Abstract
Objective
To examine the functional connectivity (fc) of hippocampal and selected frontal lobe circuits among patients with traumatic axonal injury (TAI).
Design
Echo-planar and high-resolution T1-weighted images were acquired using 3 Tesla scanners. Regions of interest (ROI) were drawn bilaterally for the hippocampus, anterior cingulate cortex (ACC), and dorsolateral prefrontal cortex (DLPFC), and were used to extract time series data. BOLD data from each ROI were used as reference functions for correlating with all other brain voxels. Interhermispheric fc was assessed for each participant by correlating homologous regions using a Pearson correlation coefficient. Patient functional and neurocognitive outcomes were assessed approximately 6 months post-injury.
Setting
Patients were recruited within days of their injury while in an inpatient traumatic brain injury unit. Imaging and neurocognitive assessments were conducted in an outpatient research facility.
Participants
25 consecutive patients with brain injuries consistent with TAI and acute subcortical white matter abnormalities were studied. Sixteen healthy volunteers of similar age and gender were recruited.
Main Outcome Measures
Interhemispheric fc for each ROI was compared between patients and controls. Spatial patterns of fc were examined for each of the three ROIs. Connectivity measures were examined for associations with functional and neurocognitive outcomes.
Results
Patients showed significantly lower interhemispheric fc for the hippocampus and ACC. Healthy controls demonstrate stronger and more focused fc for hippocampi and ACC, and a more focused recruitment of the default mode network for the DLPFC ROI. The interhemispheric fc for the hippocampus was correlated to delayed recall of verbal information.
Conclusions
Our findings suggest that traumatic axonal injury impacts interhemispheric neural activity, as patients with TAI show disrupted interhemipsheric fc. These results suggest more careful investigation of interhemisheric connectivity is warranted, as it demonstrated a modest association with outcome in chronic TBI.
INTRODUCTION
Traumatic brain injury (TBI) is a major public health problem in modern societies, with an incidence in the United States estimated between 92 and 250 per 100,000 population annually, and approximately 50,000 individuals each year are left with long-term physical and psychological limitations that limit their independence and ability to work.1,2 Diffuse axonal injury, more recently referred to as traumatic axonal injury (TAI), is a common subtype of TBI occurring in most motor vehicle collisions (MVCs) in which deccelerational and rotational forces cause shearing of the brain’s white matter.3 Computed tomography is insensitive to white matter lesions resulting from TAI4–5, but more novel neuroimaging modalities have shown sensitivity toward white matter injury6–9.
Neuroimaging studies have found that integrity of white matter after TAI is correlated with injury severity and outcome.10–17 The neurocognitive impact of TAI has been documented by Kraus et al. 18 who found a reduction in the integrity of various white matter structures was associated with poorer performance on measures of attention, memory, and executive function. It is not yet known whether degree of white matter injury (i.e., structural integrity) is associated with impairment in neuronal (i.e., functional) activity between highly interconnected cortical regions.
Functional connectivity MRI (fcMRI) is technique for analyzing functional MRI (fMRI) data to determine the functional relatedness of selected brain regions. It is based on determining brain regions that demonstrate temporally correlated blood oxygenation level-dependent (BOLD) signal.19,20 This technique demonstrates differential patterns of functional hippocampal connectivity during resting state between patients with Alzheimer’s disease and healthy volunteers, as controls showed diffuse cortical and subcortical connectivity and patients demonstrated reduced connectivity including absence of connectivity with the frontal lobes.21,22 While Alzheimer’s patients demonstrate a reduction in hippocampal functional connectivity, the association between functional connectivity and behavioral measures is not well understood, as the aforementioned studies examined patients documented to have poorer performance on tasks of memory ability than controls, but did not correlate their memory performance to connectivity measures.
The goal of the present study is to examine whether hippocampal and frontal lobe circuits of patients with suspected TAI differ from those of healthy individuals during resting state. Given hippocampal and frontal lobe injuries are common after TAI, 23, 24 and subsequent memory and executive function deficits are frequently reported,25, 26 we hypothesize patients with TAI will demonstrate distinct hippocampal and frontal lobe connectivity patterns and weaker bilateral connectivity than controls. Furthermore, we will examine whether the degree of functional connectivity in these regions correlate with test performance in their respective neurocognitive domains.
METHOD
PARTICIPANTS
Twenty-five patients with TBI were recruited from Parkland Memorial Hospital, Dallas, Texas. Inclusion criteria required that patients: 1) sustain closed head traumatic brain injury through a mechanism consistent with TAI (such as high-speed motor vehicle collision), 2) were at least 16 years old. Exclusion criteria were: 1) preexisting neurologic disorders or prior history of TBI, 2) presence of focal lesions (including contusion, extra-axial hematoma, and/or intraparenchymal hemorrhages) with volume greater than 10ml visible on cranial CT, 3) conditions which may result in abnormal MRI findings and compromise cognitive functions (i.e., prior brain tumor, epilepsy, multiple sclerosis, encephalitis/meningitis, Parkinson’s Alzheimer’s disease/mild cognitive impairment, HIV encephalopathy, vascular malformation and psychiatric disease), 4) prisoners, homeless patients, and pregnant women. All patients demonstrated subcortical white matter lesions visible on T2 FLAIR MRI. Sixteen healthy volunteers of similar age- and gender were recruited as controls. All healthy volunteers had good general health and no known neurocognitive disorders. Informed consent was obtained from all participants or their legally authorized representative.
IMAGE ACQUISITION AND PROCESSING
Functional and anatomical magnetic resonance images were obtained for each participant using either a Siemens Trio 3 Tesla (T) (Siemens AG, Erlangen, Germany) or a General Electric Signa Excite 3T (General Electric Healthcare, Milwaukee, Wisconsin) scanner. A time series of 128 echo-planar image volumes was acquired at 36 axial slice locations throughout the whole brain. Participants were asked to direct their attention to crosshairs projected onto a screen during image acquisition and not think of anything. Each scanner acquired images from normal controls and patients. The Siemens scanner acquired images from seven controls and nine patients, and the GE acquired images from nine controls and sixteen patients. The echo-planar image data acquired by the Siemens scanner were obtained with single-shot gradient-recalled pulse sequence, TR = 2 seconds, echo time = 25 milliseconds, flip angle, 90°; matrix, 64 × 64; field of view (FOV) 210 mm, and 3.5 mm slice thickness). High-resolution T1-weighted structural images acquired by the Siemens scanner were acquired using MP-RAGE with Slice thickness 1.0mm, FOV 240 mm, and TE/TI/TR 4/900/2250 ms, flip angle 9°, NEX 1. Echo-planar image data acquired by the GE scanner were obtained with single-shot gradient-recalled pulse sequence, TR = 2 seconds, echo time = 25 milliseconds, flip angle, 90°; matrix, 64 × 64; field of view 210 mm, and 3.5 mm slice thickness). High-resolution T1-weighted structural images acquired by the GE scanner were acquired using FSPGR with slice thickness 1.3 mm, FOV 240–280 mm, TR/TE 8.0/2.4 ms, flip angle 25°, and NEX 2. Patients’ neuroimaging data were acquired between 6–10 months post-injury, which coincides with the day their outcome evaluations were conducted.
PREPROCESSING OF FUNCTIONAL IMAGING DATA
The images were first converted from DICOM to Analysis of functional NeuroImages (AFNI) readable format. AFNI Software was used for selected preprocessing steps. Slice-time correction was performed to adjust for varying acquisition time for slices. Time series data were corrected for motion and linear drift artifacts. The amount of movement observed on a frame-by-frame basis did not exceed 1 voxel in size for any participant in this study. Given coherence in blood oxygenation level-dependent signal fluctuations occurs at low frequencies, 19, 27, 28 high frequency components were removed prior to analysis of functional connectivity by setting a low-pass filter at 0.12Hz. The signal to noise ratio was then increased by spatially smoothing the data with a 3-dimensional Gaussian tapering function (5 mm full width at half maximum). Functional images were coregistered to high-resolution T1 images for each participant, and masks of regions containing cerebral spinal fluid was created using FSL 4.1.4.29 These masks were used to obtain averaged time series data from regions unlikely to contribute variance of neuronal origin. The time series from these regions were later regressed out from functional data of interest.
SEED REGIONS OF INTEREST
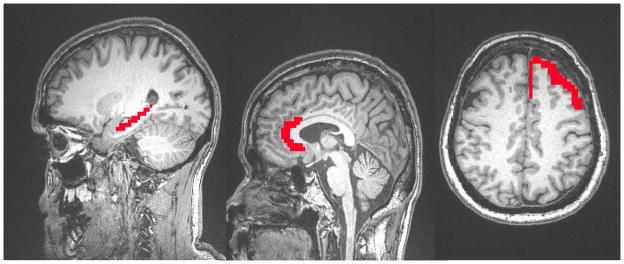
Six anatomical regions of interest (ROI) corresponding to the hippocampus, anterior cingulate, and dorsolateral prefrontal cortex were hand drawn bilaterally using AFNI’s graphical user interface by trained research assistants who used a human brain atlas for reference.30 These ROI’s were used as seed volumes to extract average time series data in subjects’ native space (see Figure 1). Due to low spatial resolution involved in fMRI, seeding the anterior cingulate unilaterally may include signal from both left and right hemispheres and ultimately make interpreting interhemispheric connectivity difficult. The ACC seed ROI was drawn by excluding slices on either side of the midline.
Figure 1.
Location of Reference Seed Regions of Interest.
Note. Location of manually drawn regions of interest. A. Hippocampus. B. Anterior cingulate cortex. C. Left Dorsolateral Prefrontal cortex (radiologic convention).
OUTCOME MEASURES
Functional Outcomes
Functional and neurocognitive outcome was assessed on the same day the neuroimaging scans were obtained (i.e., at least 6 months post-injury). Functional outcome was assessed using the Glasgow Outcome Scale – Extended (GOSE)31. The GOSE is a commonly used structured interview that assesses functional abilities in multiple domains following a head injury. Total GOSE scores range from one to eight, with higher scores associated with better outcome.
Neurocognitive Outcome
Given that information processing speed, learning and memory, and executive function deficits are common after TBI.32–36 Neurocognitive outcome assessments were conducted by a research coordinator who completed standardized training, was supervised by a neuropsychologist, and was blinded to imaging results. Demographically adjusted scores for neurocognitve measures were used when applicable.
Information Processing Speed
Processing speed was assessed using the Trail Making Test A,37 and the Digit Symbol and Symbol Search subtests of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III).38
Learning and Memory
Memory functioning is commonly affected after TBI and was also assessed approximately 6 months post-injury using the California Verbal Learning Test-II (CVLT-II).39 Total items learned across five trials were used to measure learning, while short and long delay free recall trials were used to measure memory.
Executive Functioning
Various neurocognitive tests were used to evaluate executive functions, which are largely influenced by the frontal lobes. Trail Making Test B37 was used to measure patients’ ability to shift mental sets efficiently. The Dodril Stroop Color-Naming condition40 was used to measure ability to selectively attend to meaningful information while inhibiting a pre-potent response. The Controlled Word Association Test (COWAT)41 was used to assess verbal generativity.
STATISTICAL ANALYSES
Demography
Between-group differences in age and interhemispheric functional connectivity was examined using an independent-samples t-test, as these data were suitable for analyzing with parametric tests. Group differences for gender were examined using a chi-squared test for independence.
Functional Connectivity Analyses
Spatial Distribution of Functional Connectivity
For each ROI, the averaged time courses of BOLD signal were used as the seed reference time series for calculating correlation with all other brain voxels’ time series. A false discovery rate (FDR) corrected p <0.05 was considered statistically significant for to reduce the occurrence of multiple comparison-related false positives. The result is a spatial map of correlation coefficients for every voxel in the brain representing an individual’s pattern of functional connectivity with the seed region. Fisher’s Z-transformation was applied to the individual correlation maps to adjust the variance of correlation coefficients for subsequent group level comparisons. The results were then transformed into Talairach space and separate group maps were generated for patient and normal controls. A significant cluster of correlation was defined as a group of at least 200 adjacent voxels. Spatial statistical analyses were conducted using AFNI.
Interhemispheric Connectivity Analysis
Interhemispheric functional connectivity examined synchronicity of BOLD signal fluctuations of bilateral regions over time. This analysis utilized the BOLD data extracted from the ROIs from each participant. Averaged left and right BOLD fluctuations from each ROI across 124 time points (excluded first four frames) were tested for significant associations using a Pearson correlation coefficient. The Fisher’s Z-transformation was applied to the correlation coefficients to allow for group comparisons between patient and normal controls. Between groups t-tests were used to detect significant differences in degree of interhemispheric connectedness between the respective groups for each ROI. Amplitude of BOLD fluctuation was inspected and were not determined to be statistically different between groups.
Outcomes Analyses
Spearman correlations were used to test the association between connectivity measures and functional outcome, as the GOSE is an ordinal measure. Pearson correlations were used to examine associations between connectivity measures and neurocognitive outcome. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS v11.0; SPSS, Chicago, IL).
RESULTS
DEMOGRAPHY
As expected, no differences were found between patients and controls in age M=30, SD=14, and M=37, SD=14, respectively) or gender (80% and 63% male, respectively). Patients sustained traumatic brain injuries ranging in severity from complicated mild to severe, as the mean Glasgow Coma Scale score was of 8 (SD = 5). Patients’ average GOSE scores fell in the upper moderate recovery range (see Table 1). Additionally, their neurocognitive outcomes ranged from mildly impaired to low average, with lowest scores on the Digit Symbol subtest, COWAT, Stroop-Word Reading Condition, and CVLT-II short delay recall.
Table 1.
Participant Characteristics
| NC n = 16 |
TAI n = 25 |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | |
| Age | 37.06 | 14.16 | 36.00 | 30.28 | 13.80 | 24.00 |
| Education* | 15.38 | 2.72 | 16.00 | 12.63 | 3.05 | 12.00 |
| Gender (% male) | 63% | 80% | ||||
| Handedness (% right) | 100% | 96% | ||||
| Ethnicity (% Caucasian) | 81% | 76% | ||||
| GCS | 8.04 | 5.01 | 8.00 | |||
| Days in ICU | 8.28 | 7.68 | 4.00 | |||
| Days in hospital | 13.28 | 10.09 | 12.00 | |||
| Months post-injury to scan | 7.13 | 1.12 | 7.00 | |||
| GOSE | 5.97 | 1.97 | 7.00 | |||
| Digit Symbol T score | 39.00 | 11.01 | 40.00 | |||
| Symbol Search T score | 43.78 | 13.27 | 46.67 | |||
| COWAT T score | 35.47 | 13.36 | 36.00 | |||
| TMT A T score | 42.87 | 19.40 | 43.50 | |||
| TMT B T score | 44.53 | 20.12 | 48.50 | |||
| Stroop Color-Naming T score | 44.97 | 19.72 | 53.50 | |||
| CVLT Total Learning T score | 40.42 | 20.29 | 46.00 | |||
| CVLT Short Delay T score | 38.65 | 19.16 | 45.00 | |||
Note. No significant differences between groups in age, gender, handedness, or ethnicity.
Denotes a significant between group difference in education, p <0.05. NC = Normal Control. TAI = Traumatic Axonal Injury. GCS = Glasgow Coma Scale. ICU = Intensive Care Unit. GOSE = Glasgow Outcome Scale Extended. TMT A= Trail Making Test A, TMT B= Trail Making Test B, COWAT= Controlled Oral Word Association Test, CVLT-II = California Verbal Learning Test – Second Edition.
INTER-SCANNER VARIABILITY
To investigate whether there is significant variability between the two scanners used in the present study, we examined the interhemispheric connectivity measures among controls in a between scanner fashion. Nine controls were scanned using the GE magnet and 7 using the Siemens magnet. The results of two-sample independent t-tests showed all three interhemispheric connectivity measures were similar across scanner (p > 0.05), suggesting the data from the two scanners are comparable.
HIPPOCAMPAL CONNECTIVITY
Connectivity Between Hippocampi
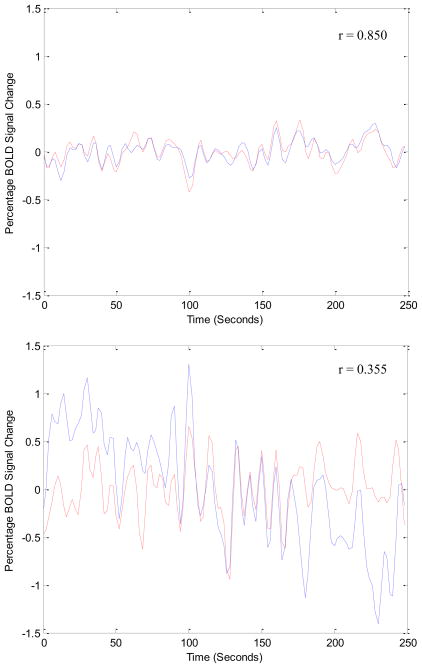
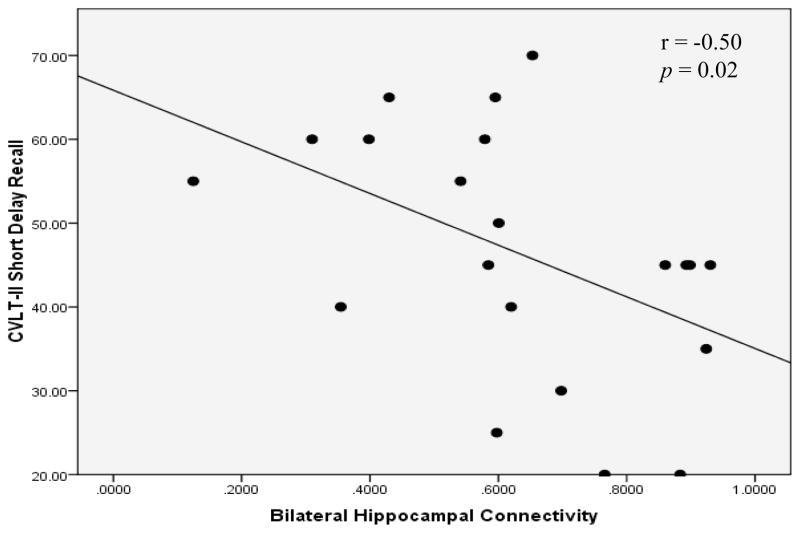
Figure 2 illustrates the fluctuation of BOLD signal in bilateral hippocampi for a representative control relative to a representative patient with TAI. Degree of interhemispheric hippocampal connectivity was significantly greater for controls than for patients with TAI (p = 0.036) (see Table 2). The degree of interhemispheric hippocampal connectivity among patients was negatively correlated to delayed recall of verbal information (see Table 3). A scatterplot of this association is displayed in Figure 3.
Figure 2.
Bilateral Hippocampal BOLD Fluctuations Over Time.
Note. Interhemispheric functional connectivity for left and right hippocampi for a healthy control (above), and for a patient with TAI (below). BOLD signal change is plotted over time. Notice the BOLD signal fluctuates significantly more synchronously in a healthy brain as opposed to an injured brain.
Table 2.
Connectivity between Bilateral Regions of Interest
| Region of Interest | NC Mean (SD) |
TAI Mean (SD) |
p-value |
|---|---|---|---|
| Hippocampus | 0.78 (0.11) | 0.59 (0.26)* | 0.036 |
| Anterior Cingulate Cortex | 0.85 (0.11) | 0.73 (0.19)* | 0.015 |
| Dorsolateral Prefrontal Cortex | 0.49 (0.21) | 0.54 (0.25) | 0.353 |
Note. Average degree of bilateral connectivity for each region of interest for patients and controls.
p < 0.05
Table 3.
Association between Functional Connectivity and Outcome Measures
| Outcome Measure | Interhemispheric Connectivity
|
|||
|---|---|---|---|---|
| Hippo | ACC | DLPFC | ||
| Functional Outcome | GOSE | r = −0.16 | 0.35 | 0.35 |
| p = 0.45 | 0.09 | 0.09 | ||
|
| ||||
| Processing Speed | Digit Symbol T score | −0.27 | 0.07 | −0.06 |
| 0.22 | 0.73 | 0.77 | ||
| Symbol Search T score | 0.02 | −0.10 | 0.04 | |
| 0.93 | 0.66 | 0.87 | ||
| TMT A T score | 0.06 | 0.26 | 0.36 | |
| 0.80 | 0.22 | 0.09 | ||
|
| ||||
| Executive Function | TMT B T score | −0.09 | 0.06 | 0.30 |
| 0.69 | 0.78 | 0.15 | ||
| COWAT T score | −0.28 | −0.35 | 0.04 | |
| 0.20 | 0.10 | 0.85 | ||
| Stroop Color Naming T score | 0.10 | 0.02 | 0.16 | |
| 0.66 | 0.92 | 0.46 | ||
|
| ||||
| Learning & Memory | CVLT-II Total learning T score | −0.34 | −0.25 | −0.10 |
| 0.13 | 0.26 | 0.67 | ||
| CVLT-II Short delay recall T score | −0.50* | −0.04 | −0.04 | |
| 0.02 | 0.86 | 0.86 | ||
Note: GOSE = Glasgow Outcome Scale Extended. TMT A= Trail Making Test A, TMT B= Trail Making Test B, COWAT= Controlled Oral Word Association Test, CVLT-II = California Verbal Learning Test – Second Edition. Hippo = Hippocampus, ACC = Anterior cingulate, DLPFC = Dorsolateral Prefrontal Cortex. Pearson correlations between measures of interhemispheric functional connectivity and neurocognitive outcome. A Spearman correlation was used for the GOSE, as it is an ordinal measure.
p < 0.05.
Figure 3.
Association between Interhemispheric Hippocampal Connectivity and Verbal Memory Outcome.
Note. A Spearman correlation coefficient shows a negative association between interhemispheric hippocampal connectivity and delayed verbal memory. CVLT-II = California Verbal Learning Test-Second Edition.
Whole Brain Connectivity
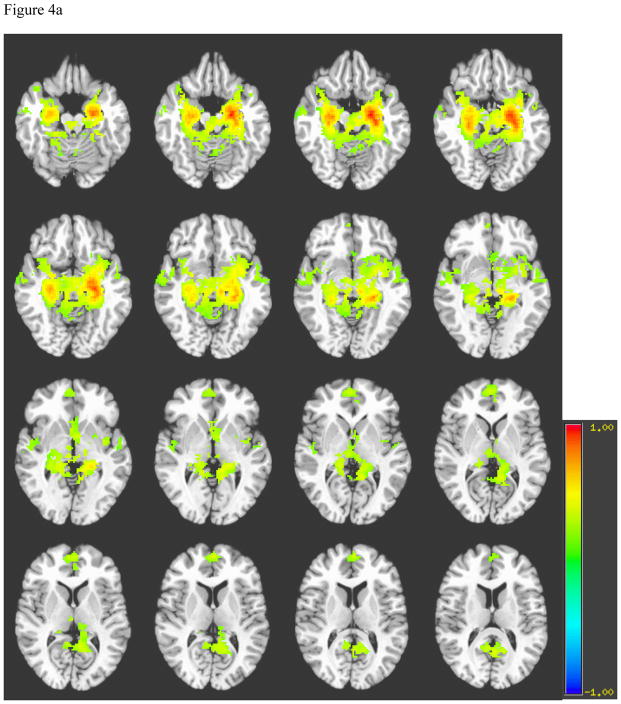
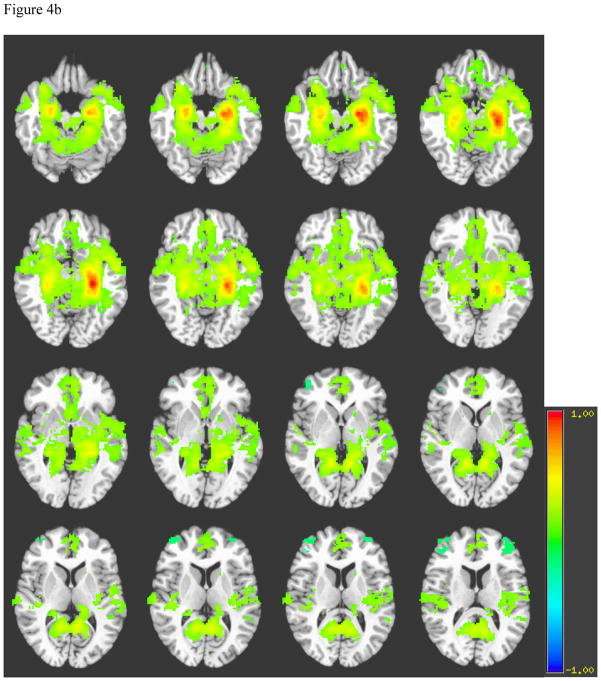
Generally, the spatial distribution of hippocampal connectivity among healthy controls showed strong and focused signal bilaterally within the body of the hippocampi (see Figure 4a). Controls also demonstrated connectivity in the septal and subthalamic neuclei. While the hippocampus appeared to have connectivity with bilateral parahippocampal gyri, the majority of correlated signal among controls occurred in the anterior medial temporal lobes. In contrast, patients demonstrated more abundant connectivity with the parahippocampal gyrus and posterior cingulate, as well as more diffuse connectivity in the temporal and frontal lobes, basal forebrain, and subthalamic nuclei than controls. Furthermore, in areas of bilateral hippocampal connectivity, patients demonstrated weaker contralateral connectivity than controls (see Figure 4b). Healthy right hippocampal connectivity showed a similar pattern of connectivity as the left hippocampus.
Figure 4.
Figure 4a. Average Left Hippocampus Connectivity among Normal Controls
Note for Figures 4a and 4b. A= Hippocampi, B= Basal forebrain, C= Frontal Lobe, D= Hypothalamus, E= Temporal lobe, F= Septal nuclei, G= Subthalamic nuclei, H= parahippocampal gyrus, I= Posterior cingulate.
Figure 4b. Average Left Hippocampus Connectivity among Patients with TAI
Note: A= Hippocampi, B= Basal forebrain, C= Frontal Lobe, D= Hypothalamus, E= Temporal lobe, F= Septal nuclei, G= Subthalamic nuclei, H= parahippocampal gyrus, I= Posterior cingulate cortex.
ANTERIOR CINGULATE CONNECTIVITY
Connectivity Between Bilateral Anterior Cingulate
Interhemispheric connectivity for the anterior cingulate was significantly different between healthy and injured brains, as the average strength of bilateral anterior cingulate interconnectivity was greater for controls than for patients with TAI (p = 0.015)(see Table 2). The degree of interhemispheric anterior cingulate connectivity among patients was not significantly associated with outcome, but showed a trend toward significance with the GOSE (p < 0.10) (see Table 3).
Whole Brain Connectivity
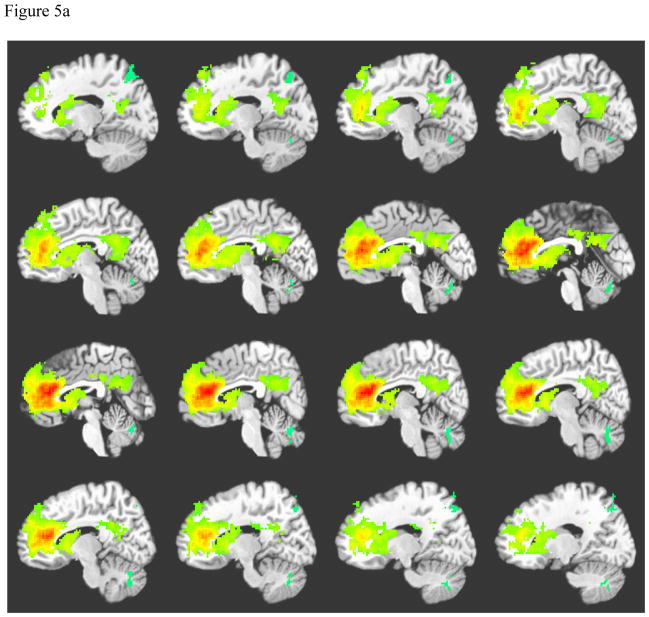
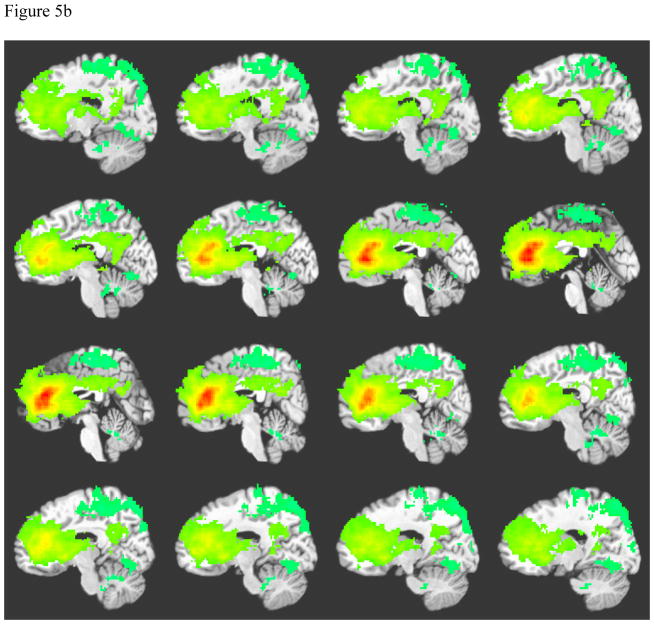
Anterior cingulate connectivity was similar for both left and right seeds. The pattern of ACC connectivity for controls is displayed on Figure 5a. Among controls, synchronous areas include focused signal in the anterior cingulate bilaterally, bilateral ventral posterior cingulate cortex, and bilateral caudate. Anterior cingulate connectivity for patients showed diffuse correlations surrounding the anterior cingulate bilaterally, bilateral caudate and thalamus, bilateral dorsal posterior cingulate cortex, and cingulate cortex connecting the anterior and posterior cingulate cortices (see Figure 5b). Patients also demonstrated negatively correlated signal in the occipital-temporal gyrus.
Figure 5.
Figure 5a. Average Left Anterior Cingulate Connectivity among Normal Controls
Note: Crosshairs were placed on slice closest to midline.
A = Right caudate, B= Anterior cingulate cortex, C= Cingulate cortex, D= Posterior cingulate cortex, E = Thalamus, F= Left caudate.
Figure 5b: Average Left Anterior Cingulate Connectivity among Patients with TAI
Note: Crosshairs were placed on slice closest to midline.
A = Right caudate, B= Anterior cingulate cortex, C= Cingulate cortex, D = Posterior cingulate cortex, E = Thalamus, F= Left caudate.
DORSOLATERAL PREFRONTAL CORTEX CONNECTIVITY
Connectivity Between Bilateral Dorsolateral Prefrontal Cortex (DLPFC)
There was no significant difference in connectivity for bilateral DLPFC between controls and patients with TAI (p = 0.353)(see Table 2). Additionally, the degree of bilateral DLPFC connectivity among patients was not significantly associated with outcome, but demonstrated a trend toward significance for two outcome measures (p = 0.10)(see Table 3).
Whole Brain Connectivity
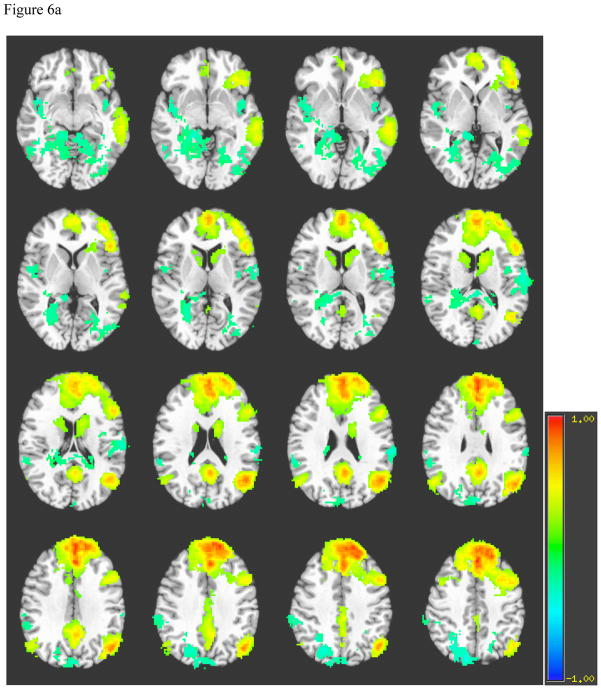
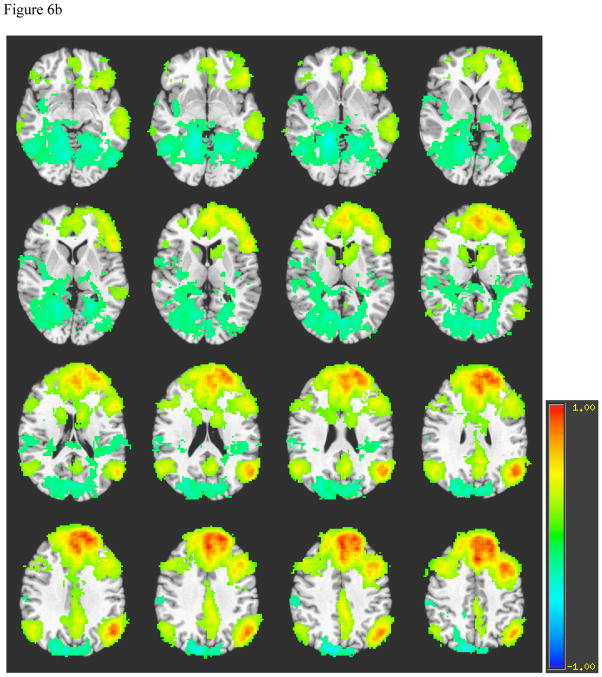
Left DLFPC connectivity for healthy individuals (see Figure 6a) includes ipsilateral inferior frontal gyrus, middle temporal gyrus, anterior cingulate, posterior cingulate, bilateral angular gyrus, dorsal aspect of superior and middle frontal gyri, and contralateral precuneus. The pattern of left DLPFC is similar between healthy volunteers and patients, but patients demonstrate stronger correlations in bilateral angular gyri, more diffuse correlations in contralateral frontal and temporal lobes, occipito-temporal and parahippocampal gyri. Additionally, while negative correlations are found in the contralateral precuneus among patients and controls, patients also demonstrate a relatively greater number of negatively correlated voxels in the ipsilateral precuneus (see Figure 6b). The pattern of right DLPFC is similar to that of the left DLPFC within patients and within controls.
Figure 6.
Figure 6a. Average Left Dorsolateral Prefrontal Cortex Connectivity among Normal Controls
Note. A = Parahippocampal gyrus, B = Occipito-temporal gyrus, C = Middle temporal gyrus, D = Inferior frontal gyrus, E = Caudate, F = Posterior cingulate cortex, G = Anterior cingulate cortex, H = Angular gyrus, I = Superior frontal gyrus, J = Precuneus.
Figure 6b. Average Left Dorsolateral Prefrontal Cortex Connectivity among Patients with TAI
Note. A = Parahippocampal gyrus, B = Occipito-temporal gyrus, C = Middle temporal gyrus, D = Inferior frontal gyrus, E = Caudate, F = Posterior cingulate cortex, G = Anterior cingulate cortex, H = Angular gyrus, I = Superior frontal gyrus, J = Precuneus.
COMMENT
The synchronicity of BOLD signal fluctuations throughout the brain has been useful in understanding functionally related brain regions/networks.42–44 Functional connectivity patterns among healthy individuals demonstrate a functional link between various regions known to communicate during various tasks and at rest. In contrast, connectivity patterns among clinical populations deviate considerably from those observed among healthy brains. For example, patients with Alzheimer’s disease have demonstrated disrupted hippocampal and frontal lobe connectivity throughout the brain, as compared to healthy peers.21,22 Furthermore, significant functional connectivity between hemispheres is found among healthy individuals; 22,45–47 whereas, interhemispheric functional connectivity is significantly reduced among clinical populations with compromise in the corpus callosum (CC).48–49 The relationship between CC integrity and functional connectivity was demonstrated by Quigley and colleagues (2003)48, who found patients with agenesis of the CC showed significantly reduced interhemispheric connectivity as compared to healthy controls. Likewise, Johnston et al. (2008)49 demonstrated dramatic reductions in interhemispheric functional connectivity of various functional systems after a complete callosotomy, while intrahemispheric connectivity was relatively preserved. These results implicate the CC as having a significant role in the degree of interhemispheric functional connectivity observed using fMRI, and suggest investigating the influence of corpus callosum damage in functional connectivity among other clinical populations with axonal damage.
Given that the CC is the most commonly injured white matter structure after traumatic axonal injury,4,23,50,51 and compromise to the integrity of the CC results in reduced interhemispheric functional connectivity among other clinical populations, it stands that patients with TAI should demonstrate reduced functional connectivity as well. In deed, the interhemispheric connectivity results in this study are commensurate with findings among other clinical populations, as patients with TAI demonstrated significantly reduced interhemispheric functional connectivity in bilateral hippocampi and anterior cingulate relative to healthy controls. The results are consistent with a prior case study MacDonald et al. (2008)52 that demonstrated compromised hippocampal connectivity in a single patient who suffered a TBI. To our knowledge, this investigation is the first to examine functional connectivity differences between a group of patients with TAI and healthy controls.
The general pattern of hippocampal functional connectivity among healthy individuals included stronger bilateral hippocampal and greater connectivity in the septal neuclei near the anterior commissure than the septal neuclei, as compared to patients. In contrast, the pattern of hippocampal connectivity among patients with TAI demonstrated reduced contralateral strength of correlation, but generally preserved correlation ipsilaterally, and greater correlation in the subthalamic nuclei near the posterior commissure, parahippocampal gyrus, and posterior cingulate cortex than controls. Although contralateral hippocampal connectivity was significantly reduced among patients, it was not absent. This is consistent with a prior report of functional connectivity before and after a complete surgical corpus callosotomy.49 Their observation of limited interhemispheric connectivity despite the complete transection of the main commissural fiber suggests that interhemispheric connectivity also occurs through other commissural fibers such as the anterior and/or the posterior commissures. Patients in the present study undoubtedly underwent varying degrees of subcortical white matter injury including injury to the CC, as evidenced by FLAIR MRI (see inclusion criteria); therefore, they presumably have varying degrees of healthy callosal axons allowing some (albeit reduced) functional connectivity with the contralateral hemisphere through this most parsimonious route. However, it is also possible that patients in this study demonstrate some interhemispheric connectivity via the use of the anterior or posterior commissures, or the dorsal hippocampal commissure in leiu of the CC.
Studies of both animals and humans have suggested that these smaller commissures play a role in interhemispheric hippocampal connectivity due to their proximity to the hippocampus.49,53,54 Our data show interheimspheric connectivity occurs near both the anterior and posterior commissures among healthy brains. In contrast, interhemispheric connectivity among injured brains occurs more posteriorly, and may rely on posterior commissural fibers (i.e., posterior commissure and/or dorsal hippocampal commissure) to a greater degree than healthy brains. Additionally, patients demonstrated more diffuse but weaker correlations throughout the medial temporal and frontal lobes, and posterior cingulate cortex as compared to controls. This appears to be evidence of neural inefficiency, and may represent the brain’s attempt to restore interhemispheric hippocampal connectivity by utilizing less direct connections in light of injured subcortical white matter.
The functional connectivity pattern of the ACC demonstrated interesting spatial differences between groups, as healthy brains showed greater correlation with the ventral posterior cingulate cortex as compared to injured brains; and injured brains had greater connectivity with most of the cingulate cortex and particularly the dorsal aspect of the posterior cingulate cortex than healthy brains. An altered connectivity pattern within the cingulate cortex has been found among clinical populations. Castellanos et al. (2008)54 reported compromised connectivity between the precuneus/posterior cingulate and areas of the default mode network including the anterior cingulate. Furthermore, Wang et al., (2006)22 found patients with early stage Alzheimer’s disease showed compromised resting state connectivity between the posterior cingulate and the hippocampus, areas involved in the default mode network. Taken together, these studies and the results from the present investigation suggest the connectivity between anterior and posterior cingulate may be sensitive to compromise in clinical populations with functional or neurocognitive deficits. Furthermore, the results of this study support examining the functional connectivity between various brain regions involved in the default mode network, as their connectedness may be a marker of cerebral integrity or compromise.
Interestingly, functional connectivity for DLPFC for patients and controls demonstrate a similar pattern observed in the default network (i.e., medial superior frontal lobe, posterior cingulate and bilateral inferior parietal lobes, parahippocampal gyrus). Furthermore, patients demonstrate significantly greater negative connectivity in occipital-temporal and parahippocampal gyri than controls, which may suggest that patients are suppressing brain activity to a greater degree than controls. While it is unclear whether the DLPFC is suppressing brain activity in the aforementioned regions or if these regions are suppressing brain activity in the DLPFC and other regions involved in the default network, given the frontal lobes play a role in modulating and coordinating complex behaviors, the DLPFC is more likely modulating activity in other regions. Greater amount of negative correlations observed when using the DLPFC as a seed among patients may suggest they are less efficient in quieting their minds during a resting state task, thus demonstrating the default mode network is sensitive to changes after TAI. Subsequent investigation into this matter should utilize a time-lag analysis of connectivity, as this may help determine whether there is a causal relationship between the DLPFC and the negatively correlated brain regions.
Relatively few studies have examined the association between measures of functional connectivity and cognitive ability. Their findings generally suggest that functional connectivity of various brain regions have a significant relationship with certain cognitive abilities.55–56 In this study, the degree of functional connectedness between hippocampi is negatively associated with performance on an auditory verbal memory task, such that patients with less bilateral connectivity recalled more words after a delay than patients with greater interhemispheric connectivity. Given the role of the hippocampus in learning and memory, we hypothesized that interhemispheric hippocampal connectivity is associated with performance in this cognitive domain. Interestingly, the observed negative association between hippocampal connectivity and memory suggests cognitive functioning is not completely dependent on the integrity of structural connections. Additionally, these results hint at neural plasticity, as the data suggests hippocampal signal is rerouted to reach its homologous contralateral region through more indirect posterior connections (i.e., posterior commissure/thalamic nuclei) after TAI, and the degree of inefficiency (i.e., diffuse connectivity) seen in the pattern of hippocampal connectivity among patients may be evidence of this plasticity. However, while this plasticity eventually results in neuronal signal reaching its contralateral destination and a relative increase in interhemispheric hippocampal connectivity, it occurs at the expense of memory ability.
While the interhemispheric connectivity for the ACC and the DLPFC was significantly lower among patients than controls, the correlation between degree of connectivity in these regions and outcome only trended toward significance with outcome. Though the associations between interheispheric functional connectivity and outcome observed in the current study do not fully support the hypothesis that frontal lobe functional brain synchronicity is associated with executive functions post-TBI, the results are not entirely surprising, as resting state interhemispheric connectivity should not be assumed to have a strong association with functional or neurocognitive outcome in this clinical population. Patients with TBI, even TAI present with very heterogeneous injury profiles including mechanism of injury, injury severity, and most importantly location of brain lesions. Although every patient in this sample was selected based on having a head injury consistent with traumatic axonal injury, the degree of injury to particular white matter structures undoubtedly varied widely. For example, Benson et al. (2007)10 examined the integrity of whole-brain white matter of twenty patients with TAI using a histogram analysis and demonstrated that the distribution of white matter fractional anisotropy (i.e. measure of the directionality of water diffusion along axons) for individual patients was significantly more variable than the distribution for healthy controls. Variability of white matter integrity in various interhemispheric structures, may in part, explain how interhemispheric connectivity can be reduced without reducing cognitive or functional ability, as it is possible certain patients suffered damage to the CC and subsequently rerouted neuronal signal between hippocampi through less direct but more intact commissural fibers (i.e., posterior cingulate, hippocampal commissure), thereby lowering their degree of interhemispheric hippocampal connectivity, but maintaining enough contralateral connectivity to approximate the desired behavior.
LIMITATIONS
A limitation of the present study is that the degree of compromise to interhemispheric white matter is not reported. Decrement in interhemispheric structural connectivity may account for degree of functional connectivity among patient populations, but DTI studies must be performed to measure the degree of structural compromise. It is highly recommended that diffusion tensor tractography (or similar analysis of diffusion tensor imaging data) be incorporated into the research design to more directly examine the association between the integrity of certain white matter structures, functional connectivity, and outcome. Another limitation of the current study, and any neuroimaging study in TBI has to do with heterogeneity inherent in TBI, as injury profiles including mechanism of injury, injury severity, and most importantly location of brain lesions can vary from patient to patient. Therefore, the results of this investigation may not be generalizable across traumatic brain injuries with different profiles.
Given that the functional connectivity measures for this study are obtained from a resting state fMRI paradigm, it is not known whether patterns of connectivity during rest should correlate to functional or neurocognitive tasks. Consequently, it is possible the association between functional connectivity measures and outcome could be significantly different had synchronicity of BOLD signal between regions been measured during a cognitive task rather than during rest. Future studies may benefit from incorporating both resting state and task related functional connectivity measures in their design. It is also important to note that the results of this study are specific to functional connectivity patterns present six-months post-injury, as results may be different among a more acutely or more chronically brain injured sample.
The use of MRI scanners from different manufacturers in this study may be perceived as a limitation, despite demonstrating equivalence in interhemispheric connectivity among controls between scanners. However, novel neuroimaging modalities must show robust differences between healthy and clinical populations across scanners to ever be useful as a clinical biomarker, as hospitals/medical centers use scanners from various manufacturers. While attempts were made to limit the influence of multiple comparisons on the results of the statistical analyses (i.e., using false discovery rate alpha correction and cluster thresholding), the present study may still be impacted by false positives due to the large number of voxel by voxel comparisons.
SUMMARY
The present investigation provides support for the use of fcMRI in clinical populations, including patients with compromised anatomical connectivity such as TAI. The results support the hypothesis that the hippocampus and frontal lobe circuits of patients with TAI have distinct patterns of interconnectedness and less connectivity with their contralateral homologue, as compared to healthy individuals. Additionally, the degree of bilateral connectivity in hippocampal circuits appears to correlate with patients’ memory-related outcome post-TAI.
Acknowledgments
We thank Evelyn Babcock, PhD of the University of Texas Southwestern Medical Center for her assistance with image acquisition. Thanks to Kaundinya Gopinath, PhD for his image processing and statistical advice. We are also very thankful to Caryn Harper for coordinating and conducting many of the outcome assessments. This research was supported by funding to C. Marquez de la Plata (NIH K23 NS060827) and R. Diaz-Arrastia (NIH R01 HD48179 and Department of Education H133 A020526).
References
- 1.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14(6):602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Langlois M, Cramer KM. The relationship between household composition and retirement stress. Guidance & Counseling. 2004;20(1):89–98. [Google Scholar]
- 3.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology. 1989;15(1):49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 4.Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: Diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82(10):1461–71. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- 5.Mittl RL, Grossman RI, Hiehle JF, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR, Am J Neuroradiology. 1994;15(8):1583–89. [PMC free article] [PubMed] [Google Scholar]
- 6.Garnett MR, Cadoux-Hudson TA, Styles P. How useful is magnetic resonance imaging in predicting severity and outcome in traumatic brain injury? Current Opinion in Neurology. 2001;14(6):753–57. doi: 10.1097/00019052-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Levine B, Fujiwara E, O’Connor C, et al. In vivo characterization of traumatic brain injury neuropathology with structural and functional neuroimaging. J Neurotrauma. 2006;23(10):1396–411. doi: 10.1089/neu.2006.23.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong KA, Ashwal S, Holshouser BA, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56(1):36–50. doi: 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- 9.Marquez de la Plata C, Ardelean A, Koovakkattu D, et al. Magnetic resonance imaging of diffuse axonal injury: quantitative assessment of white matter lesion volume. J Neurotrauma. 2007;24(4):591–98. doi: 10.1089/neu.2006.0214. [DOI] [PubMed] [Google Scholar]
- 10.Benson RR, Meda SA, Vasudevan S, et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma. 2007;24(3):446–59. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- 11.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: A pilot study. J Neurotrauma. 2007;24(9):1447–59. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 12.Wang JY, Bakhadirov K, Devous MD, Sr, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65(5):619–26. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- 13.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR, Am J Neuroradiol. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta RK, Saksena S, Agarwal A, et al. Diffusion tensor imaging in late posttraumatic epilepsy. Epilepsia. 2005;46(9):1465–71. doi: 10.1111/j.1528-1167.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 15.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 16.Salmond CH, Menon DK, Chatfield DA, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29(1):117–24. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, Wu O, Sorensen AG. Diffusion Tensor Imaging as potential biomarker of white matter injury in diffuse axonal injury. Am J Neuroradiol. 2004;25:370–376. [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(10):2508–19. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 19.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 20.Peltier SJ, Noll DC. T2* dependence of low frequency functional connectivity. Neuroimage. 2002;16(4):985–92. doi: 10.1006/nimg.2002.1141. [DOI] [PubMed] [Google Scholar]
- 21.Allen G, Barnard H, McColl R, et al. Reduced hippocampal functional connectivity in alzheimer disease. Arc Neurol. 2007;64(10):1482–87. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Zang Y, He Y, et al. Changes in hippocampal connectivity in the early stages of alzheimer’s disease: Evidence from resting state fMRI. Neuroimage. 2006;31(2):496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12(6):557–63. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 24.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR, Am J Roentgenol. 1988;150(3):663–72. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- 25.Wallesch C-W, Curio N, Kutz S, Jost S, Bartels C, Synowitz H. Outcome after mild-to-moderate blunt head injury: effects of focal lesions and diffuse axonal injury. Brain injury. 2001;15(5):401–12. doi: 10.1080/02699050010005959. [DOI] [PubMed] [Google Scholar]
- 26.Fork M, Bartels C, Ebert AD, Grubich C, Synowitz H, Wallesch CW. Neuropsychological sequelae of diffuse traumatic brain injury. Brain injury. 2005;19(2):101–08. doi: 10.1080/02699050410001726086. [DOI] [PubMed] [Google Scholar]
- 27.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am J Neuroradiol. 2001;22(7):1326–33. [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behren TE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain, ED. 2. Elsevier Academic Press; Amsterdam: 2004. [Google Scholar]
- 31.Wilson JT, Pettigrew LE, Teasdale GM. Structured interview for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale. J Neurotrotrauma. 2007;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 32.Mathias JL, Wheaton P. Changes in attention and information-processing speed following severe traumatic brain injury: A meta-analytic review. Neuropsychology. 2007;21:212–223. doi: 10.1037/0894-4105.21.2.212. [DOI] [PubMed] [Google Scholar]
- 33.Levin HS. Memory deficit after closed head injury. J Clin Exp Neuropsychol. 1990;12:129–53. doi: 10.1080/01688639008400960. [DOI] [PubMed] [Google Scholar]
- 34.Brooks N, Campsie L, Symongton C, et al. The five-year outcome of severe blunt head injury: a relative’s view. J Neurol Neruosurg Psychiatry. 1986;49:764–70. doi: 10.1136/jnnp.49.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ylvisaker M, Feeney T. Executive functions after traumatic brain injury: Supported cognition and self-advocacy. Semin Speech Lang. 1996;17:217–232. doi: 10.1055/s-2008-1064100. [DOI] [PubMed] [Google Scholar]
- 36.McAllister Thomas W, McDonald Brenna C, Flashman Laura A, Saykin Andrew J. Executive dysfunction following traumatic brain injury: Neural substrates and treatment strategies. NeuroRehabilitation. 2002;17(4):333. [PubMed] [Google Scholar]
- 37.Reitan RM. Validity of the Trail-making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–76. [Google Scholar]
- 38.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- 39.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: Psychological Corporation; 2000. (CVLT-II) [Google Scholar]
- 40.Dodrill CB. A neuropsychological battery for epilepsy. Epilepsia. 1978;19:611–23. doi: 10.1111/j.1528-1157.1978.tb05041.x. [DOI] [PubMed] [Google Scholar]
- 41.Benton AL, Hamsher K. Multilingual Aphasia Examination. AJA Associates; Iowa City, IA: 1983. [Google Scholar]
- 42.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–58. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. The Journal of Neuroscience. 2009;29(6):1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30(10):3127–41. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol. 2000;21(8):1397–401. [PMC free article] [PubMed] [Google Scholar]
- 46.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology. 2008;21(4):424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 47.Damoiseaux JS, Beckmann CF, Arigita EJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 48.Quigley M, Cordes D, Turski P, et al. Role of the corpus callosum in functional connectivity. AJNR, American Journal of Neuroradiology. 2003;24(2):208–12. [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston JM, Vaishnavi SN, Smyth MD, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28:6453–58. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng HK, Mahaliyana RD, Poon WS. The pathological spectrum of diffuse axonal injury in blunt head trauma: assessment with axon and myelin strains. Clin Neurol Neurosurg. 1994;96:24–31. doi: 10.1016/0303-8467(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 51.Amaral DG, Insausti R, Cowan WM. The commissural connections of the monkey hippocampal formation. J Comp Neurol. 1984;224:307–35. doi: 10.1002/cne.902240302. [DOI] [PubMed] [Google Scholar]
- 52.MacDonald CL, Schwarze N, Vaishnavi SN, Epstein AA, Snyder AZ, Raichle ME, Shimony JS, Brody DL. Neurology. 2008;71:1199–1201. doi: 10.1212/01.wnl.0000327521.69520.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gloor P, Salanova V, Olivier A, Quesney LF. The human dorsal hippocampal commissure. An anatomically identifiable and functional pathway. Brain. 1993;116:1249–73. doi: 10.1093/brain/116.5.1249. [DOI] [PubMed] [Google Scholar]
- 54.Castellanos FX, Margulies DS, Kelly AMC, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–37. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bosma I, Douw L, Bartolomei F, et al. Synchronized brain activity and neurocognitive function in patients with low-grade glioma: A magnetoencephalography study. Neuro-oncol. 2008;10(5):734–44. doi: 10.1215/15228517-2008-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song M, Zhou Y, Li J, et al. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41(3):1168–76. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]