Abstract
K562 mammalian cells are sorted using a highly integrated microfabricated fluorescence-activated cell sorter (μFACS). The sample cells are purified with an enrichment factor of 230 at a high throughput (>1,000 cells/sec)
The fluorescence-activated cell-sorter (FACS) is an essential bio-analysis tool that characterizes biochemical properties of biological samples such as cells by optically interrogating in a quantitative manner and sort out pure cells of interest out of various kinds of cell mixtures [1]. Commercial FACS systems are capable of detecting and sorting bio-specimen suspended in a fluid at a high throughput (> 100,000 cells/sec). However, such commercial bench top FACS systems are still bulky, heavy and expensive, and are operated by highly skilled personnel, thus preventing them from being widely used in biomedical research laboratories.
Microfluidics based lab-on-a-chip platform has been explored as an alternative, since it allows inexpensive and portable devices where many other functionalities such as optics, acoustics can be integrated together working synergistically. Significant progress has been made toward developing microfabricated FACS (μFACS) based on various principles [2], and few approaches have so far proved the performance to meet the requirements for real biomedical or clinical applications. The high screening throughput and the high purity enrichment are essential as screening of a large number of cells (> 1 million) is required in order to identify rare cells or to generate meaningful clinical results out of small volume of samples.
We demonstrate a high performance μFACS system in which microfluidics, optics and acoustics are all integrated on a single lab-on-a-chip platform in conjunction with the innovative space-time coding signal processing algorithm and the real-time control electronics system. Micro-beads and human erythroleukemic cells are sorted by the μFACS with the enrichment factor of 230 fold at a high throughput upto 1,500 cells/sec.
The μFACS device made of PDMS has the 250 μm wide main channel followed by a sorting junction where the channel is split into three 80 μm wide sub channels (Fig 1(a)). The left and right channels are for collecting samples and the center channel is for waste collection. The piezoelectric PZT actuator is mounted directly to the the actuation chamber at the sorting junction. As it bends up or down according to the applied voltage, the fluid flow and the suspended cells are deflected to one of the collecting channels. When untargeted cells are present, the PZT is not triggered by the control system, and those cells travel through the center waste channel. The integrated PZT actuator deflects sub-nanoliters per stroke within less than 0.1 ms and minimizes the fluid disturbance, making it possible manipulating a single suspended cell. The integrated PZT actuator requires low voltage (<10V) and consumes low power (~1mW), which makes it a good candidate for portable point-of-care diagnostic applications.
Fig. 1.
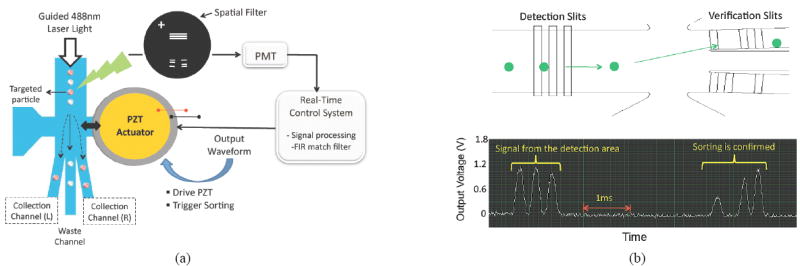
(a) Schematic of the μFACS system. As the PZT actuator bends up or down according to the output voltage waveform, the targeted particle (pink) is deflected to the collection channel. (b) Spatial filter overlapped with the image of the micro sorter (Top). Example of space-time coded signals for detection and sorting verification, which are coded differently (111 vs. 1011) due to the different slit design (Bottom).
The optofluidic waveguide is formed by coating amorphous Teflon (Teflon AF; DuPont, USA) on to the microfluidics channel walls [3]. Teflon AF is transparent and has a refractive index (n=1.31) lower than water (n=1.33), and its thin layer forms a low refractive index cladding layer, while water carrying biological samples through the same channel becomes a core of the waveguide. The Teflon AF coated waveguide enables multi-point detection critical to implementing the space-time coding algorithm, as light always traces cells in the channel. It also prevents cells from clogging the PDMS channel, as Teflon AF is chemically inert like other Teflon materials.
The spatial filter is placed in front of the PMT, and that it is overlapped with the magnified image of the device blocking all the light except for the light passing through the transparent slits as shown in Figure 1 (a) and (b). The space-time coded detection signal is followed by the differently coded sorting verification signal in the bottom of Figure 1 (b). As a result of the implementation of the spatial filter, sorting parameters to drive the PZT actuator such as the threshold voltage or time delay can be monitored and optimized in real-time during the experiment. The optimization process would result in the appearance of sorting verification signals after detection signals since this tells us targeted beads are detected and sorted successfully. The real time control system is built with the embedded field programmable gate array (FPGA) chip [4]. The timing jitter of the control system critical to the sorting accuracy is less than 10 μs that is shorter than cells’ travel time from being detected to being sorted.
In order to test the performance and to optimize the parameters of the μFACS system, the bead mixture with the initial population ratio between fluorescent 10μm and non-fluorescent 5μm beads of 1:160 is introduced to the device. After continuous automated sorting about 30 mins, the sorting result shows ~200 fold enrichment at a high throughput of 1,500 beads/sec as shown in Figure 2. Biological feasibility of the integrated micro-sorter is demonstrated using human erythroleukemic cells (K562). Under similar flow condition (~5-10 cm/sec), sample concentration (~1.1 ×107 cells/ml), and initial mixture ratio (1:150), the targeted cells are purified with an enrichment factor of ~230 fold at a high throughput of 1,000 cells/sec. Even though the cell concentration used is high, no significant cell sticking or channel clogging is observed due to the chemical inertness of the Teflon AF coating. The μFACS can sustain continuous operation for long periods of time (>2 hours), which is essential for purifying sufficient number of rare cells (<0.01% population) such as circulating tumor cells in blood.
Fig. 2.
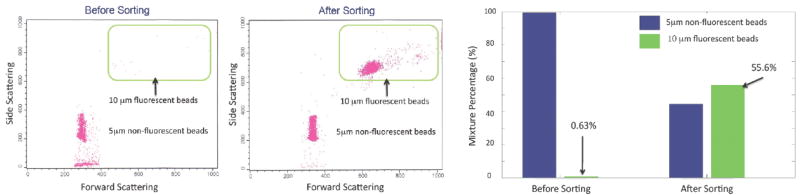
The flow cytometry scattering plots (Left and Middle) show the result of sorting fluorescent 10 μm beads from non-fluorescent 5 μm beads. The population of 10 μm fluorescent beads is enriched after sorting (Middle). The histogram (Right) shows the initial population ratio of the two beads is 1:160. After sorting, the ratio between 10 μm and 5 μm beads becomes 1.25:1, resulting in an enrichment factor of around 200.
In summary, we have demonstrated a lab-on-a-chip cell sorter with an integrated PZT actuator and the optofluidic waveguide in conjunction with space-time coding signal processing algorithm and real-time control electronics system. Polystyrene beads and human mammalian cells (K562) are purified with an enrichment factor about 200 fold at a high throughput. This holds promise for high-performance μFACS system for point-of-care applications.
Acknowledgments
This work was supported by the NIH grant under xxxxx. The authors acknowledge the technical support of the staff of the Nano3 (Nanoscience, Nanoengineering, and Nanomedicine) in Calit2 in UCSD.
References
- 1.Shapiro . Practical Flow Cytometery. 2 [Google Scholar]
- 2.Godin J, Chen CH, Cho SH, Tsai FS, Qiao W, Lo Y-H. Microfluidics and photonics for Bio-System-on-a-Chip: A review of advancements in technology towards a microfluidic flow cytometry chip. J Biophoton. 2008;1(5):355–376. doi: 10.1002/jbio.200810018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho SH, Godin J, Lo Y-H. Opfofluidic waveguides in Teflon AF-coated PDMS microfluidics channels. IEEE Photonic Tech Let. 2009;21(15):1057–1059. doi: 10.1109/LPT.2009.2022276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CH, Cho SH, Tsai FS, Erten A, Lo Y-H. Microfluidic cell sorter with integrated piezoelectric actuator. Biomed Microdevices. 2009;11(6):1223–1231. doi: 10.1007/s10544-009-9341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


