Abstract
Corticolimbic neurons express neurosteroid biosynthesis, which is altered during anabolic androgenic steroid (AAS) treatment. The brain circuits and neurons that underlie the behavioral deficits found after AAS treatment remain undefined. We studied the effects of testosterone propionate (testosterone) on fear conditioning responses and in primary output corticolimbic neurons on 5α-reductase-type-I and 3α-hydroxysteroid-dehydrogenase expression. Testosterone fails to change cued fear responses although it induces excessive contextual fear associated with corticolimbic 5α-reductase-type-I mRNA expression downregulation in the prefrontal cortex, hippocampus, and basolateral amygdala glutamatergic neurons. Increased fear responses are abolished by normalizing corticolimbic allopregnanolone levels with allopregnanolone treatment (8 µmol/kg) or selective brain steroidogenic stimulants, including S-norfluoxetine (1.8 µmol/kg). Agents that increase corticolimbic allopregnanolone levels may be beneficial in treating AAS users.
Keywords: anabolic androgenic steroids, contextual fear conditioning, gamma-aminobutyric acid A receptors, neurosteroids, selective brain steroidogenic stimulants
Introduction
The illegal use of anabolic androgenic steroids (AAS) can result in serious psychological and physical disorders [1–4]. There is evidence that AAS increase aggression in laboratory animals, [5–9], and that females exhibit enhanced susceptibility to the negative side effects of AAS [10,11], yet the molecular mechanisms and neuronal circuitries involved in AAS-induced behavioral symptoms in males and females remain unknown.
In female rodents, AAS promote the onset of stereotypical male behaviors, very likely by remodeling neurotransmission in dimorphic central nervous system circuits [12]. Testosterone, an AAS, elicits territorial aggression in socially isolated female mice and orchiectomized mice to the levels found in socially isolated male mice, which are increased over group housed male mice [7,9]. Testosterone-induced aggression is associated with a decrease in brain expression of the rate-limiting step enzyme in allopregnanolone biosynthesis (i.e. 5α-reductase-type-I) and in brain allopregnanolone content [7,9].
Allopregnanolone acts as a positive modulator of gamma-aminobutyric acid (GABA) action at several GABAA receptor subtypes and neurophysiologically, maintains GABAergic neurotransmission efficacy [13,14]. 5α-reductase and 3α-hydroxysteroid dehydrogenase, allopregnanolone biosynthetic enzymes are highly expressed in primary output neurons and are virtually absent in interneurons and glial cells [15]. Allopregnanolone is produced in key neurons of the corticolimbic circuits that regulate affective and cognitive behaviors and it is conceivable that by decreasing the biosynthesis of corticolimbic allopregnanolone levels, testosterone administration may cause a GABAergic transmission downregulation and thereby elicit altered behavioral responses.
A decrease of allopregnanolone levels in selected neuronal populations of corticolimbic circuits is implicated in the enhanced expression of fear conditioning responses in male mice [16]. We hypothesized that by decreasing the levels of corticolimbic allopregnanolone in female mice, testosterone would enhance fear responses. This study will elucidate (i) the brain circuitry, (ii) the neuronal populations in which allopregnanolone biosynthesis is affected by testosterone treatment, and (iii) how changes in corticolimbic allopregnanolone biosynthesis impact contextual and cued fear responses.
Materials and methods
Animals and drug treatment
Female adult Swiss-Webster mice (Harlan Breeders, Indianapolis, USA), 18–20 g, were housed in groups of five or individually for four weeks and subjected to daily subcutaneous injection of testosterone or vehicle. Fear conditioning tests were performed 24 h after the last dose of testosterone. Allopregnanolone or S-norfluoxetine was given by intraperitoneal injection in saline as 0.1 ml/10 g 30 and 45 min before training tests, respectively. As testosterone treatment blocks cyclicity in the metaestrus, vaginal smears were collected and vehicle-treated groups were matched to the same estrus cycle as testosterone-treated mice at the time of testing. The Internal Review Board at the University of Illinois approved these experiment protocols for Animal Welfare.
Fear conditioning test
Mice were placed in a training chamber and allowed to explore it for 2min. They received an acoustic tone (conditioned stimulus) (30 s, 85 dB) coterminated with an unconditioned stimulus (electric foot shock, 2 s, 0.5mA) [17]. The tone (cue) and the foot shock were repeated three times every 2min. Mice were placed in the contextual cage 24 h after training and freezing behavior was measured for 5min without tone or foot shock presentation. Freezing was defined by the absence of any movement [17].
Locomotor activity
A computerized AccuScan 12 Animal Activity Monitoring System (AccuScan Instruments, Columbus, Ohio, USA) (perspex box 20 × 20 × 20cm surrounded by horizontal and vertical infrared sensor beams) assisted by VERSAMAX software (AccuScan Instruments) was used to monitor locomotion [18].
In-situ hybridization
To visualize 5α-reductase-type-I mRNA, free-floating 16–20 µm coronal sections were incubated for 72 h at 42°C with 50 pmol/ml of antisense oligo probes [15,16]. For 3α-hydroxysteroid dehydrogenase mRNA, adjacent sections were used. The oligo 3′ terminals were labeled with digoxigenin. The in-situ hybridization used the avidin-biotin-peroxidase complex method [15]. The stained cells were visualized using a ×40 lens. Analyses were carried out in comparable areas (100 × 100 µm) under the same optical and illumination conditions. The staining intensity inside each cell body perimeter was measured using SCION IMAGE software (Scion, Frederick, Maryland, USA); the relative optical density measurement for each cell is the mean gray of pixels inside the perimeter of the cell body measured with a 256 gray scale as a reference [16]. For each brain region studied, we plotted the gray scale categories against the percentage of cells (frequency) that fell into each specific category. To visualize neuronal nuclear protein (NeuN), free-floating 16–20µm coronal sections were incubated for 3 days with a mouse anti-NeuN antiserum and 3-3′-diaminobenzidine tetrahydrochloride staining was performed as described [16].
Brain neurosteroids
Extraction, derivatization, and gas chromatography-mass spectrometry analyses of neurosteroids were performed as described [7]. Brain areas were homogenized in 10 volumes of distilled water containing 2–5 fmol/ml [3H]allopregnanolone to monitor the high-performance liquid chromatography retention profile and 1 pmol deuterium-labeled allopregnanolone was used as an internal standard. The supernatants were extracted with ethyl acetate and after lyophilization, they were purified with high-performance liquid chromatography. The fraction containing allopregnanolone was derivatized with heptaflurobutyric acid anhydride before gas chromatography-mass spectrometry analysis.
Statistical analysis
Data are mean ± SEM or SD as indicated. Comparisons were performed by one-way analysis of variance or two-way repeated-measures analysis of variance followed by Bonferroni t-tests with corrections for multiple testing.
Results
5α-reductase mRNA expression is specifically downregulated in glutamatergic corticolimbic neurons
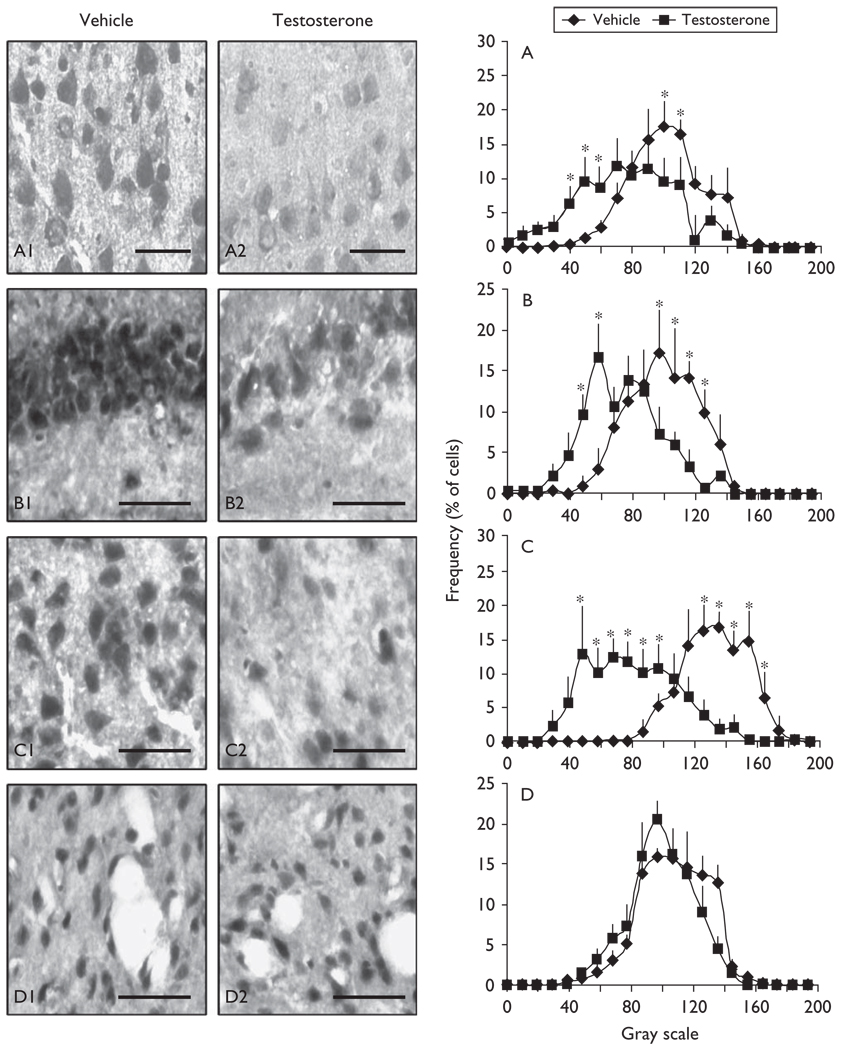
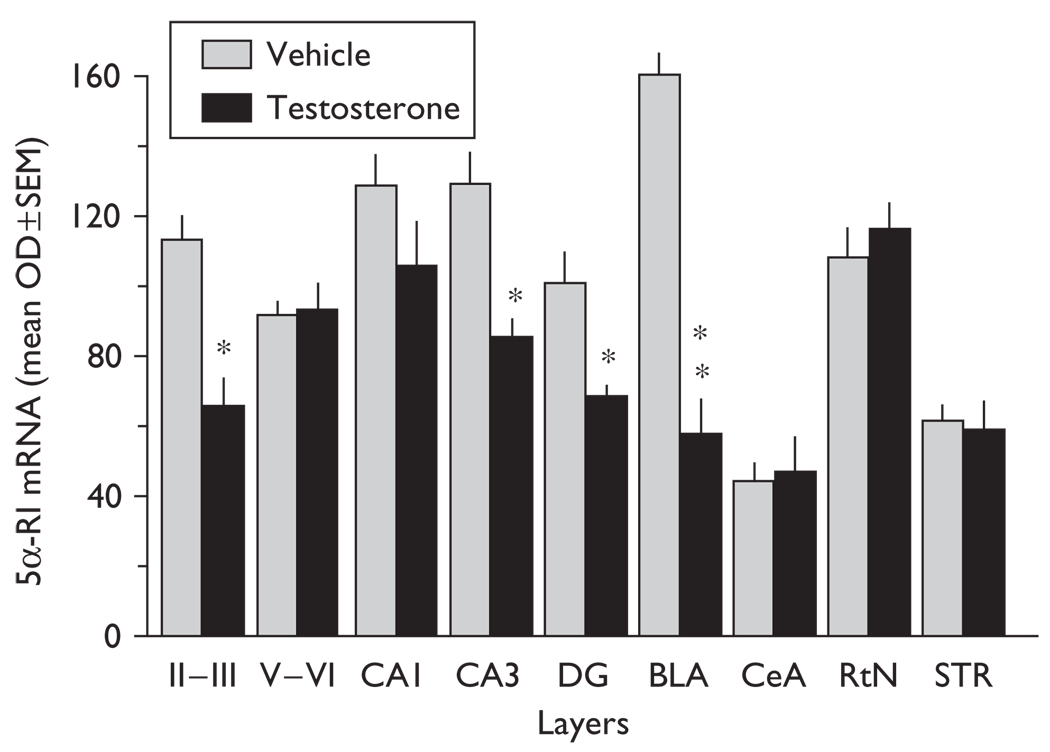
After testosterone treatment, the intensity of the 5α-reductase-type-I mRNA in-situ hybridization signal is specifically decreased in glutamatergic corticolimbic neurons of the frontal cortex, hippocampus, and amygdala but not in GABAergic neurons of the striatum and reticular thalamic nuclei (Figs 1 and 2). The intensity of the 3α-hydroxysteroid dehydrogenase mRNA in-situ hybridization signal seems to be unaffected following testosterone treatment (not shown).
Fig. 1.
5α-reductase-type-I mRNA is specifically downregulated in cortical layers II–III, CA3, and basolateral amygdala glutamatergic output neurons but not in striatal medium spiny gamma-aminobutyric acid (GABA)ergic neurons of testosterone-treated mice. Photomicrographs of 5α-reductase mRNA in-situ hybridization in cortical layer II–III pyramidal neurons (A1, A2), CA3 pyramidal neurons (B1, B2), basolateral amygdala glutamatergic pyramidal-like neurons (C1, C2), and GABAergic (GAD65/67-positive) medium spiny neurons of the striatum (D1, D2), of vehicle-treated (left panels) and of testosterone-treated (1.45µmol/kg/day/3 weeks) (right panels) mice. Comparision of the densitometric (gray scale) distribution profiles of the 5α-reductase mRNA in-situ hybridization signal in cortical layers II–III (A), CA3 (B), basolateral amygdala (C), and striatum (D), of vehicle-treated and testosterone-treated mice. Coronal sections correspond to 1.9–1.4mm anterior to the bregma (cortical layers II–III); −2.46mm to the bregma (CA3); −1.58mm to bregma (basolateral amygdala); +1.10mm to the bregma (striatum). For densitometric distribution profiles, each point is the mean ± SD of four to five mice. Two-way repeated-measure analysis of variance followed by Bonferroni t-tests adjusted for multiple-comparison test revealed a significant effect of treatment [A: F(1,167)=8.646, P<0.001; B: F(1,125)=10.117, P<0.001; C: F(1,167)=4.635, P<0.001; *P<0.01 with vehicle-treated]. (Scale bars, 50µm).
Fig. 2.
In-situ hybridization signals for 5α-reductase mRNA expression in several corticolimbic areas of vehicle-treated and testosterone-treated (1.45µmol/kg/day/3 weeks) female mice (for layers II–III, t8=3.126; CA3, t8=2.948; DG, t8=2.981; BLA, t8=5.657; Bonferroni t-test, *P<0.05; **P<0.01 with vehicle-treated mice). 5α-RI, 5α-reductase-type-I; BLA, basolateral amygdala pyramidal-like neurons; CA1, CA3, CA1, CA3 hippocampal pyramidal layer; CeA, central amygdala medium spiny neurons; DG, dentate gyrus granular cells; layer II–III and V–VI, cortical pyramidal neurons; OD, optical density; RtN, reticular thalamic nucleus neurons; STR, striatum medium spiny neurons. Mean ± SEM, n=5 mice.
Frontal cortex
In the frontal cortex, including the cingulate, prelimbic, and infralimbic cortices, the intensity of 5α-reductase mRNA staining is specifically decreased in layer II/III (Figs 1 and 2) but not in layer V/VI pyramidal neurons (Figs 1 and 2).
Hippocampal formation
The intensity of the 5α-reductase mRNA in-situ hybridization signals is decreased by ~35% in CA3 glutamatergic pyramidal neurons and in glutamatergic dentate gyrus granule cells (Figs 1 and 2) but fails to change in CA1 glutamatergic pyramidal neurons (Figs 1 and 2).
Amygdaloid nuclei
5α-reductase mRNA expression levels are decreased (~70%) in glutamatergic pyramidal-like neurons of the basolateral amygdala but not in the central amygdala (Figs 1 and 2).
Thalamus
Interestingly, 5α-reductase mRNA expression in GABAergic output neurons in the reticular thalamic nucleus (Fig. 2) and in the striatum (Figs 1 and 2) failed to change.
Neuronal counts
We used a specific marker for NeuN to perform neuronal counts in the CA3, dentate gyrus, and basolateral amygdala. There were no differences in the number of positive NeuN neurons in testosterone-versus vehicle-treated mice [mean (number of neurons/mm2) ±SEM: dentate gyrus: 2052 ± 193 (vehicle), 2182 ± 93 (testosterone); CA3: 448 ± 35 (vehicle), 405 ± 41 (testosterone); basolateral amygdala: 329 ± 53 (vehicle), 302 ± 25 (testosterone)].
Enhanced contextual fear responses induced by testosterone are abolished by drugs that stimulate neurosteroidogenesis
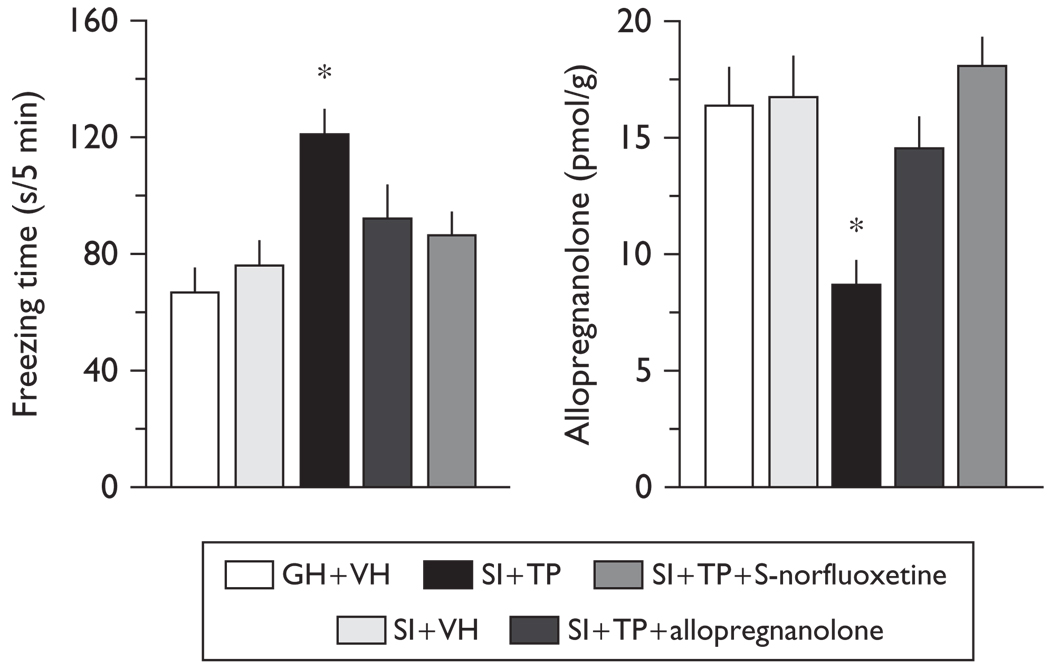
Testosterone treatment induces a 50% increase in conditioned fear responses (Fig. 3). Importantly, no differences in freezing were recorded during the training session [mean (freezing time, s/8 min) ±SEM of five mice: vehicle-treated, 35.0 ± 5.3, testosterone-treated, 46.0 ± 14.2].
Fig. 3.
Excessive contextual fear responses in testosterone-treated mice are reversed by administering allopregnanolone (8 µmol/kg, intraperitoneal) or S-norfluoxetine (1.8 µmol/kg, intraperitoneal) (left) at doses that normalize endogenous medial frontal cortical levels of allopregnanolone (right). One-way analysis of variance followed by Bonferroni t-tests revealed a significant effect of treatment for contextual fear responses (left), [F(1,19)=6.336, P=0.005; socially isolated testosterone-treated (SI+TP), t8=3.968, *P=0.007 with vehicle-treated (SI+VH)]; and for allopregnanolone levels (right), [F(1,19)=4.818, P=0.014; socially isolated testosterone-treated (SI + TP), t8=3.054, *P=0.018 with vehicle-treated (SI + VH)]. Mean ± SEM in five to six mice. GH, grouped-housed.
A single dose of allopregnanolone (8 µmol/kg) or S-norfluoxetine (1.8 µmol/kg) at concentrations that normalize the frontal cortex levels of allopregnanolone abolishes the increased fear responses in testosterone-treated mice (Fig. 3). This dose of allopregnanolone or S-norfluoxetine fails to change the contextual fear conditioning responses of untreated female mice [mean (freezing time, s/5 min) ±SEM of five mice: vehicle-treated, 82.3 ± 5.8, allopregnanolone-treated, 96.1 ± 9.2, S-norfluoxetine-treated, 88.7 ± 11.4]. Cued fear conditioning responses did not vary in the group receiving testosterone (not shown). There were no changes in the locomotor activity of mice treated with testosterone (horizontal activity counts, mean ± SEM; vehicle-treated, 4223 ± 362; testosterone-treated, 4098 ± 431).
Discussion
Testosterone treatment induces excessive contextual fear although it fails to change cued fear responses in mice. The increased contextual fear might be associated with a corticolimbic 5α-reductase expression downregulation in selected subtypes of glutamatergic neurons; the basolateral amygdala exhibits the largest decrease of 5α-reductase mRNA expression in pyramidal-like neurons, followed by pyramidal CA3 neurons and dentate gyrus granular cells and the pyramidal neurons of layers II/III in the medial prefrontal cortex. As 5α-reductase is the rate-limiting step enzyme in neurosteroid biosynthesis [19], the AAS-induced decrease of 5α-reductase expression is likely responsible for the decrease of allopregnanolone levels found in these brain areas [7].
The increase in the contextual fear response can be abolished by treatment with drugs that normalize the corticolimbic levels of allopregnanolone (Fig. 3). These results seem to be independent of changes in locomotor activity or of testosterone-induced changes in stimulus perception, shown by the similar freezing time during training sessions. As the training test was performed at least 24 h after the last testosterone dose, the direct effects of testosterone or metabolites do not contribute to the excessive fear response of these mice.
It is widely accepted that contextual fear responses are largely controlled by hippocampus and amygdala function and the interconnections of the amygdala nuclei with several main corticolimbic structures, including the infralimbic prefrontal cortex [20–22]. Thus, a reduction of the allopregnanolone content in selected glutamatergic neuronal populations of the somatosensory frontal cortex, CA3, the dentate gyrus, and basolateral amygdala could impair the function of cortico-hippocampal-amygdaloid circuits and explain the excessive contextual fear and heightened aggression observed in AAS-treated animals (Fig. 3 and [5,9]). The finding that protracted treatment with testosterone fails to change the cued responses may suggest that the fear responses in these mice are triggered by changes in allopregnanolone biosynthesis that occur primarily in hippocampal neurons. An exaggerated fear response to the context is generally regarded as an indication of impairment in cortico-limbic neurotransmission, which results in dysfunctional behavior, including anxiety disorders, altered emotions, impulsivity, and posttraumatic stress disorder [22,23].
It can be speculated that the inability to regulate emotional responses and therefore, the irrational behaviors, exaggerated fear, and impulsive aggression that often occur in testosterone abusers could be the result of a testosterone-mediated GABAergic neurotransmission deficit. As allopregnanolone is a positive modulator of the action of GABA at a variety of GABAA receptor subtypes [13,14,24], testosterone-induced allopregnanolone content downregulation may affect the fine tuning of strategically positioned corticolimbic GABAA receptors and thereby give rise to an excessive excitatory outflow.
As a proof of concept, using systemic administration of allopregnanolone or selective brain steroidogenic stimulants, such as S-norfluoxetine, to increase corticolimbic allopregnanolone content but at doses ~10 times lower than the dose inhibiting serotonin reuptake [25], we have demonstrated a reversal of the excessive fear responses of testosterone-treated mice (Fig. 3). This finding further supports the hypothesis that by facilitating GABAergic transmission, brain allopregnanolone levels regulate contextual fear responses. Thus, neurosteroidogenic drugs related to fluoxetine may provide a reliable therapy for the behavioral deficits induced by testosterone.
Understanding the molecular mechanisms underlying testosterone-induced exaggerated contextual fear expression is crucial in the development of therapeutics for the treatment of psychiatric disorders, including irrational fear, impulsivity, and posttraumatic stress disorder.
Conclusion
AAS treatment in mice may generate exaggerated contextual fear responses, likely by decreasing the allopregnanolone-mediated positive modulation of GABAA receptors located in selected corticolimbic glutamatergic neurons. Our results suggest that selective brain steroidogenic stimulants may offer a safe therapy for behavioral deficits resulting from AAS use.
Acknowledgement
This study is supported by CRB Award 2-611185 (to G.P.).
References
- 1.Gruber AJ, Pope HG., Jr Psychiatric and medical effects of anabolic–androgenic steroid use in women. Psychother Psychosom. 2000;69:19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- 2.Pearson HA. Dangerous elixir? Nature. 2004;431:500–501. doi: 10.1038/431500a. [DOI] [PubMed] [Google Scholar]
- 3.Irvin LM, Wall M, Neumark-Sztainer D, Story MN. Steroid using among adolescents: finding from project EAT. J Adolesc Health. 2002;30:243–252. doi: 10.1016/s1054-139x(01)00414-1. [DOI] [PubMed] [Google Scholar]
- 4.Sjöqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371:1872–1882. doi: 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- 5.Grimes JM, Melloni RH., Jr Prolonged alterations in the serotonin neural system following the cessation of adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behav Neurosci. 2006;120:1242–1251. doi: 10.1037/0735-7044.120.6.1242. [DOI] [PubMed] [Google Scholar]
- 6.Ricci LA, Grimes JM, Melloni RH., Jr Lasting changes in neuronal activation patterns in select forebrain regions of aggressive, adolescent anabolic/androgenic steroid-treated hamsters. Behav Brain Res. 2007;176:344–352. doi: 10.1016/j.bbr.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinna G, Costa E, Guidotti A. Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc Natl Acad Sci USA. 2005;102:2135–2140. doi: 10.1073/pnas.0409643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGinnis MY, Lumia AR, Breuer ME, Possidente B. Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids. Horm Behav. 2002;41:101–110. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- 9.Pibiri F, Nelson M, Carboni G, Pinna G. Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. Neuroreport. 2006;17:1537–1541. doi: 10.1097/01.wnr.0000234752.03808.b2. [DOI] [PubMed] [Google Scholar]
- 10.Clark AS, Costine BA, Jones BL, Kelton-Rehkopf MC, Meerts SH, Nutbrown-Greene LL, et al. Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions. Brain Res. 2006;1126:122–138. doi: 10.1016/j.brainres.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre KL, Porter DM, Henderson LP. Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABAA receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43:634–645. doi: 10.1016/s0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 12.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation on the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 13.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 14.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, et al. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 15.Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Downregulation of 5α-reductase type I mRNA expression in cortico-limbic glutamatergic neurons in socially-isolated mice. Proc Natl Acad Sci USA. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased allopregnanolone content during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci USA. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinna G, Galici R, Schneider HH, Stephens DN, Turski L. Alprazolam dependence prevented by substituting with the beta-carboline abecarnil. Proc Natl Acad Sci USA. 1997;94:2719–2723. doi: 10.1073/pnas.94.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong E, Matsumoto K, Uzunova V, Sugaya I, Costa E, Guidotti A. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 21.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–605. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 22.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research – past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Hosie AM, Wilkins ME, Da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 25.Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30. doi: 10.1016/j.coph.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]