: 10.1016/S0002-9440(10)64152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paveley RA, Aynsley SA, Cook PC, Turner JD, Mountford AP. Fluorescent imaging of antigen released by a skin-invading helminth reveals differential uptake and activation profiles by antigen presenting cells. PLoS. Negl. Trop. Dis. 2009;3:e528. doi: 10.1371/journal.pntd.0000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toliver-Kinsky TE, Lin CY, Herndon DN, Sherwood ER. Stimulation of hematopoiesis by the Fms-like tyrosine kinase 3 ligand restores bacterial induction of Th1 cytokines in thermally injured mice. Infect. Immun. 2003;71:3058. doi: 10.1128/IAI.71.6.3058-3067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008;83:64. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 31.Jung S, Unutmaz D, Wong P, Sano G, De los SK, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eaton KA, Mefford ME. Cure of Helicobacter pylori infection and resolution of gastritis by adoptive transfer of splenocytes in mice. Infect. Immun. 2001;69:1025. doi: 10.1128/IAI.69.2.1025-1031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohannon J, Fang G, Cui W, Sherwood E, Toliver-Kinsky T. Fms-like tyrosine kinase-3 ligand alters antigen-specific responses to infections after severe burn injury. Shock. 2009;32:435. doi: 10.1097/SHK.0b013e31819e2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J. Immunol. 2005;174:404. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 35.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J. Immunol. 2008;180:3038. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 36.Ward BR, Jester JV, Nishibu A, Vishwanath M, Shalhevet D, Kumamoto T, Petroll WM, Cavanagh HD, Takashima A. Local thermal injury elicits immediate dynamic behavioural responses by corneal Langerhans cells. Immunology. 2007;120:556. doi: 10.1111/j.1365-2567.2006.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibran NS, Heimbach DM, Holbrook KA. Immunolocalization of FXIIIa+ dendritic cells in human burn wounds. J. Surg. Res. 1995;59:378. doi: 10.1006/jsre.1995.1179. [DOI] [PubMed] [Google Scholar]
- 38.Molne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 2000;68:6162. doi: 10.1128/iai.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 2000;165:4515. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 40.Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J. Immunol. 1999;162:7369. [PubMed] [Google Scholar]
- 41.van Gisbergen KP, Aarnoudse CA, Meijer GA, Geijtenbeek TB, van KY. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005;65:5935. doi: 10.1158/0008-5472.CAN-04-4140. [DOI] [PubMed] [Google Scholar]
- 42.van Gisbergen KP, Sanchez-Hernandez M, Geijtenbeek TB, van KY. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005;201:1281. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ludwig IS, Geijtenbeek TB, van KY. Two way communication between neutrophils and dendritic cells. Curr. Opin. Pharmacol. 2006;6:408. doi: 10.1016/j.coph.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999;29:1617. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia-Aguilar J, Hibbs ML, Springer TA. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) J. Cell Biol. 1990;111:3129. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klaile E, Muller MM, Kannicht C, Singer BB, Lucka L. CEACAM1 functionally interacts with filamin A and exerts a dual role in the regulation of cell migration. J. Cell Sci. 2005;118:5513. doi: 10.1242/jcs.02660. [DOI] [PubMed] [Google Scholar]


