Abstract
Objectives
Adenosine is produced in response to ischemia or inflammation and protects tissues from injury. There are four adenosine receptors, which play a critical role in the physiological negative-feedback mechanism for limitation and termination of tissue-specific and systemic inflammatory responses. Accumulating evidence has focused on the anti-inflammatory and immunosuppressive role of the adenosine 2A receptor (A2AR), and we have previously reported on its’ role in the development of bronchiolitis obliterans (BO) following lung transplantation. However, few studies have reported on the role of the adenosine 2B receptor (A2BR) in BO. Data suggests that the A2BR has pro-inflammatory and profibrotic roles. We hypothesized that adenosine signaling through the A2BR is involved in the development of BO.
Methods
A murine heterotopic tracheal model across a total alloantigeneic mismatch was used to study A2BR signaling in BO. Tracheal transplants consisted of Balb/c donor tracheas transplanted into wild-type or A2BR knockout C57BL/6 recipients. Transplanted tracheas were removed 3, 7, 12, and 21 days after transplantation. The luminal obliteration was evaluated through hematoxylin and eosin staining and the cellular infiltration (macrophage, neutrophil, CD3+ and Foxp3+ regulatory T cell) was detected by immunohistochemical staining.
Results
In comparison to allografts in wild type recipients, tracheas transplanted into A2BR knockout mice displayed less BO development on day 21. A2BR knockout mice had an increase in CD3+ T cells and CD4+/CD25+/Foxp3+ regulatory T cells when compared to wild type on day 7. By day 12, more CD3+ T cells were present in the wild-type trachea compared to the A2BR KO, but the percentage of CD4+/CD25+/Foxp3+ regulatory T cells remained higher in the tracheas of A2BR KO mice.
Conclusions
A2BR stimulation may promote the development of BO via inhibiting CD4+/CD25+/Foxp3+ regulatory T cell infiltration.
Keywords: bronchiolitis obliterans, Adenosine2B Receptor, lung injury
INTRODUCTION
Lung transplantation is currently recognized as an effective treatment for selected patients with end-stage pulmonary diseases. Despite notable advances in the last decade in operative techniques, immunosuppresion and critical care, long term mortality for lung recipients remains the highest of all solid organ transplantations and is still hampered by the development of chronic rejection (1). The development of bronchiolitis obliterans (BO) following lung transplantation is a key factor contributing to patient mortality. BO is a form of chronic allograft rejection, characterized by histologic evidence of peribronchiolar leukocyte infiltration and subsequent fibro-obliteration of the small airways. Once BO develops, patients frequently experience a progressive decline in pulmonary function and most die of respiratory failure within 5 years of onset. Furthermore, BO responds poorly to immunosuppression. Thus, further understanding of the molecular mechanisms of BO development is critical to develop novel treatment strategies to prevent this devastating complication.
Extracellular adenosine elicits its’ biological effects via activation of four distinct adenosine receptors (A1, A2A, A2B and A3)(2). Accumulated data indicates that endogenous adenosine released following cell injury confers protection against ischemia-reperfusion injury (IRI) in liver(3), kidney (4), heart(5) and lung(6). Commensurate with other studies, we have previously reported on the protective role of adenosine signaling via the A2AR in IRI and BO (6, 7). Specifically, we have demonstrated that murine tracheal allografts in A2AR knockout recipients displayed increased inflammation and more severe BO development compared to wild type C57BL/6 mice (7). Alternatively, since the A2BR is the most highly expressed adenosine receptor within human donor bronchial epithelia (8) and human bronchial epithelial cell lines (9), it is plausible that it may also be involved in certain pulmonary diseases. Some reports suggest that adenosine signaling via the A2BR exerts a proinflammatory effect within pulmonary tissue (9, 10), while other studies have demonstrated an anti-inflammatory effect in mouse acute lung injury models (11, 12). The role of A2BR activation in the development of BO following lung transplantation remains unknown. Consequently, in this study, we investigated the potential role of adenosine signaling via the A2B R in a murine heterotopic tracheal transplant model of BO development.
METHODS
Animals
Balb/c mice (Jackson Laboratory, Bar Harbor, ME) were used as donors. C57BL/6 mice (Wild-Type; WT, B6) (Jackson Laboratory, Bar Harbor, ME) and A2BR knock out (A2BR KO; on a C57BL/6 background) male mice (Linden’s Laboratory) (25–35 grams) were used as recipients. The experimental protocol was reviewed and approved by the Animal Care and Use Committee at the University of Virginia.
Experimental group design
As previously described, we utilized a heterotopic subcutaneous tracheal transplant model of BO (7). Experimental mice were divided into two groups: (1) Balb/c tracheas transplanted to C57BL/6 mice; and (2) Balb/c tracheas transplanted to A2BR KO mice. A total of 96 donors and 24 recipients were used in each group: four donor tracheas were transplanted into one recipient, 3 recipients were used in each group and each time point (4 time points: day 3, 7, 12 and 21).
Histology
After sacrifice, all tracheal allografts were collected at each time point for histology and immunohistochemical staining. Transplanted tracheal tissues were processed according to our previous methods (7).
Immunohistochemical Staining of Migratory Macrophages and Neutrophils
Macrophages and neutrophils were detected by immunohistochemical staining as previously described (7, 13). We used either rat anti-mouse neutrophil (AbD Serotec, Raleigh, NC) or rat anti-mouse migratory macrophage (Mac-2, Accurate Chem, Westbury, NY) as primary antibodies. The secondary antibody was alkaline phosphatase - conjugated anti-rat IgG (Sigma, St Louis MO). Positively staining cells were detected with Fast-Red (Sigma, St Louis MO). Macrophage and neutrophil infiltration were semiquantified using Imag-Pro Plus software (7, 13). The average value of each group (at least five different tracheal sections) was obtained for statistical analysis.
Immunohistochemical staining of CD3+ T cells, CD4+/CD25+/Foxp3+ regulatory T cells and CD31 molecule in the allografts
All CD3+ T cells, CD4+/CD25+/Foxp3+ regulatory T cells and CD31 were stained and quantified according to previous methods (7). After antigen unmasking and blocking, CD4+/CD25+/Foxp 3+ regulatory T cells were detected using a polyclonal rabbit anti-mouse Foxp3 primary antibody (Abcam Inc, Cambridge, MA). The number of infiltrated CD3+ T cells and CD4+/CD25+/Foxp 3+ regulatory T cells were counted and used for statistical analysis. In addition, goat anti-mouse CD31 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) was used as primary antibody to detect CD31 expression in all allografts. The expression of CD31 was semiquantified, and the average value of each group was obtained for statistical analysis.
Measurement of the luminal obliteration
Total luminal area as well as the area of the obliterated lumens of transplanted allografts were measured using the Image-Pro Plus software as previously described(7). The percent of obliteration was calculated by dividing the area of tracheal fibrosis by the total luminal area. Eight to ten allografts were measured in each group.
Statistical analysis
Data are presented as the mean ± SEM. For comparison between two groups, the Student's t test was used. For comparison of more than two groups, the macrophage, neutrophil and CD3+ T cell counts were processed using one-way ANOVA followed by Bonferroni correction. P<0.05 was considered significant.
RESULTS
The following histopathological kinetics for untreated tracheal allografts post-transplantation have been characterized: 1) IRI within the first 3 days associated with loss of the airway epithelium, 2) AR from 3 to 14 days with early recovery then later loss of the epithelium; and 3) complete loss of epithelium and fibro-obliteration of the airway from 14 to 28 days post-transplantation(7).
Histopathological Comparison of Allografts between Two Groups at Various Time points
The histopathological staining patterns demonstrate the kinetics of allografts post-transplantation among different groups and different time points (Figure 1). Similar to our previous report (7), allografts within both A2BR KO (Balb/c into A2BR KO) and control mice (Balb/c into C57BL/6) exhibited a complete loss of the airway epithelium within the first 3 days (Figure 1A and 1B). From 3 to 7 days, lost epithelial cells were regenerated and formed a pseudoepithelial layer on the basement membrane of the allografts (Figure 1C and 1D). By day 12, the majority of the pseudoepithelial layer was absent again in both groups (Figure 1E and 1F). However, compared to controls, the intensity and timing of the epithelial loss was decreased in A2BR KO recipients on day 12 (1E). Even on day 21, some of the epithelia cells were still observed on the basement membrane of the allografts in A2BR KO recipients (Figure 1G), while the epithelia cells were completely lost and the lumen was obliterated in the C57BL/6 (Figure 1H). Within isograft control mice, epithelial cells loss similarly occurred at day 3 with regeneration at day 7; however, no further cell loss was observed up through day 21 (Figure 1I and 1J). Moreover, there was no histological evidence of BO development by day 21 among transplanted isografts (Figure 1I and 1J).
Figure 1. Comparison of histopathological characters of the allografts in A2BR KO and C57BL/6 recipients on days 3, 7 12 and 21.
A) Illustration of tracheal thickness measurement in the allografts. All the HE staining pictures were taken under microscope with 4X magnification. The donor tracheas were Balb/c mice. A, C, E and G are examples of allografts in A2BR KO recipients at days 3, 7, 12 and 21, respectively. B, D, F and H are examples of allografts in C57BL/6 recipients at days 3, 7, 12 and 21, respectively. The high magnification (40X) of the allografts are also displayed at the right-bottom corner of each representative picture to show the changes of epithelial cells post transplantation. I (4X magnification) and J (40X magnification) is a representative isograft of Balb/c mice at days 21.
Macrophage and Neutrophil Infiltration into Allografts
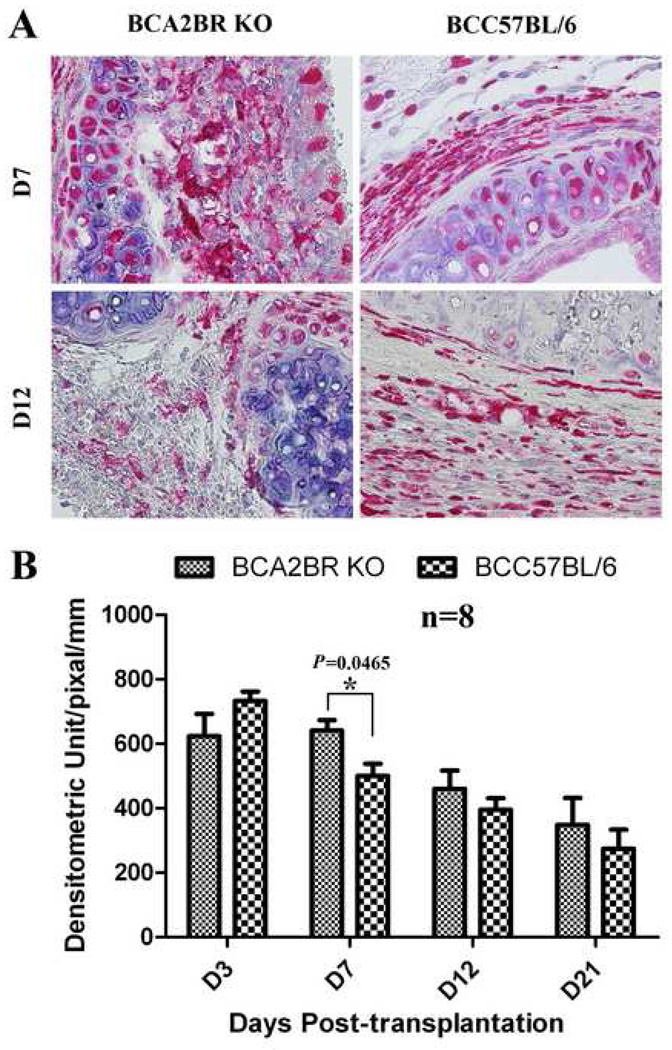
Macrophage and neutrophil infiltration is a hallmark feature of IRI and the resultant inflammatory response. Consequently, we investigated migratory macrophage and neutrophil infiltration at four different time points following transplantation. Our results (Figure 2) demonstrate that macrophage infiltration peaked on days 3 and 7 and gradually decreased thereafter, while neutrophil infiltration (Figure 3) peaked on day 3 and day 12 and decreased on day 7 and day 21 within allografts of both groups. Moreover, macrophage infiltration in the allografts of A2BR KO recipients was significantly elevated on day 7 compared to control recipients (P=0.0465), while neutrophil infiltration was decreased on days 3 (P=0.0122) and 21 (P=0.0026). These findings indicate that adenosine signaling via the A2BR is involved in the early inflammatory response following tracheal transplantation.
Figure 2. Immunohistochemical staining of migratory macrophages in the allografts of Balb/c to A2BR KO and Balb/c to C57BL/6.
A) Representative migratory macrophages staining in allografts at days 7 and 12 post-transplantation. Cells stained red indicate migratory macrophage infiltration. All sections were counterstained lightly with hematoxylin for viewing negatively stained cells. The slides were stained with rat anti-mouse Mac-2 antibody. All pictures are 40X magnification. B) The bar graph shows the analysis of positive immunostaining of migratory macrophages in the allografts from days 3 to 21.
Figure 3. Immunohistochemical staining of neutrophils in the allografts of Balb/c to A2BR KO, Balb/c and C57BL/6.
A) Representative pictures of neutrophil staining in allografts at days 7 and 12 post-transplantation. Cells stained red indicate neutrophil infiltration. All sections were counterstained lightly with hematoxylin for viewing negatively stained cells. The slides were stained with rat anti-mouse neutrophil antibody. All pictures are 40X magnification. B) The bar graph shows the analysis of positive immunostaining of neutrophils in the allografts from day 3 to day 21.
CD3+ T-lymphocyte infiltration into allografts
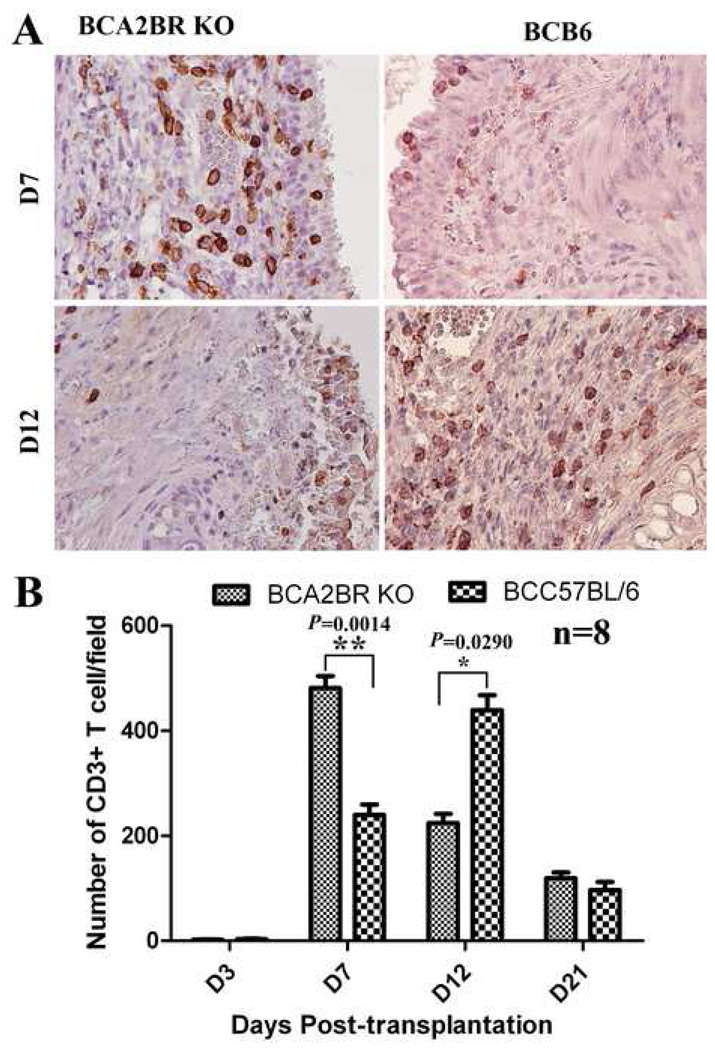
In order to determine if there was an adaptive immune role of A2BR signaling in the development of BO, infiltration of CD3+ T cells was assessed via immunohistochemistry in these two groups. As a result, few CD3+ T cells were detected within any allografts during the first 3 days after transplantation. The number of infiltrating CD3+ T cells was significantly increased in the A2BR KO recipients compared to controls on days 7 (P=0.0014), but significantly decreased on day 12 (P=0.029). These results (Figure 4) indicate that adenosine signaling via the A2BR alters CD3+ T cell infiltration, which may be critical for developing BO.
Figure 4. Immunohistochemical staining of CD3+ T-cells in the allografts of Balb/c to A2BR KO and Balb/c to C57BL/6.
A) Representative pictures of CD3+ T-cell staining in allografts at days 7 and 12 post-transplantation. Cells stained brown indicate CD3+ T-cell infiltration. All sections were counterstained lightly with hematoxylin for viewing negatively stained cells. The slides were stained with goat anti-mouse CD3 antibody. All pictures are 40X magnification. B) The bar graph shows the analysis of positive immunostaining of CD3+ T-cell in the allografts from day 3 to day 21.
Identification of CD4+/CD25+/Foxp3+ regulatory T cells in the allografts
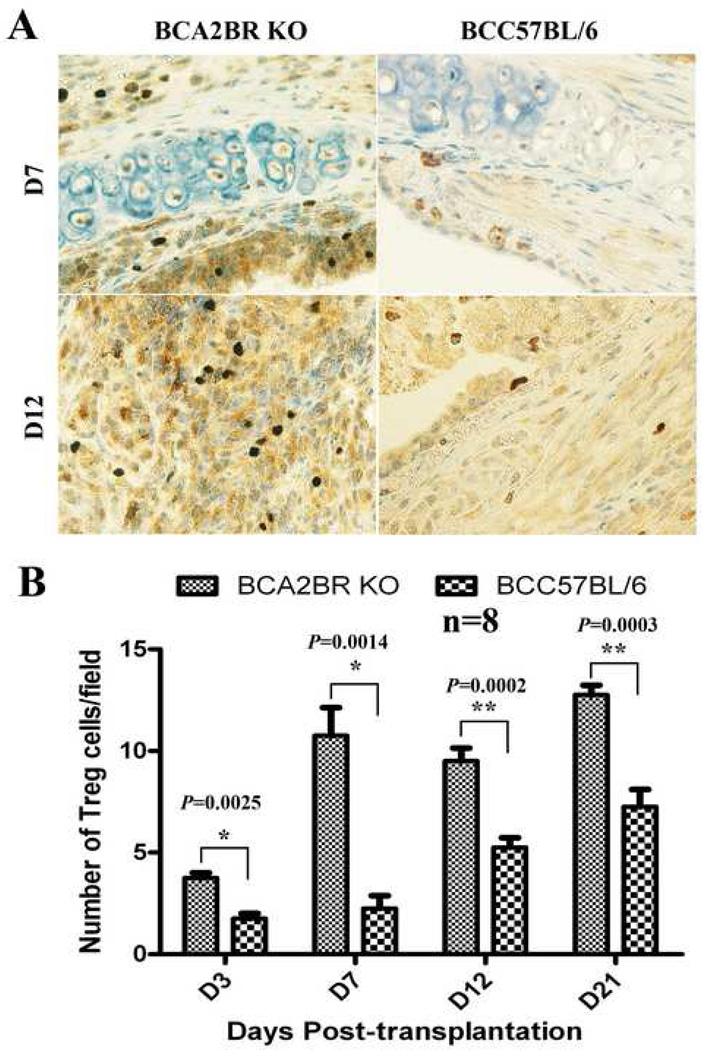
Among T cells, natural regulatory CD4+/CD25+/Foxp3+T cells are known to play an important role in maintaining immunological tolerance to both self and to non-self antigens (14, 15). To further identify regulatory T cell populations in the allografts, immunohistochemical staining of Foxp3 was employed using allografts at various time points. The results (Figure 5) showed that depletion of A2BR promoted CD3+/CD25+/Foxp3+ regulatory T cell infiltration into the allografts at days 3 (P=0.0025), 7 (P=0.0014), 12 (P=0.0002) and 21 (P=0.0003) compared to C57BL/6 recipients. These results suggest that endogenous adenosine signaling through the A2BR facilitates chronic allograft rejection via suppression of Foxp3+ regulatory T cell infiltration following transplantation.
Figure 5. Immunohistochemical staining of Foxp3+/CD4+/CD25+ regulatory T-cells in the allografts of Balb/c to A2BR KO and Balb/c to C57BL/6.
A) representative pictures of Foxp3+/CD4+/CD25+ regulatory T-cell staining in allografts at days 7 and 12 post-transplantation. Cells stained dark-brown indicate Foxp3+/CD4+/CD25+ regulatory T-cell infiltration. All sections were counterstained lightly with hematoxylin for viewing negatively stained cells. The slides were stained with polyclonal rabbit anti-mouse Foxp3 antibody. All pictures are 40X magnification. B) The bar graph shows the analysis of positive immunostaining of Foxp3+/CD4+/CD25+ regulatory T-cells in the allografts from day 3 to day 21.
Expression of CD31 in the allografts
CD31 is generally used in the evaluation of angiogenesis (16). Accumulated evidence suggests that CD31 is a key participant in the adhesion cascade leading to extravasation of leukocytes during the inflammatory process (17–19). CD31 antibodies inhibit leukocyte migration across an endothelial cell monolayer in vitro (20) as well as in-vivo(21). CD31 may also be involved in activation of the T-cell receptor (18). Therefore, we examined allograft vascularization by comparing the density of CD31 staining among allografts of the two groups at different time points (Figure 6). CD31 immunostaining demonstrated that the density of CD31 expression was significantly increased in the allografts of A2BR knockout recipients compared to control recipients on days 3 (P=0.0069), 7 (P=0.0011) and 12 (P=0.0211). However, no significant difference was observed on day 21 (P=0.3589). These results suggest that the increase of macrophage, CD3+ T cell and regulatory T cell infiltration maybe caused increased CD31 expression and density of vascularity in the allografts of A2BR knockout recipients.
Figure 6. Immunohistochemical staining of CD31 in the allografts of Balb/c to A2BR KO and Balb/c to C57BL/6.
A) representative pictures of CD31 staining in allografts at days 7 and 12 post-transplantation. Brown (black arrows) indicates CD31 positive staining. All sections were counterstained lightly with hematoxylin for viewing negatively stained cells. The slides were stained with goat anti-mouse CD31 antibody. The magnifications of all pictures were 10X. B) The bar graph shows the analysis of positive immunostaining of CD31 in the allografts from day 3 to day 21.
Luminal Obliteration
Minimum luminal fibro-obliteration was observed at 12-days but increased thereafter (Figures 1&7). There was no significant difference in luminal fibro-obliteration in the A2BR KO recipients compared to controls by 12 days (Figure 1). However, the fibro-obliteration was decreased (P=0.0426) in the A2BR KO recipients compared to controls by 21 days (Figure 7). These results suggest that adenosine signaling via the A2BR contributes to the development of BO.
Figure 7. Luminal obliteration in the transplanted tracheas from days 3 to 21.
Data shown are the mean ± S.D.
DISCUSSION
In this study, we have demonstrated that endogenous signaling via the A2BR results in enhanced development of bronchiolitis obliterans following murine tracheal allotransplantation. The heterotopic tracheal transplantation model used in the current study displays several key features that are similar to human BO. In our experimental model, the allografts in A2BR KO mice demonstrated delayed epithelial loss; alterations of CD3+ T cells, CD4+/CD25+/Foxp3+ regulatory T cells, and neutrophil infiltration; and delayed luminal fibro-obliteration compared to control allografts. As the A2BR subtype has been proven to be the predominant adenosine receptor expressed in human lung fibroblasts (22), in donor specimens of human bronchial epithelial cell (8) and in human bronchial epithelial cell lines(9), adenosine antagonism at the A2BR may be a potential therapeutic target for prevention or treatment of BO post lung transplantation.
Our experimental results implicate an important interaction between A2BR activation and CD4+/CD25+/Foxp3+ regulatory T cell activity during BO development. Accumulated evidence suggests that the CD4+/CD25+/Foxp3+ regulatory T cell population is actively involved in the negative control of a variety of physiological and pathological immune responses, and that this cell population can be exploited for the induction of immunological tolerance to non-self antigens, including organ transplantation (14). Furthermore, regulatory T cells are defined by expression of the forkhead family transcription factor (Foxp3), which is found in CD4+/CD25+ peripheral T cells and CD4+/CD25+/CD8-thymocytes (23, 24) and is essential for the development and function of regulatory T cells (25). Our results reveal that Foxp3 expressing CD4+/CD25+/ T-regulatory cells were markedly increased in the allografts in the A2BR KO recipients when compared with control mice at day 3, 7, 12, and 21. In addition, the depletion of A2BR (A2BR KO) significantly increased CD3+ T cell infiltration in the allografts on day 7; however, it prevented CD3+ lymphocyte infiltration into the trachea allografts at day 12 compared with the control group. As a result, it appears that endogenous adenosine promotes the rejection of transplanted trachea at least in part through the binding and activation of the A2BR which in turn inhibits CD4+/CD25+/Foxp3+ regulatory T cell infiltration in the allografts.
Another possible way that A2BR depletion inhibits BO development is through an attenuated IRI as evidenced by decreased neutrophil infiltration on days 3 and 21. Inflammation in general may be decreased in these recipients as we have shown neutrophil infiltration to be decreased on day 21 as well. Reports have shown that lung IRI is associated with an increased risk of developing BO, and this risk is directly related to the severity of acute primary graft dysfunction after lung transplantation (26, 27). Additionally, our experimental results are consistent with previous studies that have shown that synthesized A2BR-selective antagonist CVT-6694 attenuated Adenosine-5'-N-ethyluronamide (NECA) induced interleukin (IL)-6 and monocyte chemotactic protein-1 (MCP-1) in bronchoalveolar lavage smooth muscle cells (9). Collectively, these results suggest a novel mechanism whereby adenosine functions as a proinflammatory mediator in the bronchiole airways. Similarly, the A2BR subtype is the predominant adenosine receptor expressed in human lung fibroblasts, which upon activation by NECA increases the release of IL-6 and induces the differentiation of fibroblast into myofibroblasts (22). Several other reports have corroborated the anti-inflammatory effects of A2BR antagonism. In mice, A2BR-selective antagonism with CVT-6883 significantly reduced AMP-induced airway reactivity (28) and bleomycin-induced pulmonary fibrosis and inflammation (10), and the antagonist MRE-2029-F20 has been shown to inhibit the levels of cAMP in neutrophils and lymphocytes (29).
Leukocyte infiltration and transmigration is a hallmark inflammatory event in the early and intermediate stages of BO development. This process is regulated at multiple levels by the expression and activation of different molecules, including platelet endothelial cell adhesion molecule-1 (PECAM-1 or CD31)(18, 19). In the current study, we have demonstrated that depletion of the A2BR induced expression of CD31 may lead to increase macrophage, CD3+ T cell and CD4+/CD25+/Foxp3+ regulatory T cells infiltration in allografts on day 7 post-transplantation.
Despite our significant results, there are some limitations with the use of the heterotopic tracheal model. Although our experimental model is well described, technically simple, and reproducible in its production of obliterative airway disease, we have described some of its limitations in previous studies (7). A recently reported novel murine model for BO (30) may prove additionally valuable to investigate the role of A2BR in BO development.
Conclusion
Based upon our experimental results, we conclude that depletion of A2BR in mice confers delayed development of bronchiolitis obliterans compared to control mice and altered T-lymphocyte infiltration with increased T-regulatory cells migration in tracheal allografts. Manipulation of the A2BR with a selective synthetic antagonist (31) may provide a therapeutic option for the prevention and treatment of bronchiolitis obliterans.
Acknowledgements
This study was supported by Award Number T32HL007849 (DJL) from the National Heart, Lung, And Blood Institute and the Thoracic Surgery Foundation for Research and Education Research Grant (GA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. CLL is supported by a grant sponsored by the National Heart, Lung, and Blood Institute (1K08HL094704-01), the CVRC Partner’s Grant, and was the AATS John W. Kirklin Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Poster Session of the 46th Annual Meeting of The Society of Thoracic Surgeons, Fort Lauderdale, Florida, January 25–27, 2010.
REFERENCES
- 1.Granton J. Update of early respiratory failure in the lung transplant recipient. Current Opinion in Critical Care. 2006;12(1):19–24. doi: 10.1097/01.ccx.0000198995.44943.63. [DOI] [PubMed] [Google Scholar]
- 2.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death & Differentiation. 2007;14(7):1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 3.Lappas CM, Day Y-J, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. Journal of Experimental Medicine. 2006;203(12):2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Huang L, Sung S-sJ, Lobo PI, Brown MG, Gregg RK, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. Journal of Immunology. 2007;178(9):5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Day Y-J, Toufektsian M-C, Xu Y, Ramos SI, Marshall MA, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114(19):2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 6.Gazoni LM, Laubach VE, Mulloy DP, Bellizzi A, Unger EB, Linden J, et al. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. Journal of Thoracic & Cardiovascular Surgery. 2008;135(1):156–165. doi: 10.1016/j.jtcvs.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Lau CL, Zhao Y, Kron IL, Stoler MH, Laubach VE, Ailawadi G, et al. The role of adenosine A2A receptor signaling in bronchiolitis obliterans. Annals of Thoracic Surgery. 2009;88(4):1071–1078. doi: 10.1016/j.athoracsur.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollins BM, Burn M, Coakley RD, Chambers LA, Hirsh AJ, Clunes MT, et al. A2B adenosine receptors regulate the mucus clearance component of the lung's innate defense system. American Journal of Respiratory Cell & Molecular Biology. 2008;39(2):190–197. doi: 10.1165/rcmb.2007-0450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong H, Wu Y, Belardinelli L, Zeng D. A2B adenosine receptors induce IL-19 from bronchial epithelial cells, resulting in TNF-alpha increase. American Journal of Respiratory Cell & Molecular Biology. 2006;35(5):587–592. doi: 10.1165/rcmb.2005-0476OC. [DOI] [PubMed] [Google Scholar]
- 10.Sun C-X, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, et al. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. Journal of Clinical Investigation. 2006;116(8):2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. Journal of Clinical Investigation. 2008;118(10):3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Mohsenin A, Morschl E, Young HWJ, Molina JG, Ma W, et al. Enhanced airway inflammation and remodeling in adenosine deaminase-deficient mice lacking the A2B adenosine receptor. Journal of Immunology. 2009;182(12):8037–8046. doi: 10.4049/jimmunol.0900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau CL, Zhao Y, Kim J, Kron IL, Sharma A, Yang Z, et al. Enhanced fibrinolysis protects against lung ischemia-reperfusion injury. Journal of Thoracic & Cardiovascular Surgery. 2009;137(5):1241–1248. doi: 10.1016/j.jtcvs.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature Immunology. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Greene MI. Special regulatory T-cell review: FOXP3 biochemistry in regulatory T cells--how diverse signals regulate suppression. Immunology. 2008;123(1):17–19. doi: 10.1111/j.1365-2567.2007.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aroca F, Renaud W, Bartoli C, Bouvier-Labit C, Figarella-Branger D. Expression of PECAM-1/CD31 isoforms in human brain gliomas. Journal of Neuro-Oncology. 1999;43(1):19–25. doi: 10.1023/a:1006233816724. [DOI] [PubMed] [Google Scholar]
- 17.Woodfin A, Voisin M-B, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 2009;113(24):6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodfin A, Voisin M-B, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arteriosclerosis, Thrombosis & Vascular Biology. 2007;27(12):2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 19.Newman PJ. The biology of PECAM-1. Journal of Clinical Investigation. 1997;100(11) Suppl:S25–S29. [PubMed] [Google Scholar]
- 20.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. Journal of Experimental Medicine. 1993;178(2):449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, et al. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262(5139):1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 22.Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, et al. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension. 2004;44(5):649–654. doi: 10.1161/01.HYP.0000144800.21037.a5. [DOI] [PubMed] [Google Scholar]
- 23.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 25.Khattri R, Cox T, Yasayko S-A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunology. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 26.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. American Journal of Respiratory & Critical Care Medicine. 2007;175(5):507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 27.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Annals of Thoracic Surgery. 2002;73(4):1041–1047. doi: 10.1016/s0003-4975(01)03606-2. discussion 1047–1048. [DOI] [PubMed] [Google Scholar]
- 28.Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D. Effect of a specific and selective A(2B) adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. Journal of Pharmacology & Experimental Therapeutics. 2007;320(3):1246–1251. doi: 10.1124/jpet.106.112250. [DOI] [PubMed] [Google Scholar]
- 29.Gessi S, Varani K, Merighi S, Cattabriga E, Pancaldi C, Szabadkai Y, et al. Expression, pharmacological profile, and functional coupling of A2B receptors in a recombinant system and in peripheral blood cells using a novel selective antagonist radioligand, [3H]MRE 2029-F20. Molecular Pharmacology. 2005;67(6):2137–2147. doi: 10.1124/mol.104.009225. [DOI] [PubMed] [Google Scholar]
- 30.Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A new murine model for bronchiolitis obliterans post-bone marrow transplant. American Journal of Respiratory & Critical Care Medicine. 2007;176(7):713–723. doi: 10.1164/rccm.200702-335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalla RV, Zablocki J, Tabrizi MA, Baraldi PG. Recent developments in A2B adenosine receptor ligands. Handbook of Experimental Pharmacology. 2009;193:99–122. doi: 10.1007/978-3-540-89615-9_4. [DOI] [PubMed] [Google Scholar]