Abstract
An efficient synthetic methodology for 3-hydroxy-2,2-dimethyloctynoic acid (DHOYA) and several variants, which are increasingly common fragments encountered in bioactive marine cyanobacterial metabolites, was developed. These fragments were obtained in three steps via a tertiary aldol reaction utilizing an Evans’ chiral auxiliary to afford the desired stereochemistry at the β-hydroxy carbon. Thus far, this methodology has been successfully applied in determination of the absolute stereochemistry of eight cyanobacterial natural products, including the VGSC activator palymramide A.
Keywords: DHOYA, Evans’ chiral auxiliaries, Titanium Lewis acid, Terminal alkyne, Tertiary aldol
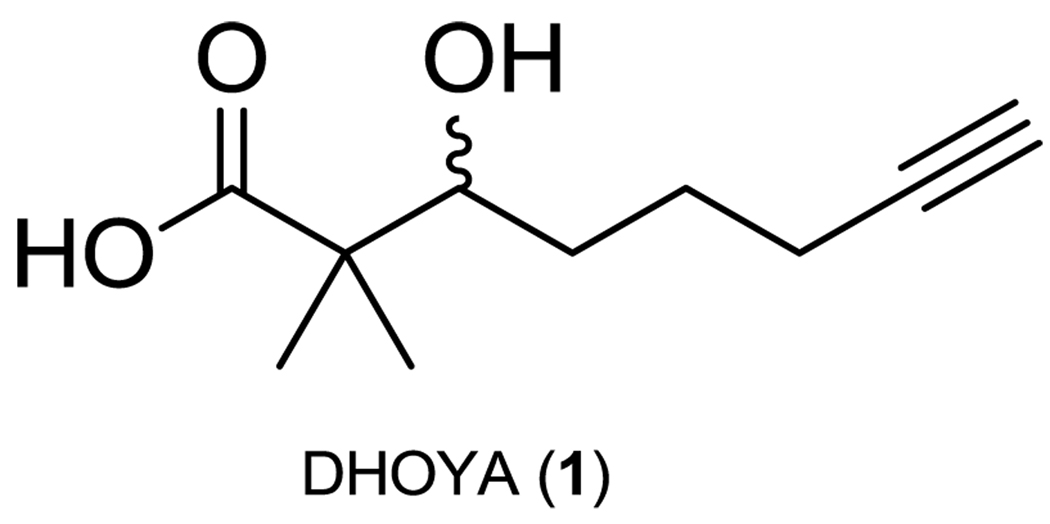
The α,α-dimethyl-β-hydroxy carbonyl functionality is a unique structural feature of a number of bioactive natural products, including the epothilones,1 mycalamide A,2 and peloruside A.3 Recently a number of marine cyanobacterial natural products possessing an α,α-dimethyl-β-hydroxy carbonyl functionality in the form of a 3-hydroxy-2,2-dimethyloctynoic acid (DHOYA, 1) fragment, or a derivative thereof, have been described (Figure 1). Naturally occurring modifications of the structural motif represented by 1 include varying degrees of saturation, chain shortening, replacement of the α-hydroxy group by an amine, and mono-methylation at the α-carbon. Other derivatives of 1 feature multiple modifications; examples include antanapeptin B,4 onchidin,5 and malevamide B.6 Natural products featuring 1 or a variant on this motif display a variety of biological activities including cytotoxicity to a number of cancer cell lines,7–9 anti-microbial activity to mycobacteria,8 anti-parasitic activity10 and brine shrimp toxicity.11,12 Given the range of biological activities of these molecules and the diversity of the modifications observed for fragment 1, there is a compelling need for efficient synthetic methodologies to access this structural class as well as produce synthetic analogues for drug development purposes.
Figure 1.
3-hydroxy-2,2-dimethyloctynoic acid (DHOYA, 1).
Moreover, one of the more difficult aspects of the structure elucidation of depsipeptides containing 1 is the determination of absolute configuration of the β-hydroxy ester linkage of fragment 1. Chiral GCMS comparison of the hydrogenated and methyl esterified derivative of 1 (i.e. methyl 3-hydroxy-2,2-dimethyloctanoate) to synthetic standards affords a pragmatic approach for determining the absolute configuration of this stereocenter.9 However, a critical requirement for this methodology is the availability of enantiomerically pure synthetic standards.
Fragment 1 has previously been synthesized in 9 steps using an asymmetric Kiyooka-Muikaiyama aldol reaction during total synthesis of yanucamide A13 and pitipeptolide A14 and to provide standards for chiral GCMS analysis of the wewakpeptins.9 Additional approaches for construction of the α,α-dimethyl-β-hydroxy carbonyl backbone via a tertiary enolate have been applied in partial and total syntheses of peloruside A,15,16 and in the total synthesis of pasteurestins A and B;17 in the latter case, a Reformatsky reaction employing a bromoacyl derivative of Evans’ chiral auxiliary and a TMS-protected terminal alkyne was employed.
However, we conceived a more efficient synthetic route in which fragment 1 could be achieved enantioselectively via a tertiary aldol reaction with an acylated Evans’ chiral auxiliary and an aldehyde possessing an unprotected terminal alkyne. As the terminal alkyne of fragment 1 is unstable to acid hydrolysis, which is usually the first step in the stereochemical determination of depsipeptides, saturated 3-hydroxy-2,2-dimethyloctanoic acid (DHOAA, 2) is preferred for chiral GCMS analysis.7,9 Thus, our initial studies focused on the synthesis of fragment 2.
Since the key reaction step in this scheme requires an Evans’ chiral auxiliary that is not commercially available, the desired starting material was obtained via acylation of (R)-4-benzyl-2-oxazolidinone with isobutyryl chloride to give (R)-4-benzyl-3-isobutyryloxazolidin-2-one (3) in good yield (86%). Initially, we attempted the aldol reaction of (4′R)-3 and hexanal using LDA alone; however, the expected aldol product was not formed based on LR-ESI-LCMS and 1H NMR analyses. We suspected that a stronger Lewis acid was required for formation and stabilization of the tertiary enolate. We initially selected a titanium Lewis acid as we thought the titanium enolate would best facilitate the coordination of the aldehyde via the Zimmerman-Traxler transition state (see Table 1).18,19 A similar strategy has been employed previously by the Kobayashi group in which LDA was used in combination with TiCl(O-i-Pr)3 to form the titanium enolate in preparation of a 1,2-diol with a quaternary chiral center.19
Table 1.
Optimization of aldol reaction in the synthesis of 2.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | S.M.a | Scaleb | Aldehyde (equiv) |
Temperature conditions |
Product | Yieldc (%) |
| 1 | (4’R)-3 | 3.8 | 1.2 | −40°C, 1.5 h | (3R,4’R)-4 | 35 |
| 2 | (4’S)-3 | 1.7 | 1.2 | −40°C, 1 h | (3S,4’S)-4 | 32 |
| 3 | (4’S)-6 | 0.2 | 1.2 | −40°C, 3 h | (3S,4’S)-7 | 41 |
| 4 | (4’S)-6 | 0.1 | 1.2 | −78°C→ 22°C, 18 h | (3S,4’S)-7 | 9 |
| 5 | (4’S)-6 | 0.2 | 3.0 | −40°C, 3 h | (3S,4’S)-7 | 58 |
| 6 | (4’S)-6 | 0.2 | 3.0 | −40°C, 3 h; 0°C, 2 h | (3S,4’S)-7 | 98d |
| 7 | (4’S)-6 | 0.2 | 3.0 | −40°C, 3 h; 0°C, 2 h | (3S,4’S)-7 | 91 |
| 8 | (4’R)-6 | 0.2 | 3.0 | −40°C, 3 h; 0°C, 2 h | (3R,4’R)-7 | 82 |
S.M.: starting material.
Scale based on mmol of starting material.
Isolated yield following flash column chromatography.
Enantiomeric ratio ≥ 50:1 according to 1H NMR and chiral GCMS analyses.
Thus, we attempted the aldol addition of hexanal (1.2 eq) and (4′R)-3 using LDA (1.5 eq) and TiCl(O-i-Pr)3 (4 eq). The reaction was stirred for 1.5 h at −40°C and proceeded favorably to give (3R,4′R)-4 in 35% yield (entry 1, Table 1). The configuration of the β-alcohol in this intermediate was confirmed as R by Mosher’s analysis, which was consistent with the predicted Zimmerman-Traxler transition state model.19 The Evans’ chiral auxiliary was removed via hydrolysis to furnish (3R)-2 with an overall yield of 29%. The synthesis was also conducted starting with (S)-4-benzyl-2-oxazolidinone. The aldol reaction with (4′S)-3 gave (3S,4′S)-4 with a 32% yield (entry 2, Table 1), while hydrolysis afforded the final product (3S)-2 with an overall yield of 24%.
These initial results verified that this three step synthesis was indeed a viable route for the generation of (3R)-2 and (3S)-2. These products are invaluable for use as standards in the chiral GCMS analysis of the β-hydroxy chiral center of marine natural products featuring 1 or a saturated derivative. One such application was in the case of mantillamide A where 5.2 mg was isolated from field collections; however, only 0.2 mg of the natural product was required for chiral GCMS analysis. Comparison of the methyl esterified fragment derived from mantillamide A to methyl esterified (3R)-2 and (3S)-2 obtained using the described synthetic route revealed that mantillamide A possesses an R-DHOYA unit. The retention time for the natural product was 53.67, while the retention time for the R-enantiomer was 53.64 and the retention time for the S-enantiomer was 52.08. Co-injections of the natural product with the R- and S-enantiomers confirmed the assignment as R. While the route was efficient for preparation of standards for stereochemical analyses, the overall yield was quite low. Thus, we investigated optimization of the aldol reaction to improve the overall yield making the route more attractive for utilization in total syntheses.
Suspecting that an additional deprotonation may be occurring on C-5, resulting in decomposition of the starting material and therefore contributing to the observed low yield, an Evans’ chiral auxiliary [(4′S)-5], featuring gem-dimethyl substitution at C-5, was employed as the starting material in subsequent reactions. Acylation of (4′S)-5 gave (4′S)-6 (97% yield), which was then utilized in the aldol reaction, and resulted in an increase in yield from 32% for (3S,4′S)-4 to 41% for (3S,4′S)-7 (entry 3). In subsequent reactions, 1M solutions in THF were prepared from neat TiCl(O-i-Pr)3 due to concerns that hexanes present in the commercial 1M preparation may be adversely affecting solvation of the enolate. Additionally, the amount of aldehyde was increased from 1.2 to 3 equivalents and the reaction time was adjusted to 3 h, resulting in an increase in yield from 41% to 58% (entry 5). Next, the final temperature was increased to 0°C after 3 h and the reaction was run for an additional 2 h, resulting in an excellent yield of 98% (entry 6). While 1H NMR analysis of the crude aldol product did not indicate the presence of diastereomers, the aldol product was hydrolyzed to give (3S)-2, which was methyl esterified and analyzed by chiral GCMS analysis. The enantiomeric ratio was determined as 50:1 for the desired enantiomer based on peak area. Repetition of these favorable aldol reaction conditions affirmed the observed result and high yield (a 91% yield was achieved, entry 7). Subsequently, the aldol reaction was conducted with (4′R)-6 and an 82% yield was obtained.
Additional reactions substituting butanal for hexanal were conducted to provide (S)- and (R)-3-hydroxy-2,2-dimethylhexanoic acid (DMHHA) for use as standards for chiral analysis of the β-hydroxy stereocenter in palmyramide A,21 a natural product which possesses the DMHHA residue. In both of these reactions, 3 equivalents of aldehyde were used and the final reaction temperature was −40°C. The S-enantiomer was obtained in 54% yield (entry 1, Table 2), while the R-enantiomer was obtained in 35% yield (entry 2). Comparison of the yield for the S-enantiomer from this aldol reaction to its two carbon extended counterpart reveals that the yields are not significantly different, 54% for the former compared to 58% for the latter, suggesting that chain length does not substantially impact yield.
Table 2.
Tertiary aldol reaction with additional aldehydes.
 | |||||
|---|---|---|---|---|---|
| Entry | S.M.a | Aldehydeb | Temperature conditions |
Product | Yieldc (%) |
| 1 | (4’S)-6 | Butanal | −40°C, 3 h | (3S,4’S)-8 | 54 |
| 2 | (4’R)-6 | Butanal | −40°C, 3 h | (3R,4’R)-8 | 35 |
| 3 | (4’S)-6 | 5-hexynal | −40°C, 3 h | (3S,4’S)-9 | 27 |
| 4 | (4’S)-6 | 5-hexynal | −40°C, 3 h; 0°C, 2 h | (3S,4’S)-9 | 58 |
| 5 | (4’R)-6 | 5-hexynal | −40°C, 3 h; 0°C, 2 h | (3R,4’R)-9 | 74 |
S.M.: starting material.
1.2 equivalents of aldehyde were used for entry 3, whereas 3 equivalents of aldehyde were used for all other entries
Isolated yield following flash column chromatography.
Having successfully developed a route for generating synthetic (3R)-and (3S)-2, as well as (3R)- and (3S)-DMHHA, as standards for stereochemical determination at the β position of fragment 1 and the DMHHA fragment, we wanted to explore the applicability of this route for generation of chiral fragment 1 itself. Thus, the aldol reaction was conducted with (4′S)-6 utilizing 1.2 equivalents of 5-hexynl-1-al (prepared from 5-hexyn-1-ol according to Böttcher and Sieber)22 with the final temperature at −40°C for 3 h, resulting in a 27% yield (entry 3, Table 2) as compared to a 41% yield under the same conditions using hexanal (entry 3, Table 1). Due to the considerable lability of the alkynyl aldehyde, in comparison with the saturated aldehyde, a lower yield was expected for this reaction; however this result revealed that the reaction conditions were amenable for use with unprotected alkynyl aldehydes. Using these improved reaction conditions with 3 equivalents of aldehyde and increasing the final temperature to 0°C, the aldol reactions were conducted with 5-hexyn-1-al and both (4′S)- and (4′R)-6 to give yields of 58% and 74%, respectively (entries 4 and 5, Table 2).
Next, we verified that the hydrolysis conditions were suitable for use with these various substrates to provide the desired free acids. Hydrolysis of (3R,4′R)-7 and (3S,4′S)-7 with LiOH and H2O2 gave the desired products with a 68% yield for (3R)-2 and an 87% yield for (3S)-2, resulting in an overall yield of 52% for the R-enantiomer and 83% for the S-enantiomer. This is a substantial increase in yield in comparison with the initial yields of 29% for (3R)-2 and 24% for (3S)-2. Hydrolysis of the aldol products featuring a terminal alkyne afforded (3S)-1 with a yield of 84% (47% overall yield) and (3R)-1 with a yield of 81% (56% overall yield). This represents a significant improvement in the efficiency and yield for the synthesis of fragment 1 compared to previously published synthesis.13,14
In summary, we have developed a new synthetic route for the α,α-dimethyl-β-hydroxy carbonyl motif exemplified by fragment 1. This represents a significant advance in methodology for the total synthesis of compounds featuring fragment 1 or related residues. Future directions of this research include exploring the utility of thiazolidinethiones and oxazolidinethiones to afford the desired (R) and (S) aldol products from a single chiral auxiliary, and total synthesis of biologically active cyanobacterial secondary metabolites featuring fragment 1.
Supplementary Material
Figure 2.
Mantillamide A, a secondary metabolite featuring fragment 1 (highlighted in red).
Scheme 1.
Optimized reaction conditions for generation of fragments 1 and 2 via tertiary aldol reaction.
Acknowledgments
We thank Y. Su (UCSD Chemistry and Biochemistry Mass Spectrometry Facility) for HRMS data. Financial support for this work was provided by NIH TW006634 and NIH GM067550.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material
Experimental procedures and spectroscopic data for all new compounds presented in this article can be found in the online version.
References and notes
- 1.Höfle G, Bedorf N, Steinmetz H, Schomburg D, Gerth K, Reichenbach H. Angew. Chem., Int. Ed. Engl. 1996;35:1567. [Google Scholar]
- 2.Perry NB, Blunt JW, Munro MHG, Pannell LK. J. Am. Chem. Soc. 1988;110:4850. [Google Scholar]
- 3.West LM, Northcote PT, Battershill CN. J. Org. Chem. 2000;65:445. doi: 10.1021/jo991296y. [DOI] [PubMed] [Google Scholar]
- 4.Nogle LM, Gerwick WH. J. Nat. Prod. 2002;65:21. doi: 10.1021/np010348n. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez J, Fernández R, Quiñoá E, Riguera R, Debitus C, Bouchet P. Tetrahedron Lett. 1994;35:9239. [Google Scholar]
- 6.Horgen FD, Yoshida WY, Scheuer PJ. J. Nat. Prod. 2000;63:461. doi: 10.1021/np990449+. [DOI] [PubMed] [Google Scholar]
- 7.Reese MT, Gulavita NK, Nakao Y, Hamann MT, Yoshida WY, Coval SJ, Scheuer PJ. J. Am. Chem. Soc. 1996;118:11081. [Google Scholar]
- 8.Luesch H, Pangilinan R, Yoshida WY, Moore RE, Paul VJ. J. Nat. Prod. 2001;64:304. doi: 10.1021/np000456u. [DOI] [PubMed] [Google Scholar]
- 9.Han B, Goeger D, Maier CS, Gerwick WH. J. Org. Chem. 2005;70:3133. doi: 10.1021/jo0478858. [DOI] [PubMed] [Google Scholar]
- 10.Linington RL, Nunnery JK, Suyama TL, Ureña L-D, Almanza A, Gonzalez J, Romero LI, Ortega-Barria E, Kyle DE, Gerwick WH. Manuscript in preparation. [Google Scholar]
- 11.Sitachitta N, Williamson RT, Gerwick WH. J. Nat. Prod. 2000;63:197. doi: 10.1021/np990466z. [DOI] [PubMed] [Google Scholar]
- 12.Bunyajetpong S, Yoshida WY, Sitachitta N, Kaya K. J. Nat. Prod. 2006;69:1539. doi: 10.1021/np050485a. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z, Peng Y, Ye T. Org. Lett. 2003;5:2821. doi: 10.1021/ol034803d. [DOI] [PubMed] [Google Scholar]
- 14.Peng Y, Pang H, Xu Z, Ye T. Lett. Org. Chem. 2005;2:703. [Google Scholar]
- 15.Taylor RE, Jin M. Org. Lett. 2003;5:4959. doi: 10.1021/ol0358814. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh AK, Xu X, Kim J-H, Xu C-X. Org. Lett. 2008;10:1001. doi: 10.1021/ol703091b. [DOI] [PubMed] [Google Scholar]
- 17.Kögl M, Brecker L, Warrass R, Mulzer J. Eur. J. Org. Chem. 2008:2714. [Google Scholar]
- 18.Nerz-Stormes M, Thornton ER. J. Org. Chem. 1991;56:2489. [Google Scholar]
- 19.Murata Y, Kamino T, Hosokawa S, Kobayashi S. Tetrahedron Lett. 2002;43:8121. [Google Scholar]
- 20.Davies DG, Sanganee HJ. Tetrahedron: Asymmetry. 1995;6:671. [Google Scholar]
- 21.Taniguchi M, Nunnery JK, Engene N, Esquenazi E, Byrum T, Dorrestein PC, Gerwick WH. J. Nat. Prod. 2010;73:393. doi: 10.1021/np900428h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Böttcher T, Sieber SA. Angew. Chem., Int. Ed. Engl. 2008;47:4600–4603. doi: 10.1002/anie.200705768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.