Abstract
Objective
Following cardiopulmonary bypass (CPB), elaboration of cytokines, and subsequent interstitial proteases induction, such as matrix metalloproteinases (MMPs), can result in a complex postoperative course. The serine protease inhibitor, aprotinin, which has been used in congenital heart surgery putatively for modulating fibrinolysis, is now unavailable, necessitating the use of lysine analogues, such as tranexamic acid (TXA). The present study tested the hypothesis that distinctly different plasma profiles of signaling molecules and proteases, would be differentially affected following the administration of aprotinin or TXA in the context of congenital cardiac surgery and CPB.
Methods
37 Patients (age 4.8±0.3 mos) undergoing corrective surgery for ventricular septal defect (VSD) and Tetralogy of Fallot (TOF) received either aprotinin (n=22), or TXA (n=15). Using a high throughput multiplex suspension immunoassay, plasma was serially quantified for cytokines and MMPs: before aprotinin or TXA (baseline), after separation from CPB, and 4, 12, 24, and 48 hours post-CPB.
Results
Tumor necrosis factor-alpha (TNF) increased initially following CPB in both the aprotinin and TXA groups, but at 24 and 48 hours post-CPB was approximately 50% lower in the aprotinin group (p<0.05). IIL-10 levels were 3-fold higher in the TXA group compared to the aprotinin group immediately post-CBP (p<0.05). Plasma levels of MMP types associated with inflammation, MMP-8 and -9, were 2-fold higher in the late post-CPB period in the TXA group when compared to the aprotinin group.
Conclusions
Following VSD or TOF repair in children, cytokine induction occurs, which is temporally related to the emergence of a specific MMP profile. Moreover, these unique findings demonstrated differential effects between the serine protease inhibitor aprotinin, and the lysine analogue TXA with respect to cytokine and MMP induction in the early post-operative period. The different cytokine-proteolytic profile between these anti-fibrinolytics may in turn influence biological processes in the post-operative period.
Introduction
Surgical correction of common congenital malformations such as ventricular septal defect (VSD) and Tetralogy of Fallot (TOF) require the use of cardiopulmonary bypass (CPB), and as a consequence can cause abnormalities in coagulation cascades and subsequently increased bleeding in the post-operative period.1–3 In adult cardiac surgery requiring CPB, a pharmacological approach in addressing this post-operative hematological issue is through administration of what are generically defined as anti-fibrinolytics.4–7 The fundamental mechanism of action of these anti-fibrinolytics with respect to the coagulation cascade is through the inhibition of plasmin activity, which in turn stabilizes nascent clot formation. These anti-fibrinolytics such as the serine protease inhibitor aprotinin, and the lysine analogue tranexamic acid (TXA) have been adopted for use in congenital cardiac surgery.8–11 However, past restrospective as well as prospective studies have called into question the safety and overall efficacy of these anti-fibrinolytics in adult cardiac surgery.4–7 For example, past studies have identified increased incidence of post-operative renal dysfunction and potentially higher long term mortality in patients receiving aprotinin. Indeed, the outcomes from these studies resulted in the suspension of aprotinin for clinical use in November, 2007. In contrast, other studies have suggested that lysine analogues such as TXA may not be as effective in reducing early post-operative bleeding as aprotinin.4,5,7 Finally, in light of the fact that aprotinin is a non-specific serine protease inhibitor, then multiple biological pathways may be affected independent of the anti-fibrinolytic effects.12–16 Accordingly, the central hypothesis of the present study was that in those patients undergoing VSD/TOF surgical repair, that distinctly different plasma profiles of signaling molecules and proteases, which have been identified to potentially contribute to a complex post-operative course,14–20 would be differentially affected following the administration of aprotinin or TXA.
One of the ubiquitous consequences with the use of CPB, is a heightened pro-inflammatory state, and the post-operative period following congenital cardiac surgery is no exception.12–23 For example, increased elaboration of pro-inflammatory molecules such as interleukin-6 (Il-6) and tumor necrosis factor alpha (TNF) have been identified following congenital cardiac surgery and CPB.17,18,22 Increased cytokine activation in turn will provoke a number of cellular and extracellular processes which include the induction of proteases such as the matrix metalloproteinases (MMPs).20,21,24,25 Acute increases in plasma MMP levels have also been reported immediately following myocardial infarction, ischemia/reperfusion, and inflammatory states.24,25 In addition, past studies have identified increased elaboration of MMPs in both adults and children following CPB.20,21 However, comprehensive studies which serially quantify a full profile of both cytokines and MMPs before and following surgical correction of a VSD or TOF, have been limited. Moreover, whether and to what degree differential effects on the cytokine and MMP cascade occur with the administration of aprotinin or TXA in this clinical context has not been examined. Accordingly, the objectives of the present study were 2-fold. First, develop a plasma profile for a large portfolio of cytokines and MMPs using a high sensitivity multiplex array system,13,26 in children undergoing elective surgical repair of a VSD or TOF. Second, since aprotinin was uniformly utilized for these procedures prior to November 2007, and subsequent to this date lysine analogues uniformly utilized, then perform a comparative analysis of these cytokine/MMP profiles in the early post-operative period.
Methods
Patients
Following approval by the Medical University of South Carolina Institutional Review Board (HR#16107), infants undergoing isolated VSD or TOF repair from January 2006 to December 2008 were evaluated for participation. Entry criteria included a planned complete surgical repair of the cardiac defect, and ages from 1 to 9 months. Exclusion criteria included recognized chromosomal abnormalities, prior intra-cardiac surgery, prior exposure to aprotinin or TXA, family or patient history of coagulopathic diseases or thrombosis, or additional complex cardiac defects. Presences of atrial septal defect and/or patent ductus arteriosus were not considered exclusion criteria. Following which informed, written consent was obtained from the parents and/or legal guardians. The pediatric cardiac surgeons (TYH,FAC,SMB) remained constant throughout this study period.
The present study was dichotomized with respect to anti-fibrinolytic utilized. Prior to November 2007, patients undergoing VSD or TOF repair routinely received aprotinin at this institution. The aprotinin dose consisted of both an intravenous and pump prime load of 240 mg/m2 BSA (1.7 × 106 kIU/ m2 BSA), and a continuous infusion at 56 mg/m2 BSA/hr (4 × 105 kIU/m2 BSA/hr) until the completion of the primary procedure. Following the suspension of aprotinin, (November 2007), TXA was uniformly administered instead of aprotinin, and the dose consisted of an intravenous load of 100 mg/kg, followed by a 10mg/kg/hr continuous infusion. Of the 37 patients, 22 patients received aprotinin, and 15 received TXA.
Anesthesia and Surgery
Anesthesia was induced with ketamine or sevoflurane and maintained using a combination of fentanyl, midazolam, and isoflurane. Systemic anticoagulation was achieved with a heparin dose of 400 U/kg, with additional doses administered to maintain kaolin-based activated clotting time >500s. Standard non-pulsatile CPB was utilized, and the circuit primed with Plasmalyte A (Baxter Healthcare Corporation, Deerfield, IL, USA), and 1 unit of fresh-frozen plasma. Banked, packed red blood cells (PRBC) were added to achieve a hematocrit of approximately 28–30% during CPB. No steroids were administered preoperatively or in the CPB prime. Moderate (25°C to 28°C) hypothermia was employed, and myocardial preservation was obtained with cold blood cardioplegia at 20 min. intervals. The pH-stat regimen was used during cooling, and alpha-stat for rewarming. Modified ultrafiltration was performed after separation from CPB. Protamine was given at 0.6:1 protamine to heparin ratio. Blood product transfusions following CPB were administered as necessary to achieve satisfactory hemostasis and a target hematocrit of >30%. Standard transatrial closure of the VSD was employed in all patients. TOF repairs were performed through trans-atrial/trans-pulmonary technique with preservation of the pulmonary valve in half of the patients, and transannular patch repair in the other half.
Clinical Data
Clinical characteristics such as age, weight, gender, and preoperative systemic arterial oxygen saturation were recorded. During the 48 hour post-operative period, the following data were collected: aortic cross clamp and cardiopulmonary bypass times, blood product utilization, white blood cell count at 4 hours post-CPB, and maximum white blood cell count.
Protocol
Blood samples (1 mL) were obtained from an arterial line for MMPs and cytokines at the following times: just before induction of anesthesia (Baseline), following modified ultrafiltration (0 hours), and 4, 12, 24, and 48 hours post-CPB. All samples were placed in chilled EDTA tubes, centrifuged, and plasma stored at −70°C until assay. At the time of assay, plasma samples were allowed to thaw on ice, and subjected to multiplex suspension array for cytokines (Human MAP Base Kit LUH000, R&D Systems) and MMPs (Human MMP Base Kit LMP000, R&D Systems) in which all samples could be measured simultaneously, thereby minimizing inter-assay variability. The multiplex array was previously validated and calibrated using internal controls for each measured MMP and cytokine.13,26 Representative MMPs from each of the MMP classes were measured, including the collagenases (MMP-8, -13), the gelatinases (MMP-2, -9), stromelysins (MMP-3, -7). For the cytokines, plasma levels of interleukins 1-beta (IL-1b), IL-2, IL-6, IL-8, IL-10 (IL-10), tumor necrosis factor alpha (TNF), and interferon gamma (INF). Plasma samples (20 µL) were undiluted for cytokines, and 1:2 for all the MMPs except for the gelatinases, for which a 1:100 dilution was utilized. The relative fluorescence obtained for each distinct cytokine/MMP (Bio-Plex 200, BioRad Laboratories) was converted to an absolute concentration using calibration curves generated from known concentrations of recombinant standards. Average sensitivities for cytokines and MMPs were 0.3 pg/mL and 5 pg/mL, respectively. The coefficient of variation for these assays was 15% or less. Readings from all samples were within the targeted dynamic range defined by the standards.
Data Analysis
Patient demographics, including age and weight, were compared between the TXA and aprotinin groups using the t-test. Categorical variables (patient gender, ethnicity, blood product utilization) were compared using chi-square analysis. Plasma concentrations of the cytokines/MMPs were corrected for hemodilution using the hematocrit recorded at each post-operative time point. The distributions for each of the cytokines and MMP levels were checked for normality using the Shapiro-Wilk test. Since the distribution for the cytokines and MMPs failed the normality test, a logarithmic transformation was applied. Comparisons over time and type of anti-fibrinolytic agent (aprotinin vs. TXA) were performed using two-way analysis of variance (ANOVA) on the log-transformed values. Post hoc mean separation was performed using Tukey-adjusted pairwise comparisons (module prcomp, STATA). Results presented in the graphs represent mean ± standard error of the mean (SEM) of the raw data values; significance markers are based on the normally distributed log-transformed values. All statistical procedures were performed using STATA statistical software (STATA Intercooled V 8.0. College Station, TX). Values of p<0.05 were considered to be statistically significant.
Results
Preoperative white blood cell count, platelet counts, and coagulation profiles (prothrombin time and activated partial thromboplastin time) were within normal range for all 37 patients. Demographic data for the 37 patients enrolled in this study are presented in Table 1, where 22 patients received aprotinin and 15 received TXA. Aortic cross clamp and cardiopulmonary bypass (CPB) times were longer in TOF than VSD patients. Baseline cytokine and MMP levels were not different between VSD and TOF patients, and therefore the composite baseline values for plasma cytokines are shown in Table 2 and for MMPs in Table 3. Initial baseline IL-4 and MMP-12 levels were below the level of detection, and therefore these 2 analytes were excluded from further analysis. When the VSD and TOF patients were considered in terms of aprotinin and tranexamic acid (TXA) administration, there were no differences in age, weight, and preoperative arterial oxygen saturation. CPB and aortic cross clamp times were also similar (122±13 vs. 126±6 min. p=0.79, and 73±4 vs. 71±7 min. p=0.77, respectively) between the aprotinin and TXA groups. The patients enrolled in this study were then dichotomized across anti-fibrinolytic treatment with respect to post-operative white blood cell count and blood product utilization (Table 1). Early perioperative white blood cell count was lower in the aprotinin group, but the maximal white blood cell count over the first 48 hour interval was equivalent between groups. The median number of blood products utilized and the number of patients in each group receiving individual blood products are summarized in Table 1. The use of packed red blood cells, fresh frozen plasma, cryoprecipitate and platelets were similar across all groups (p>0.70).
Table 1.
Demographics and intraoperative characteristics of patients presenting for surgical repair of either a ventricular septal defect (VSD) or tetralogy of Fallot (ToF)
| Demographics and intraoperative profiles | ||
| Age (months) | 4.8±0.3 | |
| Weight (kg) | 5.6±0.2 | |
| Arterial oxygen saturation (%) | 96±1 | |
| Gender (M/F) | 18 / 19 | |
| Ethnicity (Caucasian / African American / Hispanic) | 21 / 15 / 1 | |
| Sample Size (n) | 37 | |
| Lesion type (n, %) | VSD | 17 (46%) |
| TOF | 20 (54%) | |
| Cross clamp time (min) | VSD | 56±5 |
| TOF | 86±3+ | |
| Cardiopulmonary bypass time (min) | VSD | 91±6 |
| TOF | 151±6+ | |
| Postoperative outcomes dichotomized by anti-fibrinolytic | ||
| WBC count at 4 hours (×103 / mL) | TXA | 10.9±0.7 |
| Aprotinin | 9.0±0.5* | |
| Maximum WBC count in the 48 hours perioperative period (×103 / mL) | TXA | 12.9±0.9 |
| Aprotinin | 13.2±0.7 | |
| Packed red blood cells (units) | TXA | 2 (1) |
| Aprotinin | 2 (1) | |
| Fresh frozen plasma (units) | TXA | 1 (1) |
| Aprotinin | 1 (1) | |
| Cryoprecipitate (units) | TXA | 0 (1) |
| Aprotinin | 0 (0) | |
| Platelets (units) | TXA | 1 (1) |
| Aprotinin | 1 (0) | |
| Sample size | TXA | 15 |
| Aprotinin | 22 | |
TXA: tranexamic acid
Continuous variables presented as Mean±SEM. Categorical variables presented as either number of subjects or median (interquartile range)
p<0.05 vs. VSD
p<0.05 vs. TXA.
t-test or ANOVA (as appropriate) used for continuous variables; χ2 test used for categorical variables
Table 2.
Baseline and perioperative levels of cytokines in infants presenting for surgical repair of either a ventricular septal defect or tetralogy of Fallot
| Time post-cardiopulmonary bypass (hours) | ANOVA results (p-values) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Group | 0 | 4 | 12 | 24 | 48 | Time | Anti- fibrinolytic |
Interaction | |
| IL-2 | 1.6±0.4 | TXA | 0.4±0.2# | 1.2±0.6 | 1.3±0.6 | 0.5±0.3# | 0.4±0.2# | 0.01 | <0.001 | 0.73 |
| Aprotinin | 2.5±0.4*# | 5.7±2.6*# | 2.3±0.4*# | 1.7±0.3* | 2.0±0.3* | |||||
| IL-6 | 2.1±0.4 | TXA | 43.0±16.4# | 184.4±64.2# | 102.3±18.2# | 60.2±9.6# | 27.3±6.1# | <0.001 | 0.16 | 0.85 |
| Aprotinin | 36.3±12.9# | 152.2±24.3# | 85.4±18.7# | 61.7±12.8# | 16.1±2.3*# | |||||
| IL-8 | 6.4±0.7 | TXA | 77.9±21.1# | 78.8±18.1# | 36.4±6.7# | 21.3±3.5# | 17.7±4.4# | <0.001 | 0.06 | 0.56 |
| Aprotinin | 69.3±28.4# | 89.2±19.4# | 26.0±4.6# | 17.6±2.1# | 10.6±1.5 | |||||
| IL-10 | 1.7±0.5 | TXA | 166.1±38.3# | 18.2±6.6# | 9.8±2.6# | 3.7±1.0# | 1.9±0.4 | <0.001 | <0.001 | 0.10 |
| Aprotinin | 51.4±10.9*# | 10.3±3.4# | 4.6±0.7# | 3.6±1.0# | 2.5±1.6 | |||||
| IL-1β | 0.6±0.3 | TXA | 0.7±0.1 | 0.7±0.1 | 0.5±0.1 | 0.6±0.1 | 1.1±0.4# | 0.03 | <0.001 | 0.07 |
| Aprotinin | 0.2±0.1* | 0.6±0.2 | 0.5±0.3 | 1.5±0.9 | 0.1±0.1* | |||||
| TNF-α | 3.7±0.5 | TXA | 8.2±1.6# | 9.2±2.0# | 3.7±0.8 | 2.9±0.6 | 3.3±0.7 | <0.001 | 0.10 | 0.29 |
| Aprotinin | 9.1±3.7# | 10.4±1.7# | 2.5±0.5 | 1.9±0.3# | 1.4±0.3*# | |||||
| INF-γ | 0.5±0.1 | TXA | 0.1±0.1# | 0.1±0.1# | 0.1±0.1# | 0.1±0.1# | 0.1±0.1# | 0.003 | <0.001 | 0.47 |
| Aprotinin | 0.6±0.2* | 0.9±0.3*# | 0.5±0.2* | 0.3±0.1 | 0.5±0.1* | |||||
Values represent concentrations in pg/mL and are presented as Mean±SEM.
TXA: tranexamic acid
IL: interleukins; TNF: tumor necrosis factor; INF: interferon; GM-CSF: granulocyte macrophage-colony stimulating factor Sample size: n=37 (TXA: n=15; Aprotinin: n=22)
p<0.05 vs. Baseline.
p<0.05 vs. TXA.
Table 3.
Baseline and perioperative levels of cytokines in infants presenting for surgical repair of either a ventricular septal defect or tetralogy of Fallot
| Time post-cardiopulmonary bypass (hours) | ANOVA results (p-values) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Group | 0 | 4 | 12 | 24 | 48 | Time | Anti- fibrinolytic |
Interaction | |
| MMP-2 | 401.8±23.3 | TXA | 333.1±21.9# | 271.6±17.0# | 368.8±31.6 | 329.0±19.4# | 322.8±29.4# | 0.005 | 0.11 | 0.51 |
| Aprotinin | 284.1±16.7# | 283.6±17.5# | 316.7±30.4# | 285.8±17.9# | 327.4±25.3# | |||||
| MMP-3 | 1.5±0.2 | TXA | 4.0±0.3# | 2.3±0.2 | 1.9±0.2 | 5.8±0.5# | 8.9±1.2# | <0.001 | <0.001 | 0.61 |
| Aprotinin | 2.5±0.5* | 1.3±0.2* | 1.5±0.3 | 3.0±0.5*# | 5.3±0.8*# | |||||
| MMP-7 | 1.6±0.3 | TXA | 1.5±0.2 | 1.9±0.2 | 0.7±0.2# | 1.4±0.2 | 0.8±0.1# | 0.001 | 0.98 | 0.02 |
| Aprotinin | 1.5±0.3 | 1.8±0.3 | 1.5±0.4 | 1.4±0.3 | 0.9±0.1 | |||||
| MMP-8 | 2.0±0.2 | TXA | 4.8±0.8# | 4.2±0.8# | 7.7±1.8# | 5.6±1.1# | 19.7±13.3# | <0.001 | 0.01 | 0.95 |
| Aprotinin | 4.1±0.8# | 3.5±0.6# | 4.8±0.9* | 5.3±1.4# | 4.6±0.8* | |||||
| MMP-9 | 54.1±5.3 | TXA | 376.9±49.4# | 180.0±31.1# | 184.9±27.6# | 128.5±19.5# | 140.3±40.6# | <0.001 | <0.001 | 0.64 |
| Aprotinin | 189.2±26.9*# | 114.0±17.2*# | 105.6±20.5# | 80.0±9.5* | 81.9±11.9 | |||||
| MMP-13 | 0.3±0.2 | TXA | 2.9±1.1# | 0.4±0.1# | 0.3±0.1 | 0.4±0.9# | 0.1±0.1 | <0.001 | <0.001 | 0.13 |
| Aprotinin | 4.5±0.9*# | 0.2±0.1 | 0.1±0.1 | 0.1±0.1* | 0.2±0.1 | |||||
Values represent concentrations in ng/mL and are presented as Mean±SEM.
TXA: tranexamic acid
p<0.05 vs. Baseline.
p<0.05 vs. TXA.
Sample size: n=37 (TXA: n=15; Aprotinin: n=22)
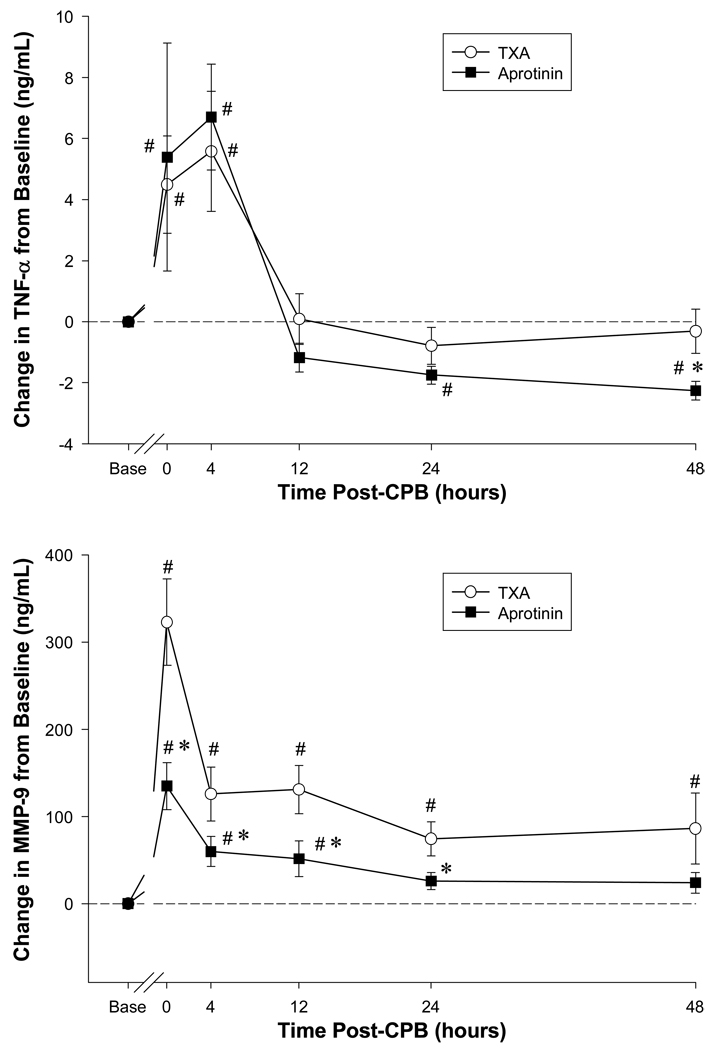
Plasma cytokine levels immediately following CPB and up to 48 hours post-CPB are summarized in Table 2. Based upon the 2-way ANOVA model, significant time dependent changes in all of the cytokines measured were observed within this perioperative period. With respect to the anti-fibrinolytic treatment effect on cytokine levels, this was observed for IL-2, IL-10, IL1-b, and INF. An anti-fibrinolytic treatment effect by ANOVA neared statistical significance for IL-8 (p=0.06) and a time-treatment interaction for IL1-b (p=0.07). With respect to individual pairwise comparisons, IL-2 levels were 2-fold higher across the entire perioperative interval in the aprotinin group when compared to the TXA group. In the aprotinin group, IL-10 levels were 3-fold lower immediately following CPB. INF levels were significantly higher in the aprotinin group when compare to the TXA group. With respect to TNF, late post-operative values were reduced by 50% in the aprotinin group. The time and treatment dependent effects are illustrated further for the cytokine TNF as a function of baseline values (Figure 1). While a robust and early increase in TNF was observed for both TXA and aprotinin, the relative levels were lower in the aprotinin group at the later post-operative time points. Thus, a diverse portfolio of cytokines was altered in the perioperative period following congenital cardiac surgery requiring CPB, and was differentially affected by anti-fibrinoltyic treatment.
Figure 1.
Plasma tumor necrosis factor alpha (TNF-TOP PANEL) and matrix metalloproteinase-9 (MMP-9-BOTTOM PANEL) levels were computed as a change from baseline values (Base) in congential cardiac surgery patients receiving either tranexamic acid (TXA) or aprotinin. TNF plasma levels were significantly increased immediately following cardiopulmonary bypass (CPB), and at late post-CPB time points, were lower in the aprotinin group. The change in plasma MMP-9 levels peaked in both the TXA and aprotinin groups immediately following CPB. However, the relative MMP-9 levels within this post-CPB period were lower in the aprotinin group than that of the TXA group. #p<0.05 vs. Baseline; *p<0.05 vs. TXA.
Serial plasma MMP levels for the perioperative interval under study and dichotomized with respect to anti-fibrinolytic therapy are summarized in Table 3. There were significant changes in all plasma MMP levels with respect to time. In addition, a significant anti-fibrinolyotic treatment effect was observed for plasma MMP-3, MMP-8, MMP-9 and MMP-13. For example, plasma MMP-9 levels increased by approximately 4-fold immediately following CPB. These robust changes in MMP-9 levels, with respect to baseline values and with respect to anti-fibrinolytic treatment are shown in Figure 1. While MMP-9 levels increased significantly in both the TXA and aprotinin groups in the early post-CPB period, the relative levels were significantly lower in the aprotinin group. Indeed, by 48 hours post-CPB, plasma MMP-9 levels had returned to near baseline values in the aprotinin group, but remained significantly elevated in the TXA group. Plasma MMP-3 levels were approximately 2-fold lower in the aprotinin group immediately following CPB and at 48 hours post-CPB when compared to the TXA group. By 48 hours post-CPB, plasma MMP-8 values were reduced by over 4-fold in the aprotinin group when compared to TXA values. Plasma MMP-13 values were initially higher in the aprotinin group post-CPB, but then fell below relative TXA values at later post-CPB time points. Thus a non-uniform temporal profile of plasma MMPs were detected in the early perioperative period following congenital cardiac surgery requiring CPB. In addition, certain MMP profiles were significantly affected by anti-fibrinolytic treatment in this perioperative interval.
Discussion
While cardiopulmonary bypass (CPB) is essential for the conduct of a large number of cardiac surgical procedures, the post-CPB period is associated with a heightened inflammatory response as demonstrated by cellular and non-cellular indices of immune activation.14–18,22,23 One of the fundamental events in this early post-CPB inflammatory response is the release of cytokines such as the interleukins (ILs) and tumor necrosis factor alpha (TNF). Through binding to cognate receptors, the ILs and TNF can result in a number of cellular and extracellular events such as cell death an induction of proteolytic pathways. For example, cytokine activation is an upstream pathway for the induction of proteolytic enzymes such as the matrix metalloproteinases (MMPs). Increased MMP induction in and of itself can lead to significant changes in tissue structure and function, and have been demonstrated to play a contributory role in a number of acute and chronic cardiovascular disease states.20,25,27,28–31 Specifically, MMP induction can directly affect wound healing, alter vascular permeability and reactivity, and contribute to multi-organ dysfunction.20,28–32 Thus, cytokine activation and MMP induction would hold particular relevance in the context of surgical repair of congenital defects requiring CPB. Past studies have focused upon a small number of cytokines, at a limited number of time points following pediatric heart surgery requiring CPB.22,23 However, a full temporal cytokine and MMP plasma profile have not been simultaneously performed in this patient population previously. Of more relevance, it remained unknown whether and to what degree different anti-fibrinolytic agents would affect this cytokine/MMP profile in the early post-CPB period. The unique results of the present study demonstrated in children following VSD or TOF repair, inflammatory cytokine induction occurs, which is temporally related to the emergence of specific MMP profile. In children who received aprotinin, cytokine induction was reduced and associated with attenuated levels of specific MMP types, such as MMP-8 and -9 up to 2 days post-operatively; an effect that was not realized with TXA.
Cytokine Levels Following Congenital Cardiac Surgery
Past studies that have examined cytokine release, particularly that in congenital cardiac surgery, have been limited either by cytokine type or time points post-CPB.17,18,22,23 One likely rate limiting step in these past studies is that cytokines were performed using a high sample volume immunoassay approach, and therefore blood sample volumes would be problematic. In the present study, a validated high-sensitivity approach was utilized which provided the ability to measure multiple analytes using a single, small volume plasma sample.26 Through this approach, multiple cytokines could be measured simultaneously, which thereby reduces intrinsic assay variability and allows for sequential analysis. The outcomes from this analysis demonstrated that a robust increase in certain cytokines occurred immediately following CPB, such as IL-6, IL-8 and IL-10, whereas other cytokines such as TNF increased transiently and then fell from baseline values. The peak levels of IL-6 and IL-10 which were observed in the present study are consistent with those reported previously following congenital cardiac surgery.18,23 In comparison to adults undergoing cardiac surgery, the relative increase in the magnitude for these cytokines following CPB were similar,13–16 but in contrast to adults, remained elevated for a much greater period post-CPB. This suggests that the effects of these cytokines would be more prolonged in children undergoing cardiac surgery when compared to adults. Both IL-6 and IL-8 are potent pro-inflammatory cytokines which are potent chemoattractants for inflammatory cells such as neutrophils and lymphocytes.17,22 On the other hand, IL-10 can be considered an anti-inflammatory molecule and is often modulated in a concordant fashion to the pro-inflammatory cytokines such as IL-6, IL-8, IL-1beta, and TNF.17,14–18,22,23 In the present study, IL-10 plasma levels increased early in the post-CPB period, but fell towards baseline values at later time points. In contrast, the relative levels for IL-6 and IL-8 remained substantially elevated up to 48 hours post-CPB. This change in the stoichiometric balance between pro and anti-inflammatory signaling molecules, would favor a heightened and more prolonged proinflammatory state in the early post-operative period. The present study was not designed for measuring the relationship between cytokines to post-operative outcomes, but increased cytokine induction following congenital cardiac surgery has been associated previously with pulmonary dysfunction, capillary leak syndrome, and oxidative stress resulting in changes in endothelial and vascular reactivity and function.17,22
MMP Levels Following Congenital Cardiac Surgery
One of the downstream events which occurs following cytokine activation is the induction and release of proteases such as MMPs.24,25 In addition, oxidative stress, neurohormonal stimulation such as the release of catecholamines, and neutrophil/macrophage degranulation are also contributory mechanisms for the induction of MMPs.24,25 Since all of these systems and pathways are present immediately following cardiac surgery and CPB, then it would be anticipated that MMP release would occur in this post-operative period. Indeed, past studies in adults following cardiac surgery have clearly demonstrated a significant increase in certain MMP types following CPB.13,19–21 Specifically, past studies in adults have identified a robust increase of plasma MMP-8 and -9 following CPB.13,19,21 However, there has been no systematic study to date that has examined a large portfolio of MMPs following congenital cardiac surgery. The present study addressed this issue by measuring a number of soluble MMP types at baseline and at sequential time points post-CPB and yielded several important observations. First, not all MMP types were uniformly increased in the peri-operative period, and the temporal pattern of release appeared to be also different between MMP types. For example, relative MMP-2 levels actually decreased following CPB, whereas MMP-7 only changed marginally in the late post-CPB periods and MMP-13 levels only changed in the early post-CPB periods. This would imply that there are different regulatory pathways for specific MMP types, which would be operative in children undergoing cardiac surgery. Second, MMP types which are most associated with an inflammatory process, such as MMP-8 and MMP-9 increased dramatically in the early post-CPB time points and were temporally related to the release of pro-inflammatory cytokines such as IL-6,-8 and TNF. Thus, while only associative, this would suggest that the early and robust increase in these cytokines would facilitate the egress of neutrophils and subsequent release of MMP-8 and MMP-9. Indeed, the primary source of MMP-8 is neutrophils, and has been consequently termed neutrophil collagenase. The increased levels of these specific MMP types would likely have significant consequences on tissue structure and function in the early post-CPB period. For example, changes in MMP levels can directly affect the wound healing response, and changes in levels of MMP-8 can directly affect the time course of wound healing.29–31 Indeed, levels of MMP-3 and MMP-9, both of which changed in the present study, have been associated with wound healing following burn injury in children.32 Changes in MMP levels will alter endothelial integrity and facilitate the vascular and organ function response with infection.27.28 Since the relative magnitude of MMP release would likely play a contributory factor in endothelial-matrix interaction and stability, then it is likely that this proteolytic system contributes to the loss of capillary integrity which can often occur following congenital cardiac surgery.1,10,17,22
Effects of Anti-Fibrinolytic Treatment on Cytokine and MMP Release Following Congenital Cardiac Surgery
A number of past studies in adults have demonstrated that the serine protease inhibitor aprotinin, reduces relative cytokine release in the early post-CPB period.12–16 In addition, there is indirect evidence to suggest that this anti-cytokine effect of aprotinin may be independent of fibrin proteolysis.3,7,12–15 For example, the relative reduction in cytokine release which is observed with aprotinin, does not occur with the lysine analogue TXA.13–15 Thus, there are likely to be a number of differential pathways which are influenced by aprotinin, and not by lysine analogues such as TXA. In order to address this issue more carefully and in the context of congenital cardiac surgery, the present study examined a balanced group of pediatric patients that presented for surgical correction of VSD or TOF, which received either aprotinin or TXA. The results from this analysis demonstrated that differential cytokine and MMP profiles occurred in pediatric patients receiving aprotinin when compared to TXA. Specifically, aprotinin administration was associated with a relative reduction in the pro-inflammatory cytokines IL-6, IL-8, IL-1beta and TNF in the post-CPB period. The relative reductions in these pro-inflammatory cytokines with aprotinin administration are consistent with past reports in adult cardiac surgery patients.12–16 However, unlike past studies, the present study demonstrated that this relative cytokine suppression with aprotinin was not uniform. Specifically, the IL-2 and INF levels were actually higher in the aprotinin group when compared to the TXA group. This observation would suggest that a different induction pathway exists for these specific cytokines exist which was unaffected by aprotinin. Indeed, a polarization of cytokine expression occurs early in development with subsets of lymphocytes where as T-helper 1 cells express IL-2 and INF, whereas T-helper 2 cells do not.33
There would likely be a number of downstream biological consequences with the relative reduction in cytokine release realized by aprotinin, which would include an attenuation of MMP induction. In adults following cardiac surgery, the relative reduction in IL-6 levels by aprotinin was associated with a concordant reduction in MMP-9 release.13 In the present study, the effects of aprotinin were particularly pronounced with respect to suppressing the release of MMP-8 and MMP-9, when compared to TXA values. These observations underscore the tight interaction between the inflammatory response following congenital cardiac surgery and the induction of specific MMPs. Moreover, the present study demonstrated that aprotinin caused a relative reduction in the early release of inflammatory cells in the post-CPB period, which was likely due to the attenuation of the chemoattractant cytokines IL-6 and IL-8. In turn, the relative reduction in this early inflammatory cell recruitment likely contributed to the reduced MMP-8 and MMP-9 levels observed with aprotinin administration. However, similar to the cytokine profile, the effects of aprotinin on MMP release were not uniform. For example, early post-CPB levels of MMP-13 were higher with aprotinin, whereas levels of MMP-2 and MMP-7 were relatively unaffected. Finally, past studies have suggested that MMPs, such as MMP-3 may play a functional role in facilitating an appropriate wound healing response.30–32 Thus, the suppression of MMPs such as MMP-3 by aprotinin may not necessarily provide beneficial effects. However, future studies which more carefully examine the relationship between changes in MMP levels following congenital cardiac surgery to long term post-operative outcomes will be necessary to address this issue.
Limitations and Conclusions
The present study exploited the fact that aprotinin was suspended from clinical use in order to examine the differential effects of aprotinin and TXA on cytokine and MMP release following congenital cardiac surgery. While this study is the first to provide evidence that these anti-fibrinolytic agents differentially affect cytokine and MMP profiles in the post-operative period, a number of issues remain to be addressed. First, empirical dosing strategies of aprotinin have been associated with long term adverse effects in adults following cardiac surgery.4–7 Indeed, these adverse events resulted in the discontinuation of aprotinin for use in cardiac surgery. However, the use of aprotinin in the pediatric cardiac surgery context has not demonstrated these adverse effects.8–11 For example, while the use of aprotinin in adult cardiac surgery patients was associated with renal dysfunction, several studies in pediatric cardiac surgery patients have not demonstrated this effect.8–11 Moreover, the use of aprotinin in pediatric cardiac surgery has been associated with reduced blood loss and improved pulmonary function,34 both of which can contribute to a complex post-operative course in this patient population.1,2 The present study demonstrated that aprotinin attenuated a specific cytokine and MMP release following congenital cardiac surgery, but a direct cause-effect relation to relevant post-operative outcomes was not demonstrated. Thus, it remains unknown whether the selective effects of aprotinin on cytokine release and MMP induction would impart beneficial effects in congenital cardiac surgery. One of the important steps to full MMP activation is the proteolytic cleavage of the propeptide domain by serine proteases.24,25 Since aprotinin can inhibit a wide spectrum of serine proteases, it may interfere in MMP proteolytic activation. However, the immunoassay approach utilized in the present study could not differentiate the pro-form and active form of the MMP subtypes. Thus, whether aprotinin specifically modifies MMP activational cascades, and that this process is than that of TXA remains speculative. The present study utilized an aprotinin dosing protocol for children using a body surface area algorithm, and past studies have identified that this dosing approach can be highly variable and may not provide sustained serine protease inhibition post-CPB. Thus, whether the effects of cytokine and MMP release observed with aprotinin in the present study would be dose dependent remains to be established.
In light of the fact that the heightened inflammatory response in children undergoing cardiac surgery requiring CPB appears to be associated with a greater incidence of adverse events,1–3 then pharmacological strategies which interrupt the cytokine cascade such as the use of glucocorticoids have been implemented.17,22,35 The present study provides a unique insight into how different anti-fibrinolytic strategies influence the cytokine and protease response following surgical repair of VSD or TOF, and underscore that aprotinin and lysine analogues affect biological processes which may be independent of hemostatic actions. Thus, in light of these observations and the findings of the present study, future investigations to identify the mechanism of action by which blood conservation strategies, including other lysine analogs and next generation serine protease inhibitors, alter biological pathways are warranted.
Acknowledgements
This study was supported by NIH grants HL059165-09, HL057952-08, a Merit Award from the Veterans’ Affairs Health Administration, and the Children’s Cardiomyopathy Foundation, USA.
Footnotes
Presented at the Southern Thoracic Surgical Association 56th Annual Meeting, November 4–7, 2009, Marco Island, FL.
References
- 1.Székely A, Cserép Z, Sápi E, Breuer T, Nagy CA, Vargha P, Hartyánszky I, Szatmári A, Treszl A. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. 2009 Jan;87(1):187–197. doi: 10.1016/j.athoracsur.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Jonas RA, Soul J, Kussman BD, Bellinger DC, Laussen PC, Robertson R, Mayer JE, Jr, del Nido PJ, Bacha EA, Forbess JM, Pigula F, Roth SJ, Visconti KJ, du Plessis AJ, Farrell DM, McGrath E, Rappaport LA, Wypij D. Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008 Feb;135(2):347–354. doi: 10.1016/j.jtcvs.2007.01.051. 354.e1-4. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan S, Das SN, Bisoi A, Kale S, Kiran U. Comparison of epsilon aminocaproic acid and tranexamic acid in pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 2004 Apr;18(2):141–143. doi: 10.1053/j.jvca.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Ngaage DL, Cale AR, Cowen ME, Griffin S, Guvendik L. Aprotinin in primary cardiac surgery: operative outcome of propensity score-matched study. Ann Thorac Surg. 2008 Oct;86(4):1195–1202. doi: 10.1016/j.athoracsur.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 5.Olenchock SA, Jr, Lee PH, Yehoshua T, Murphy SA, Symes J, Tolis G., Jr Impact of aprotinin on adverse clinical outcomes and mortality up to 12 years in a registry of 3,337 patients. Ann Thorac Surg. 2008 Aug;86(2):560–566. doi: 10.1016/j.athoracsur.2008.04.048. discussion 566-7. [DOI] [PubMed] [Google Scholar]
- 6.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008 Feb 21;358(8):771–783. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 7.Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussières JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008 May 29;358(22):2319–2331. doi: 10.1056/NEJMoa0802395. Epub 2008 May 14. [DOI] [PubMed] [Google Scholar]
- 8.Székely A, Sápi E, Breuer T, Kertai MD, Bodor G, Vargha P, Szatmári A. Aprotinin and renal dysfunction after pediatric cardiac surgery. Paediatr Anaesth. 2008 Feb;18(2):151–159. doi: 10.1111/j.1460-9592.2007.02398.x. [DOI] [PubMed] [Google Scholar]
- 9.Guzzetta NA, Evans FM, Rosenberg ES, Fazlollah TM, Baker MJ, Wilson EC, Kaiser AM, Tosone SR, Miller BE. The impact of aprotinin on postoperative renal dysfunction in neonates undergoing cardiopulmonary bypass: a retrospective analysis. Anesth Analg. 2009 Feb;108(2):448–455. doi: 10.1213/ane.0b013e318194007a. [DOI] [PubMed] [Google Scholar]
- 10.Manrique A, Jooste EH, Kuch BA, Lichtenstein SE, Morell V, Munoz R, Ellis D, Davis PJ. The association of renal dysfunction and the use of aprotinin in patients undergoing congenital cardiac surgery requiring cardiopulmonary bypass. Anesth Analg. 2009 Jul;109(1):45–52. doi: 10.1213/ane.0b013e3181a7f00a. [DOI] [PubMed] [Google Scholar]
- 11.Backer CL, Kelle AM, Stewart RD, Suresh SC, Ali FN, Cohn RA, Seshadri R, Mavroudis C. Aprotinin is safe in pediatric patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2007 Dec;134(6):1421–1426. doi: 10.1016/j.jtcvs.2007.08.006. discussion 1426-8. [DOI] [PubMed] [Google Scholar]
- 12.Brown JR, Toler AW, Kramer RS, Landis RC. Anti-inflammatory effect of aprotinin: a meta-analysis. J Extra Corpor Technol. 2009 Jun;41(2):79–86. [PMC free article] [PubMed] [Google Scholar]
- 13.Dorman BH, Stroud RE, Wyckoff MM, Zellner JL, Botta D, Leonardi AH, et al. Differential effects of epsilon-aminocaproic acid and aprotinin on matrix metalloproteinase release in patients following cardiopulmonary bypass. J Cardiovasc Pharmacol. 2008 Apr;51(4):418–423. doi: 10.1097/FJC.0b013e318168400a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greilich PE, Okada K, Latham P, Kumar RR, Jessen ME. Aprotinin but not epsilon-aminocaproic acid decreases interleukin-10 after cardiac surgery with extracorporeal circulation: randomized, double-blind, placebo-controlled study in patients receiving aprotinin and epsilon-aminocaproic acid. Circulation. 2001 Sep 18;104(12) Suppl 1:I265–I269. doi: 10.1161/hc37t1.094781. [DOI] [PubMed] [Google Scholar]
- 15.Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001 Feb;71(2):745–754. doi: 10.1016/s0003-4975(00)02218-9. [DOI] [PubMed] [Google Scholar]
- 16.Greilich PE, Brouse CF, Whitten CW, Chi L, Dimaio JM, Jessen ME. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double-blind, placebo-controlled study of epsilon-aminocaproic acid and aprotinin. J Thorac Cardiovasc Surg. 2003 Nov;126(5):1498–1503. doi: 10.1016/s0022-5223(03)00946-2. [DOI] [PubMed] [Google Scholar]
- 17.Brix-Christensen V. The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand. 2001 Jul;45(6):671–679. doi: 10.1034/j.1399-6576.2001.045006671.x. [DOI] [PubMed] [Google Scholar]
- 18.Berdat PA, Eichenberger E, Ebell J, Pfammatter JP, Pavlovic M, Zobrist C, Gygax E, Nydegger U, Carrel T. Elimination of proinflammatory cytokines in pediatric cardiac surgery: analysis of ultrafiltration method and filter type. J Thorac Cardiovasc Surg. 2004 Jun;127(6):1688–1696. doi: 10.1016/j.jtcvs.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Lin TC, Li CY, Tsai CS, Ku CH, Wu CT, Wong CS, et al. Neutrophil-mediated secretion and activation of matrix metalloproteinase-9 during cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2005 Jun;100(6):1554–1560. doi: 10.1213/01.ANE.0000154307.92060.84. [DOI] [PubMed] [Google Scholar]
- 20.Mayers I, Hurst T, Puttagunta L, Radomski A, Mycyk T, Sawicki G, et al. Cardiac surgery increases the activity of matrix metalloproteinases and nitric oxide synthase in human hearts. J Thorac Cardiovasc Surg. 2001 Oct;122(4):746–752. doi: 10.1067/mtc.2001.116207. [DOI] [PubMed] [Google Scholar]
- 21.Joffs C, Gunasinghe HR, Multani MM, Dorman BH, Kratz JM, Crumbley AJ, 3rd, et al. Cardiopulmonary bypass induces the synthesis and release of matrix metalloproteinases. Ann Thorac Surg. 2001 May;71(5):1518–1523. doi: 10.1016/s0003-4975(01)02442-0. [DOI] [PubMed] [Google Scholar]
- 22.Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006 Jun;81(6):S2347–S2354. doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 23.Madhok AB, Ojamaa K, Haridas V, Parnell VA, Pahwa S, Chowdhury D. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006 Jul–Aug;27(4):408–413. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 24.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003 May 2;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 25.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007 Oct;87(4):1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 26.Ford RL, Mains IM, Hilton EJ, Reeves ST, Stroud RE, Crawford FA, Jr, et al. Endothelin-A receptor inhibition after cardiopulmonary bypass: cytokines and receptor activation. Ann Thorac Surg. 2008 Nov;86(5):1576–1583. doi: 10.1016/j.athoracsur.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorente L, Martín MM, Solé-Violán J, Blanquer J, Páramo JA. Matrix metalloproteinases and their inhibitors as biomarkers of severity in sepsis. Crit Care. 2010 Jan 19;14(1):402. doi: 10.1186/cc8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cena JJ, Lalu MM, Cho WJ, Chow AK, Bagdan ML, Daniel EE, Castro MM, Schulz R. Inhibition of matrix metalloproteinase activity in vivo protects against vascular hyporeactivity in endotoxemia. Am J Physiol Heart Circ Physiol. 2010 Jan;298(1):H45–H51. doi: 10.1152/ajpheart.00273.2009. [DOI] [PubMed] [Google Scholar]
- 29.Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009 Nov–Dec;17(6):832–839. doi: 10.1111/j.1524-475X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 30.Gutiérrez-Fernández A, Inada M, Balbín M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noël A, Werb Z, Krane SM, López-Otín C, Puente XS. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007 Aug;21(10):2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirilä E, Korpi JT, Korkiamäki T, Jahkola T, Gutierrez-Fernandez A, Lopez-Otin C, Saarialho-Kere U, Salo T, Sorsa T. Collagenase-2 (MMP-8) and matrilysin-2 (MMP-26) expression in human wounds of different etiologies. Wound Repair Regen. 2007 Jan–Feb;15(1):47–57. doi: 10.1111/j.1524-475X.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 32.Dasu MR, Spies M, Barrow RE, Herndon DN. Matrix metalloproteinases and their tissue inhibitors in severely burned children. Wound Repair Regen. 2003 May–Jun;11(3):177–180. doi: 10.1046/j.1524-475x.2003.11305.x. [DOI] [PubMed] [Google Scholar]
- 33.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004 Jul;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 34.Mössinger H, Dietrich W, Braun SL, Jochum M, Meisner H, Richter JA. High-dose aprotinin reduces activation of hemostasis, allogeneic blood requirement, and duration of postoperative ventilation in pediatric cardiac surgery. Ann Thorac Surg. 2003 Feb;75(2):430–437. doi: 10.1016/s0003-4975(02)04412-0. [DOI] [PubMed] [Google Scholar]
- 35.Checchia PA, Bronicki RA, Costello JM, Nelson DP. Steroid use before pediatric cardiac operations using cardiopulmonary bypass: an international survey of 36 centers. Pediatr Crit Care Med. 2005 Jul;6(4):441–444. doi: 10.1097/01.PCC.0000163678.20704.C5. [DOI] [PubMed] [Google Scholar]