Abstract
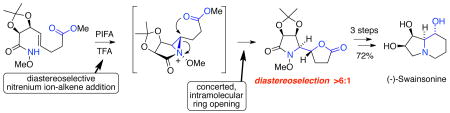
The total synthesis of (-)-swainsonine from 2,3-O-isopropylidene-D-erythrose in 12 steps and an overall yield of 28% is reported. The pivotal transformation in our route to this indolizidine alkaloid is the formation of the pyrrolidine ring and C-8a/8 stereodiad through the diastereoselective, bis-cyclofunctionalization of an γ,δ-unsaturated O-alkyl hydroxamate. This transformation is believed to proceed via the intramolecular capture of an N-acyl-N-alkoxyaziridinium ion generated by the diastereoselective addition of a singlet acylnitrenium ion to the pendant alkene.
(-)-Swainsonine (1), an alkaloid isolated first from the fungal plant pathogen Rhizoctonia leguminicola1 and subsequently from a range of other sources,2 has become one of the most keenly studied of all naturally occurring azasugars (Scheme 1). Interest in this indolizidine stems principally from its role as an inhibitor of Golgi α-mannosidase II (GMII)3,4 and, to a lesser extent, lysosomal α-mannosidase (LM).5 While the latter enzyme is involved in the catabolism of N-linked oligosaccharides, GMII plays a key role in the biosynthesis of glycoproteins, specifically in the formation of their trimannose core.6 Since alterations in the normal glycosylation patterns of such proteins are often found on the surface of tumor cells and are associated with metathesis and disease progression, inhibition of GMII offers potential as a cancer treatment. In this context, swainsonine is notable as being the first glycoprotein processing inhibitor selected for clinical evaluation.7 Most recently, 1 has also been found to be a strain-selective inhibitor of infectious mammalian prions,8 which are associated with a variety of neurodegenerative disorders including Creutzfeldt-Jakob disease and kuru.9 Although the mechanism of action in this case awaits clarification, swainsonine's activity does not appear to be associated its glycosidase inhibitory properties.
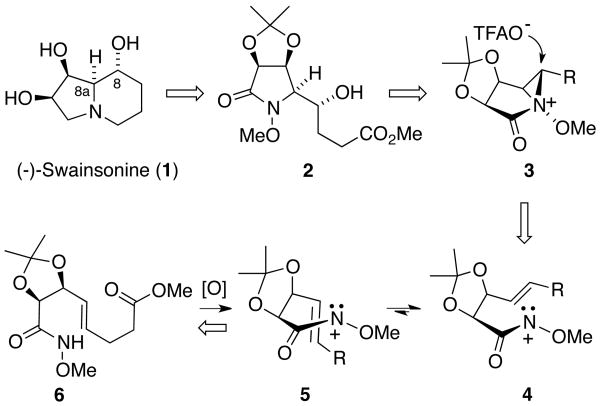
Scheme 1. Retrosynthetic Analysis of (-)-Swainsonine (1).
Since its isolation, swainsonine has proven a highly popular target of both total10 and formal syntheses.11,12 Furthermore, the search for more potent glycosidase inhibitors, which display improved GMII vs. LM selectivity has spurred the preparation of a large number of analogs.13 That swainsonine has recently been the subject of a process research study, further underscores the continued relevance of this natural product as a synthetic target.11n
As part our ongoing study of the chemistry of nitrenium ions,14 we recently reported a versatile method for the preparation of α-hydroxyalkyl lactams involving the intramolecular addition of acylnitrenium ions to alkenes.15 Having successfully utilized this methodology in the stereocontrolled synthesis of the piperidine core of the azasugar castanospermine,16 we were prompted to consider whether this chemistry might also be brought to bear on (-)-swainsonine. Herein we report the successful development of an efficient route to this indolizidine alkaloid, in which the pyrrolidine ring and C-8a/8 threo stereodiad of the target are simultaneously established in a substrate-controlled nitrenium ion oxamidation reaction. Furthermore, we demonstrate that interception of the putative bicyclic aziridinium ion in this key transformation by a proximate carboxylate group offers a novel means of alkene bis-cyclofunctionalization.
From a retrosynthetic perspective, we initially envisioned that the indolizidine skeleton of 1 could be generated from α-hydroxyalkyl lactam 2 through a sequence of functional group reductions and N-alkylative ring closure (Scheme 1). In turn, this compound would be accessed through the intramolecular oxamidation of unsaturated hydroxamate 6. On the basis of our earlier studies,17 we anticipated that this reaction would proceed via aziridinium ion 3, which upon regioselective ion-pair collapse at the external (α) position18 and hydrolysis of the resulting trifluoroacetate ester adduct would provide δ-lactam 2, thereby establishing the C-8/8a stereodiad of the natural product. With regard to the diastereoselectivity of the addition process, we anticipated that cyclization of the singlet nitrenium ion generated from 6 would preferentially proceed via a transition state resembling pseudo-chair 4, thereby avoiding the 1,3-allylic strain19 present in boat-like conformer 5.20
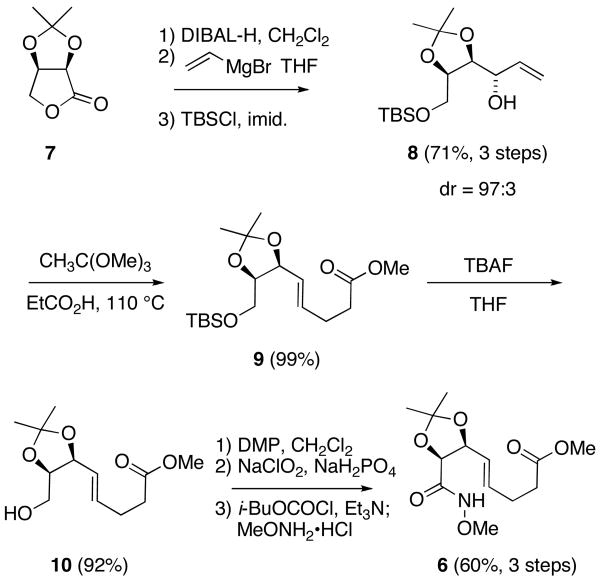
Our route to (-)-swainsonine (1) began from 2,3-O-isopropylidene-D-erythronolactone (7), which is readily available from the oxidative cleavage of sodium D-isoascorbate with hydrogen peroxide (Scheme 2).21 Following a sequence of reactions developed by Pearson and Hembre during their synthesis of 1,10c reduction of 7 with DIBAL-H yielded the corresponding D-erythrose derivative,22 which through stereoselective addition of vinylmagnesium bromide23 and selective O-silylation of the primary alcohol, was converted to allylic 8 alcohol is excellent overall yield. Upon heating with trimethyl orthoacetate in the presence of propionic acid, compound 8 underwent Johnson-Claisen rearrangement to provide β,γ-unsaturated ester 9 as the E-isomer.24
Scheme 2.
Preparation of Oxamidation Substrate 6.
Removal of the TBS ether from 9 using TBAF provided primary alcohol 10, which through a sequence of Dess-Martin and Pinnick oxidations was transformed to the corresponding carboxylic acid. Conversion of this material to methyl hydroxamate 6 was accomplished by treatment with isobutyl chloroformate, to generate the corresponding mixed anhydride, and coupling with methoxylamine.
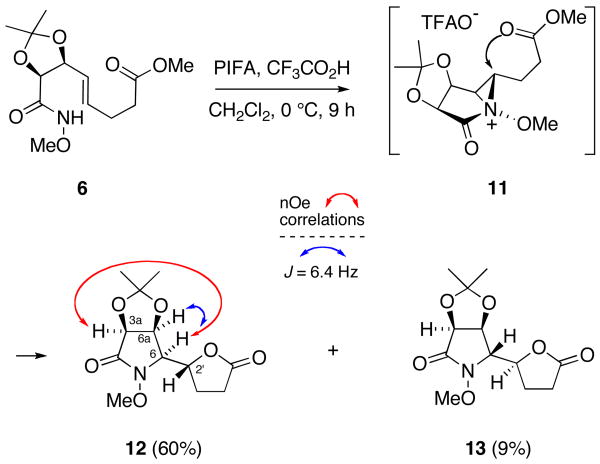
Employing our previously reported oxamidation conditions,15 cyclization of substrate 6 was now accomplished by treatment with phenyliodine(III) bis(trifluoroacetate) (PIFA, 1.2 equiv) and trifluoroacetic acid (1 equiv) in methylene chloride at 0 °C (Scheme 3). After 9 h, the reaction was simply concentrated to provide a 6:1 mixture of bis-cyclization products 12 and 13. Notably, no products arising from alkene trifluoroacetoxyamidation were observed in this mixture suggesting that interception of aziridinium ion 11 (in the case of product 12) by the proximate carboxylate group occurs significantly faster than ion pair collapse.25 Fortuitously, separation of 12 from unwanted diastereomer 13 was readily accomplished by flash chromatography on silica gel.
Scheme 3.
Bis(cyclofunctionalization) of Substrate 6.
The relative stereochemistry at the C6 position of the cyclization products was established by nOe analysis. In the case of compound 12, the 2D NOESY spectrum displayed a strong correlation between H-6 and H-3a, which was conspicuously absent in the spectrum of 13 (Scheme 3). Further confirmation of the desired cis-cis stereochemistry of the lactam ring in 12 was gleaned from the vicinal coupling constant between H6 and H6a (6.4 Hz), which is diagnostic of the cis relationship between these protons.26 At this point, assignment of the relative C2′ stereochemistry in 12 was made difficult by the conformational freedom of the C6-C2′ bond, although was assumed to be R in view of the stereospecificity of the oxamidation process with respect to alkene geometry.15
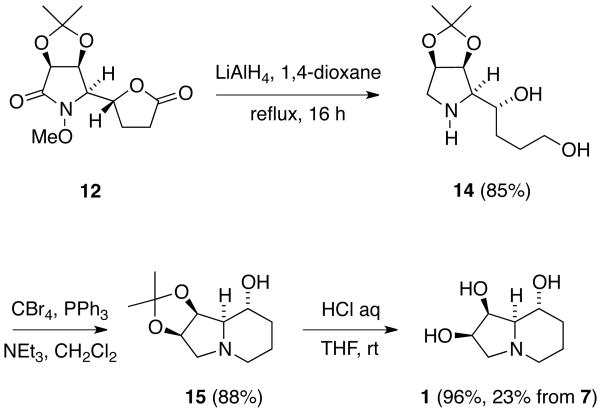
In order to progress towards swainsonine, we now sought to globally reduce 12 to gain compound 14 (Scheme 4). While treatment of 12 with excess LiAlH4 in THF heated at reflux led to lactam and lactone reduction, cleavage of the N-O bond under these conditions proved to be rather sluggish.27 Fortunately, reaction of 12 with LiAlH4 at higher temperature, in 1,4-dioxane at reflux (b.p. 101 °C), proved to be more fruitful and lead to the reduction of all three functional groups and formation of 1-amino-2,5-diol 14 in excellent yield.
Scheme 4.
Total Synthesis of (-)-Swainsonine (1).
Formation of the indolizidine ring system was now accomplished through use of an Appel reaction.28 Thus, selective bromination of the primary alcohol in 14 was accompanied by spontaneous cyclization to generate 15. Finally, removal of the acetonide group, using 6 M HCl,10c provided (-)-swainsonine (1), after purification by ion exchange chromatography. The 1H and 13C NMR spectral data obtained from this material were identical to those reported for the natural product.1b In addition, the optical rotation of synthetic 1 ([α]24D -86.0; c 0.1, MeOH) closely matched that of the natural product ([α]25D -87.2; c 2.1, MeOH).1b
In conclusion, we have developed a 12-step, azide-free synthesis of (-)-swainsonine (1) in which the C-8/8a stereodiad and pyrrolidine ring of the target are simultaneously established through the diastereoselective bis-cyclization of an unsaturated O-alkyl hydroxamate. Although lost through reduction in our current synthesis, the bis-cyclic lactone-lactam framework accessible through this nitrenium ion cyclization is present in a number of alkaloids, most recognizably in the tuberostemospironine subgroup of the Stemona alkaloid family,29 their biogenetic relatives, the pandamarilactonines,30 and the Securinega alkaloid (-)-norsecurinine.31 Extension of this type of transformation to the preparation of these alkaloids is now in progress.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM-67176) and the University of Illinois at Chicago for financial support of this work.
Footnotes
Supporting Information Available Experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Guengerich FP, DiMari SJ, Broquist HP. J Am Chem Soc. 1973;95:2055. [Google Scholar]; (b) Schneider MJ, Ungemach FS, Broquist HP, Harris TM. Tetrahedron. 1983;39:29. [Google Scholar]
- 2.(a) Colegate SM, Dorling PR, Huxtable CR. Aust J Chem. 1979;32:2257. Swainsona canescens. [Google Scholar]; (b) Molyneux RJ, James LF. Science. 1982;216:190. doi: 10.1126/science.6801763. Astragalus lentiginosus. [DOI] [PubMed] [Google Scholar]; (c) Hino M, Nakayama O, Tsurumi Y, Adachi K, Shibata T, Terano H, Kohsaka M, Aoki H, Imanaka H. J Antibiot. 1985;38:926. doi: 10.7164/antibiotics.38.926. Metarhizium anisoplia. [DOI] [PubMed] [Google Scholar]; (d) Molyneux RJ, McKenzie RA, O'Sullivan BM, Elbein AD. J Nat Prod. 1995;58:878. doi: 10.1021/np50120a009. Ipomoea calobra. [DOI] [PubMed] [Google Scholar]; (e) Braun K, Romero J, Liddell C, Creamer R. Mycol Res. 2003;107:980. doi: 10.1017/s095375620300813x. Undifilum oxytropis. [DOI] [PubMed] [Google Scholar]
- 3.(a) Elbein AD, Solf R, Dorling PR, Vosbeck K. Proc Natl Acad Sci USA. 1981;78:7393. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tulsiani DR, Harris TM, Touster O. J Biol Chem. 1982;257:7936. [PubMed] [Google Scholar]
- 4.For a comprehensive review of the enzyme inhibitory activity of swainsonine, see reference 12c.
- 5.Dorling PR, Huxtable CR, Colegate SM. Biochem J. 1980;191:649. doi: 10.1042/bj1910649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubbard SC, Ivatt RJ. Ann Rev of Biochem. 1981;50:555. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- 7.(a) Goss PE, Baptista J, Fernandes B, Baker M, Dennis JW. Cancer Res. 1994;54:1450. [PubMed] [Google Scholar]; (b) Baptista JA, Goss PE, Nghiem M, Krepinsky JJ, Baker M, Dennis JW. Clin Chem. 1994;40:426. [PubMed] [Google Scholar]; (c) Goss PE, Reid CL, Bailey D, Dennis JW. Clin Cancer Res. 1997;3:1077. [PubMed] [Google Scholar]
- 8.Li JL, Browning S, Mahal S, Oelschlegel AM, Weissmann C. Science. 2010;327:869. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shorter J. Mol Biosyst. 2010;6:1115. doi: 10.1039/c004550k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For selected total syntheses of (-)-swainsonine see: Bashyal BP, Fleet GWJ, Gough MJ, Smith PW. Tetrahedron. 1987;43:3083.Bennett RB, Choi JR, Montgomery WD, Cha JK. J Am Chem Soc. 1989;111:2580.Pearson WH, Hembre EJ. J Org Chem. 1996;61:7217. doi: 10.1021/jo961101b.Mukai C, Sugimoto Yi Y, Miyazawa K, Yamaguchi S, Hanaoka M. J Org Chem. 1998;63:6281. doi: 10.1021/jo980598h.Buschmann N, Ruckert A, Blechert S. J Org Chem. 2002;67:4325. doi: 10.1021/jo025589u.Lindsay K, Pyne SG. J Org Chem. 2002;67:7774. doi: 10.1021/jo025977w.Martin R, Murruzzu C, Pericas MA, Riera A. J Org Chem. 2005;70:2325. doi: 10.1021/jo048172s.Guo H, O'Doherty GA. Org Lett. 2006;8:1609. doi: 10.1021/ol0602811.Ceccon J, Greene A, Poisson J. Org Lett. 2006;8:4739. doi: 10.1021/ol0617751.Au CW, Pyne SG. J Org Chem. 2006;71:7097. doi: 10.1021/jo0610661.Hakansson AE, Van Ameijde J, Horne G, Nash RJ, Wormald MR, Kato A, Besra GS, Gurcha S, Fleet GWJ. Tetrahedron Lett. 2008;49:179.Guo H, O'Doherty GA. Tetrahedron. 2008;64:304. doi: 10.1016/j.tet.2007.10.109.Ajish Kumar KS, Chaudhari VD, Dhavale DD. Org Biomol Chem. 2008;6:703. doi: 10.1039/b712753g.Sharma PK, Shah RN, Carver JP. Org Process Res Dev. 2008;12:831.Kwon H, Park C, Lee S, Youn JH, Kang S. Chem Eur J. 2008;14:1023. doi: 10.1002/chem.200701199.Alam MA, Kumar A, Vankar YD. Eur J Org Chem. 2008:4972.Shi GF, Li JQ, Jiang XP, Cheng Y. Tetrahedron. 2008;64:5005.Tian YS, Joo JE, Kong BS, Pham VT, Lee KY, Ham WH. J Org Chem. 2009;74:3962. doi: 10.1021/jo802800d.Li X, Zhu Z, Duan K, Chen H, Li Z, Li Z, Zhang P. Tetrahedron. 2009;65:2322.Chooprayoon S, Kuhakarn C, Tuchinda P, Reutrakul V, Pohmakotr M. Org Biomol Chem. 2011;9:531. doi: 10.1039/c0ob00388c.
- 11.For recent formal syntheses of (-)-swainsonine see: Déchamps I, Pardo DG, Cossy J. Tetrahedron. 2007;63:9082.Kwon H, Park C, Lee S, Youn JH, Kang S. Chem Eur J. 2008;14:1023. doi: 10.1002/chem.200701199.Bates RW, Dewey MR. Org Lett. 2009;11:3706. doi: 10.1021/ol901094h.Choi HG, Kwon JH, Kim JC, Lee WK, Eum H, Ha HJ. Tetrahedron Lett. 2010;51:3284.Oxenford SJ, Moore SP, Carbone G, Barker G, O'Brien P, Shipton MR, Gilday J, Campos KR. Tetrahedron: Asymmetry. 2010;21:1563.
- 12.For reviews of recent total syntheses of (-)-swainsonine and related glycosidase inhibitors see: López M, Cobo J, Nogueras M. Curr Org Chem. 2008;12:718.Pyne S. Curr Org Synth. 2005;2:39. doi: 10.2174/1570179416666190126100312.El Nemr A. Tetrahedron. 2000;56:8579.
- 13.For recent examples of swainsonine analogs see: Kuntz DA, Nakayama S, Shea K, Hori H, Uto Y, Nagasawa H, Rose DR. ChemBioChem. 2010;11:673. doi: 10.1002/cbic.200900750.Abrams JN, Babu RS, Guo H, Le D, Le J, Osbourn JM, O'Doherty GA. J Org Chem. 2008;73:1935. doi: 10.1021/jo702476q.Bi J, Aggarwal VK. Chem Commun. 2008:120. doi: 10.1039/b713447a.Ajish Kumar KS, Chaudhari VD, Dhavale DD. Org Biomol Chem. 2008;6:703. doi: 10.1039/b712753g.Murray AJ, Parsons PJ, Hitchcock P. Tetrahedron. 2007;63:6485.
- 14.(a) Wardrop DJ, Basak A. Org Lett. 2001;3:1053. doi: 10.1021/ol015626o. [DOI] [PubMed] [Google Scholar]; (b) Wardrop DJ, Zhang WM. Org Lett. 2001;3:2353. doi: 10.1021/ol0161514. [DOI] [PubMed] [Google Scholar]; (c) Wardrop DJ, Zhang WM, Landrie CL. Tetrahedron Lett. 2004;45:4229. [Google Scholar]; (d) Wardrop DJ, Burge MS. Chem Commun. 2004:1230. doi: 10.1039/b403081h. [DOI] [PubMed] [Google Scholar]; (e) Wardrop DJ, Burge MS. J Org Chem. 2005;70:10271. doi: 10.1021/jo051252r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardrop DJ, Bowen EG, Forslund RE, Sussman AD, Weerasekera SL. J Am Chem Soc. 2010;132:1188. doi: 10.1021/ja9069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowen EG, Wardrop DJ. Org Lett. 2010;12:5330. doi: 10.1021/ol102371x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Reference 15; Rudchenko VF, Ignatov SM, Kostyanovsky RG. Chem Commun. 1990:261.Vedejs E, Sano H. Tetrahedron Lett. 1992;33:3261.Hoffman RV, Christophe NB. J Org Chem. 1988;53:4769.
- 18.For regioselective ring opening of a 1-azabicyclo[4.1.0]heptane-based aziridinium ions, see: D'hooghe MD, Vanlangendonck T, Törnroos KW, De Kimpe N. J Org Chem. 2006;71:4678. doi: 10.1021/jo060313y.
- 19.Hoffmann RW. Chem Rev. 1989;89:1841. [Google Scholar]
- 20.For a related conformational made in reference to an intramolecular Huisgen diene-azide cycloaddition see: Hudlicky T, Seoane G, Lovelace TC. J Org Chem. 1988;53:2094.Hudlicky T, Luna H, Price JD, Rulin F. J Org Chem. 1990;55:4683.
- 21.(a) Cohen N, Banner BL, Laurenzano AJ, Carozza L. Organic Syntheses. IV. Wiley & Sons; New York: 1990. pp. 432–435. [Google Scholar]; (b) Dunigan J, Weigel LO. J Org Chem. 1991;56:6225. [Google Scholar]
- 22.Cohen N, Banner BL, Lopresti RJ, Wong F, Rosenberger M, Liu YY, Thorn E, Liebman AA. J Am Chem Soc. 1983;105:3661. [Google Scholar]
- 23.Mekki B, Singh G, Wightman RH. Tetrahedron Lett. 1991;32:5143. [Google Scholar]
- 24.Johnson WS, Werthemann L, Bartlett WR, Brocksom TJ, Li TT, Faulkner DJ, Petersen MR. J Am Chem Soc. 1970;92:741. [Google Scholar]
- 25.For the formation of a related bis-cyclic lactone-lactam via intramolecular capture of an 1-azabicyclo[4.1.0]heptane-based aziridinium ion by a carboxylate group, see: Williams DR, Fromhold MG, Earley JD. Org Lett. 2001;3:2721. doi: 10.1021/ol016336a.
- 26.Comin MJ, Parrish DA, Deschamps JR, Marquez VE. Org Lett. 2006;8:705. doi: 10.1021/ol052886n. [DOI] [PubMed] [Google Scholar]
- 27.For an example of the formation of a N-methoxylamine through the incomplete reduction of an N-methoxy-γ-lactam, see reference 14a.
- 28.Appel R. Angew Chan Int Ed. 1975;14:801. [Google Scholar]
- 29.(a) Pilli R, Rosso G, De Oliveira M. Nat Prod Rep. 2010;27:1908. doi: 10.1039/c005018k. [DOI] [PubMed] [Google Scholar]; (b) Alibes R, Figueredo M. Eur J Org Chem. 2009:2421. [Google Scholar]
- 30.(a) Takayama H, Ichikawa T, Kuwajima T, Kitajima M, Seki H, Aimi N, Nonato MG. J Am Chem Soc. 2000;122:8635. [Google Scholar]; (b) Tan MA, Kitajima M, Kogure N, Nonato MG, Takayama H. Tetrahedron Lett. 2010;51:4143. [Google Scholar]
- 31.(a) Weinreb SM. Nat Prod Rep. 2009;26:758. doi: 10.1039/b902265a. [DOI] [PubMed] [Google Scholar]; (b) Alibés R, Figueredo M. Eur J Org Chem. 2009:2421. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.